High Brightness Organic Light-Emitting Diodes with Capillary-Welded Hybrid Diameter Silver Nanowire/Graphene Layers as Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Silver Nanowire/Graphene Electrode
2.3. Organic Light-Emitting Diode (OLED) Fabrication
2.4. Film and Device Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Availability of Data and Materials
References
- Olle, I. Organic photovoltaics: Avoiding indium. Nat. Photonics 2011, 5, 201–202. [Google Scholar]
- Miao, J.L.; Liu, H.H.; Li, C.B.; Zhang, X.X. Graphene-based film reduced by a chemical and thermal synergy method as a transparent conductive electrode. Sci. Adv. Mater. 2016, 8, 1066–1073. [Google Scholar] [CrossRef]
- Yabuki, A.; Okumura, K.; Fathona, I.W. Transparent conductive coatings of hot-pressed ito nanoparticles on a plastic substrate. Chem. Eng. J. 2014, 252, 275–280. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, H.; Ji, T.; Huang, L.; Chen, Y. High conductive pedot via post-treatment by halobenzoic for high-efficiency ito-free and transporting layer-free organic solar cells. Org. Electron. 2016, 33, 316–323. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive pedot: PSS electrode with optimized solvent and thermal post-treatment for ito-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Reynaud, O.; Nasibulin, A.G.; Anisimov, A.S.; Anoshkin, I.V.; Jiang, H.; Kauppinen, E.I. Aerosol feeding of catalyst precursor for CNT synthesis and highly conductive and transparent film fabrication. Chem. Eng. J. 2014, 255, 134–140. [Google Scholar] [CrossRef]
- Rowell, M.W.; Topinka, M.A.; Mcgehee, M.D.; Prall, H.J.; Dennler, G.; Sariciftci, N.S.; Hu, L.; Gruner, G. Organic solar cells with carbon nanotube network electrodes. Appl. Phys. Lett. 2006, 88, 233506. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Lin, M.; Feng, T.; Qi, C.; Wang, Y.; Ye, F.; Lei, C.; Li, Y.; Dan, W.; Zheng, C. Ambient stable large-area flexible organic solar cells using silver grid hybrid with vapor phase polymerized poly(3,4-ethylenedioxythiophene) cathode. Sol. Energy Mater. Sol. Cells 2015, 143, 354–359. [Google Scholar]
- Oh, Y.; Lee, H.; Choi, D.Y.; Lee, S.U.; Kim, H.; Yoo, S.H.; Park, I.; Sung, H.J. High-performance, solution-processed, embedded multiscale metallