Design and Fabrication Challenges of a Highly Sensitive Thermoelectric-Based Hydrogen Gas Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Design of the Sensor
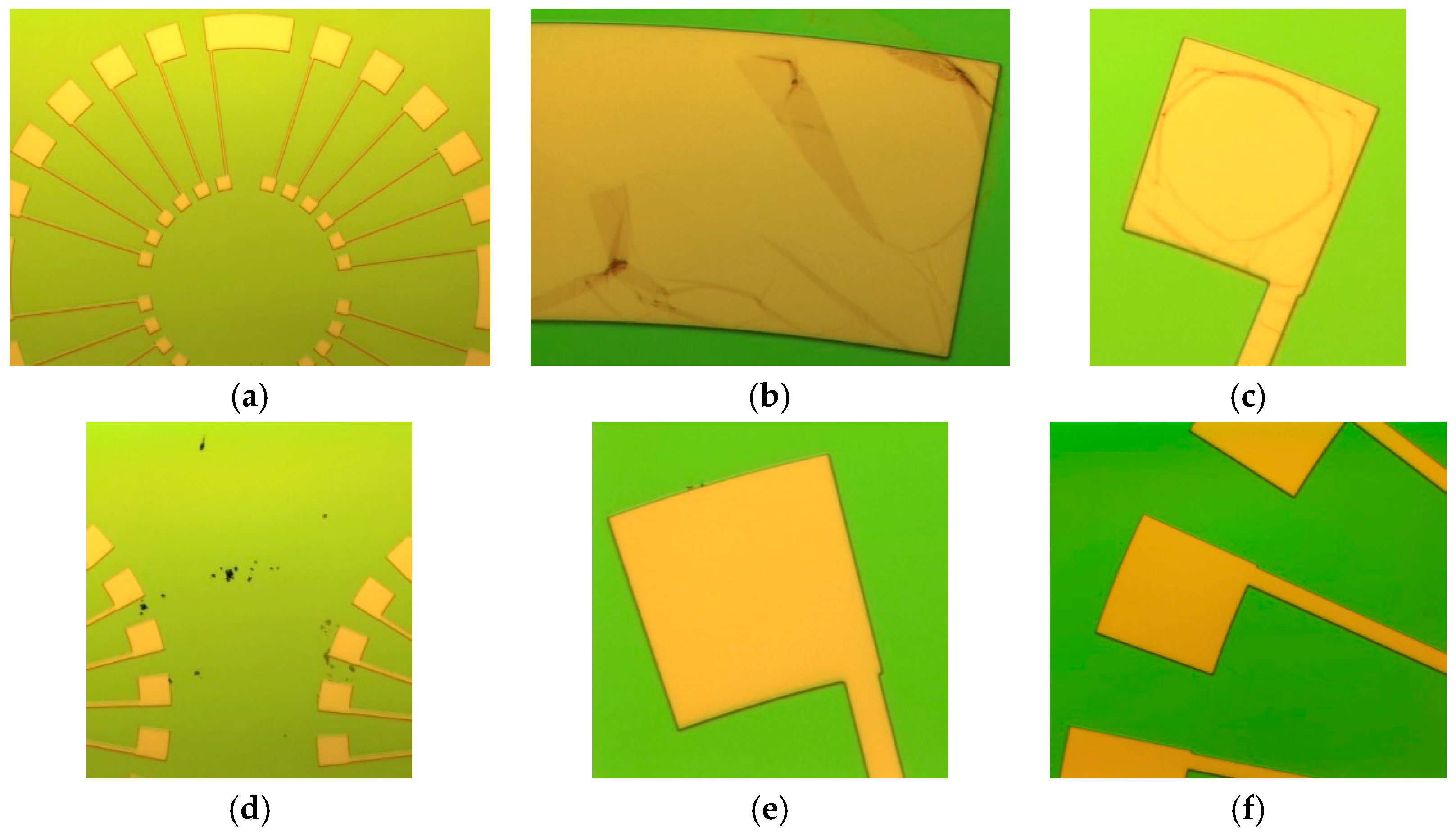
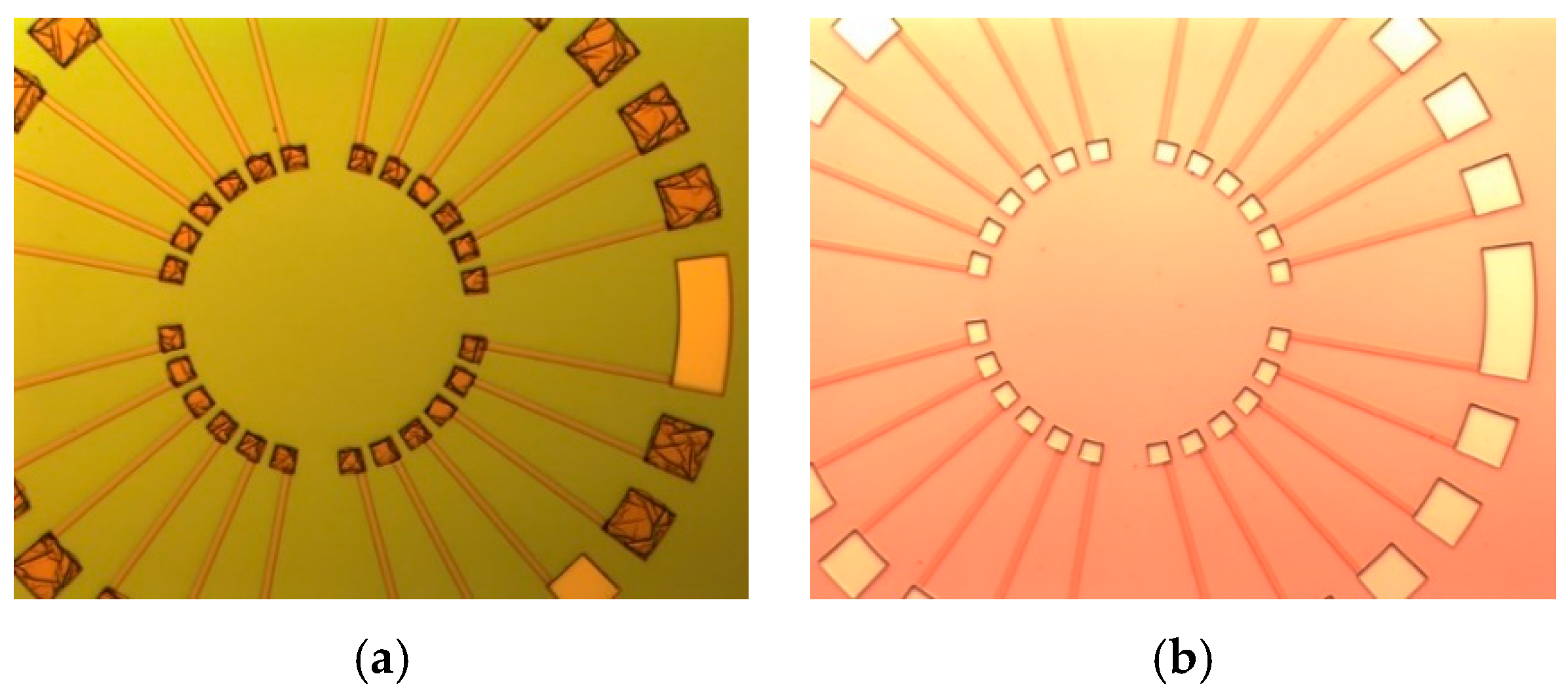
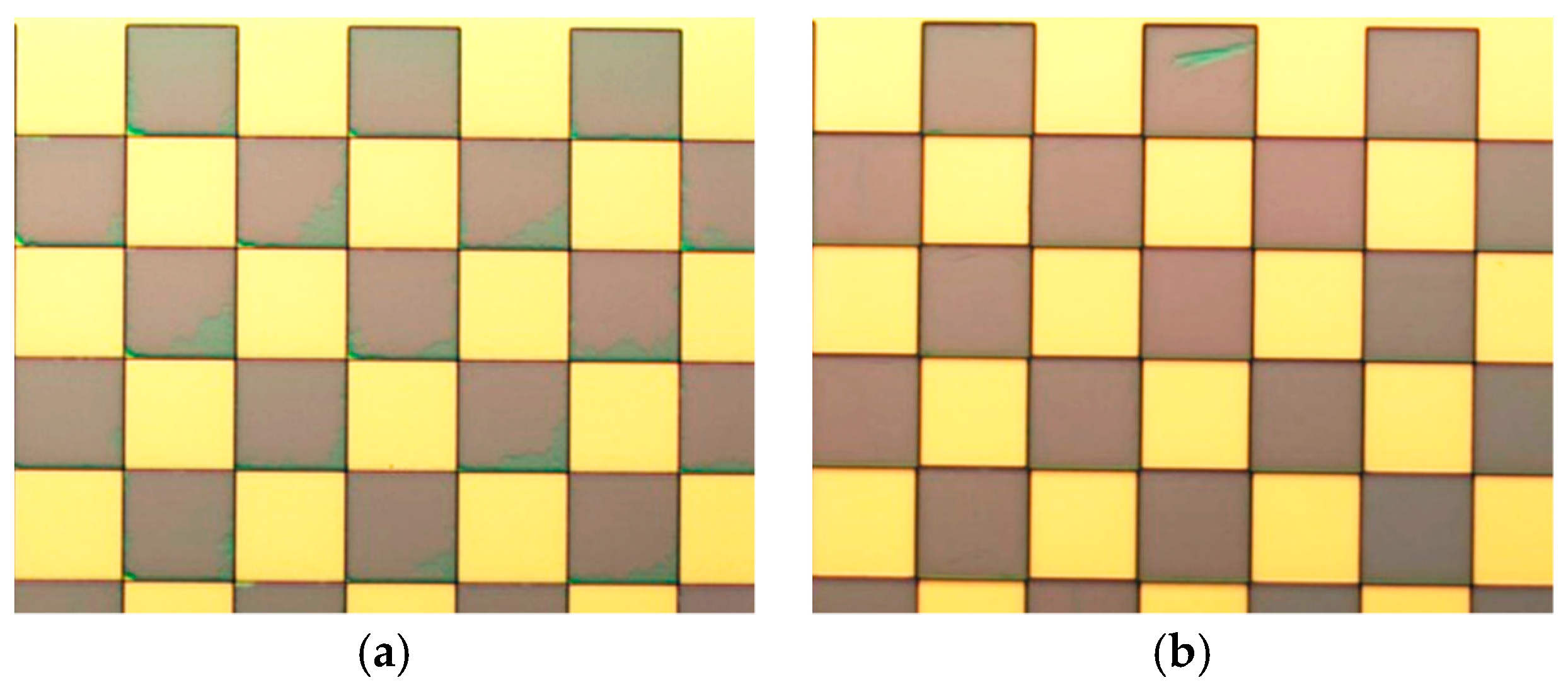
2.2. Fabrication Challenges of the Sensor and Solutions
2.3. Measurement Processes
3. Results and Discussion
3.1. Design Criteria
3.2. Temperature Distribution on the Membrane
3.3. Thermal Characteristic of the Sensor and the Response in Hydrogen Gas
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tajima, K.; Qiu, F.; Shin, W.; Izu, N.; Matsubara, I.; Murayama, N. Micromechanical fabrication of low-power thermoelectric hydrogen sensor. Sens. Actuators B 2005, 108, 973–978. [Google Scholar] [CrossRef]
- Samaeifar, F.; Afifi, A.; Abdollahi, H. Simple Fabrication and Characterization of a Platinum Microhotplate Based on Suspended Membrane Structure. Exp. Tech. 2016, 40, 755–763. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in gas sensor technology for hydrogen safety. Int. J. Hydrog. Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, G.; Wang, Y.; Yao, J. Thermal Performance of Micro Hotplates with Novel Shapes Based on Single-Layer SiO2 Suspended Film. Micromachines 2018, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Harley-Trochimczyk, A.; Rao, A.; Long, H.; Zettl, A.; Carraro, C.; Maboudian, R. Low-power catalytic gas sensing using highly stable silicon carbide microheaters. J. Micromech. Microeng. 2017, 27, 045003. [Google Scholar] [CrossRef]
- Monika, D.; Arora, A. Design and Simulation of MEMS based Microhotplate as Gas Sensor. Int. J. Adv. Res. Comput. Eng. Technol. 2013, 2, 2487–2492. [Google Scholar]
- Behera, B.; Chandra, S. A MEMS based acetone sensor incorporating ZnO nanowires synthesized by wet oxidation of Zn film. J. Micromech. Microeng. 2014, 25, 015007. [Google Scholar] [CrossRef]
- Matsumiya, M.; Shin, W.; Qiu, F.; Izu, N.; Matsubara, I.; Murayama, N. Poisoning of platinum thin film catalyst by hexamethyldisiloxane (HMDS) for thermoelectric hydrogen gas sensor. Sens. Actuators B 2003, 96, 516–522. [Google Scholar] [CrossRef]
- Nagai, D.; Nishibori, M.; Itoh, T.; Kawabe, T.; Sato, K.; Shin, W. Ppm level methane detection using micro-thermoelectric gas sensors with Pd/Al2O3 combustion catalyst films. Sens. Actuators B 2015, 206, 488–494. [Google Scholar] [CrossRef]
- Herwaarden, A.W.V.; Sarro, P.M. Thermal Sensors based on The Seebeck effect. Sens. Actuators 1986, 10, 321–346. [Google Scholar] [CrossRef]
- Yoo, B.Y.; Huang, C.-K.; Lim, J.R.; Herman, J.; Ryan, M.A.; Fleurial, J.-P.; Myung, N.V. Electrochemically deposited thermoelectric n-type Bi2Te3 thin films. Electrochim. Acta 2005, 50, 4371–4377. [Google Scholar] [CrossRef]
- Goldsmid, H. Bismuth Telluride and Its Alloys as Materials for Thermoelectric Generation. Materials 2014, 7, 2577–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fardindoost, S.; Iraji zad, A.; Rahimi, F.; Ghasempour, R. Pd doped WO3 films prepared by sol–gel process for hydrogen sensing. Int. J. Hydrog. Energy 2010, 35, 854–860. [Google Scholar] [CrossRef]
- Lichtenwalner, D.J.; Hydrick, A.E.; Kingon, A.I. Flexible thin film temperature and strain sensor array utilizing a novel sensing concept. Sens. Actuators A 2007, 135, 593–597. [Google Scholar] [CrossRef]
- Tajima, K.; Qiu, F.; Shin, W.; Sawaguchi, N.; Izu, N.; Matsubara, I.; Murayama, N. Thermoelectric Properties of RF-Sputtered SiGe Thin Film for Hydrogen Gas Sensor. Jpn. J. Appl. Phys. 2004, 43, 5978–5983. [Google Scholar] [CrossRef]
- Shin, W.; Nishibori, M.; Matsubara, I. Thermoelectric hydrogen gas sensor. Synthesiology 2011, 4, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Strasser, M.; Aigner, R.; Franosch, M.; Wachutka, G. Miniaturized thermoelectric generators based on poly-Si and poly-SiGe surface micromachining. Sens. Actuators A 2002, 97, 535–542. [Google Scholar] [CrossRef]
- Takashiri, M.; Shirakawa, T.; Miyazaki, K.; Tsukamoto, H. Fabrication and characterization of bismuth–telluride-based alloy thin film thermoelectric generators by flash evaporation method. Sens. Actuators A 2007, 138, 329–334. [Google Scholar] [CrossRef]
- Matsumiya, M.; Shin, W.; Izu, N.; Murayama, N. Nano-structured thin-film Pt catalyst for thermoelectric hydrogen gas sensor. Sens. Actuators B 2003, 93, 309–315. [Google Scholar] [CrossRef]
- Nagai, D.; Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. Thermal Balance Analysis of a Micro-Thermoelectric Gas Sensor Using Catalytic Combustion of Hydrogen. Sensors 2014, 14, 1822–1834. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.; Matsumiya, M.; Qiu, F.; Izu, N.; Murayama, N. Thermoelectric gas sensor for detection of high hydrogen concentration. Sens. Actuators B 2004, 97, 344–347. [Google Scholar] [CrossRef]
- Goto, T.; Itoh, T.; Akamatsu, T.; Izu, N.; Shin, W. CO sensing properties of Au/SnO2–Co3O4 catalysts on a micro thermoelectric gas sensor. Sens. Actuators B 2016, 223, 774–783. [Google Scholar] [CrossRef]
- Nishibori, M.; Shin, W.; Houlet, L.F.; Tajima, K.; Itoh, T.; Izu, N.; Murayama, N.; Matsubara, I. New Structural Design of Micro-Thermoelectric Sensor for Wide Range Hydrogen Detection. J. Ceram. Soc. Jpn. 2006, 114, 853–856. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Kim, J.-S. MEMS based highly sensitive dual FET gas sensor using graphene decorated Pd-Ag alloy nanoparticles for H2 detection. Sci. Rep. 2018, 8, 5902. [Google Scholar] [CrossRef]
- Photolithography Procedure. Available online: http://louisville.edu/micronano/files/documents/standard-operating-procedures/PhotolithographyProcess.pdf (accessed on 20 May 2019).
- Mack, C. The Basics of Microlithography. Available online: http://www.lithoguru.com/scientist/lithobasics.html (accessed on 20 May 2019).
- Wu, H.; Cargo, J.; Serpiello, J.; Mcginn, J. Characterization of Reactive Ion Etching of Poly Silicon over Gate Oxide for Failure Mode Analysis Deprocessing. In Proceedings of the 9th IPFA, Singapore, 8–12 July 2002; pp. 91–96. [Google Scholar]
- Seo, H.; Kim, S.B.; Song, J.; Kim, Y.; Soh, H.; Kim, Y.C.; Jeon, H. Low temperature remote plasma cleaning of the fluorocarbon and polymerized residues formed during contact hole dry etching. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2002, 20, 1548. [Google Scholar] [CrossRef]
- Mao, H.; Wu, D.; Wu, W.; Xu, J.; Hao, Y. The fabrication of diversiform nanostructure forests based on residue nanomasks synthesized by oxygen plasma removal of photoresist. Nanotechnology 2009, 20, 445304. [Google Scholar] [CrossRef]
- Pranti, A.; Loof, D.; Kunz, S.; Zielasek, V.; Bäumer, M.; Lang, W. Ligand-Linked Nanoparticles-Based Hydrogen Gas Sensor with Excellent Homogeneous Temperature Field and a Comparative Stability Evaluation of Different Ligand-Linked Catalysts. Sensors 2019, 19, 1205. [Google Scholar] [CrossRef]
- Shin, W.; Nishibori, M.; Houlet, L.F.; Itoh, T.; Izu, N.; Matsubara, I. Fabrication of thermoelectric gas sensorson micro-hotplates. Sen. Actuators B 2009, 139, 340–345. [Google Scholar] [CrossRef]
- Matsumiya, M.; Qiu, F.; Shin, W.; Izu, N.; Murayama, N.; Kanzaki, S. Thin-film Li-doped NiO for thermoelectric hydrogen gas sensor. Thin Solid Film. 2002, 419, 213–217. [Google Scholar] [CrossRef]
- Foggiato, J. Chemical Vapor Deposition of Silicon Dioxide Films. In Handbook of Thin Film Deposition Processes and Techniques, 2nd ed.; Seshan, K., Ed.; Noyes publications/William Andrew Publications: New York, NY, USA, 2001; pp. 111–150. [Google Scholar] [CrossRef]
- TEOS Deposition. Available online: http://www.iue.tuwien.ac.at/phd/holzer/node96.html (accessed on 18 June 2019).
- Qiu, F.; Matsumiya, M.; Shin, W.; Izu, N.; Murayama, N. Investigation of thermoelectric hydrogen sensor based on SiGe film. Sens. Actuators B 2003, 94, 152–160. [Google Scholar] [CrossRef]
- US20040229762A1-Polymer Remover-Google Patents. Available online: https://patents.google.com/patent/US20040229762 (accessed on 18 June 2019).
- Kitis, M.; Akcil, A.; Karakaya, E.; Yigit, N.O. Destruction of cyanide by hydrogen peroxide in tailings slurries from low bearing sulphidic gold ores. Miner. Eng. 2005, 18, 353–362. [Google Scholar] [CrossRef]
- What Is the World’s Strongest Superacid? Available online: https://www.thoughtco.com/the-worlds-strongest-superacid-603639 (accessed on 20 April 2019).
- Shin, W.; Imai, K.; Izu, N.; Murayama, N. Thermoelectric Thick-Film Hydrogen Gas Sensor Operating at Room Temperature. Jpn. J. Appl. Phys. 2001, 40, L1232–L1234. [Google Scholar] [CrossRef]
- Buchner, R.; Sosna, C.; Maiwald, M.; Benecke, W.; Lang, W. A high-temperature thermopile fabrication process for thermal flow sensors. Sens. Actuators A 2006, 130, 262–266. [Google Scholar] [CrossRef]
- Lang, W. Heat transport from a chip. IEEE Trans. Electron Devices 1990, 37, 958–963. [Google Scholar] [CrossRef]
- Briand, D.; GrŽtillat, M.-A.; Van der Schoot, B.; de Rooij, N.F. Thermal Management of Micro-Hotplates using MEMCAD as Simulation Tool. In Proceedings of the International Conference on Modeling and Simulation of Microsystems-MSM, San Diego, CA, USA, 27–29 March 2000; pp. 640–643. [Google Scholar]
- Brauns, E.; Morsbach, E.; Kunz, S.; Bäumer, M.; Lang, W. A fast and sensitive catalytic gas sensors for hydrogen detection based on stabilized nanoparticles as catalytic layer. Sens. Actuators B 2014, 193, 895–903. [Google Scholar] [CrossRef]
- Huang, H.; Luan, W.; Zhang, J.; Qi, Y.; Tu, S. Thermoelectric hydrogen sensor working at room temperature prepared by bismuth–telluride P–N couples and Pt/-Al2O3. Sen. Actuators B 2008, 128, 581–585. [Google Scholar] [CrossRef]
- Park, S.; Yoon, S.; Lee, C.; Kim, Y.; Song, S. A micro-thermoelectric gas sensor for detection of hydrogen and atomic oxygen. Analyst 2009, 134, 236–242. [Google Scholar] [CrossRef]
- Houlet, L.; Shin, W.; Tajima, K.; Nishibori, M.; Izu, N.; Itoh, T.; Matsubara, I. Thermopile Sensor-Devices for the Catalytic Detection of Hydrogen Gas. Sens. Actuators B 2008, 130, 200–206. [Google Scholar] [CrossRef]












| Design Sample | Design Criteria |
|---|---|
| A1 | Main design (explained in Section 2.1) |
| A2 | Half width of the thermopile structures |
| A3 | Different heater design (round) |
| A4 | Higher number of thermocouples |
| A5 | Larger membrane |
| A6 | Smaller membrane |
| Sensor Type | A1 | A2 | A3 | A4 | A5 | A6 | A1 |
|---|---|---|---|---|---|---|---|
| R Thermopile at 27 °C (kΩ) | 194 | 438 | 210 | 212 | 192 | 196 | 194 |
| Output of one thermocouple/°C (µV/K) | 273 | 196 | 138 | 160 | 92.2 | 336 | 273 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pranti, A.S.; Loof, D.; Kunz, S.; Zielasek, V.; Bäumer, M.; Lang, W. Design and Fabrication Challenges of a Highly Sensitive Thermoelectric-Based Hydrogen Gas Sensor. Micromachines 2019, 10, 650. https://doi.org/10.3390/mi10100650
Pranti AS, Loof D, Kunz S, Zielasek V, Bäumer M, Lang W. Design and Fabrication Challenges of a Highly Sensitive Thermoelectric-Based Hydrogen Gas Sensor. Micromachines. 2019; 10(10):650. https://doi.org/10.3390/mi10100650
Chicago/Turabian StylePranti, Anmona Shabnam, Daniel Loof, Sebastian Kunz, Volkmar Zielasek, Marcus Bäumer, and Walter Lang. 2019. "Design and Fabrication Challenges of a Highly Sensitive Thermoelectric-Based Hydrogen Gas Sensor" Micromachines 10, no. 10: 650. https://doi.org/10.3390/mi10100650





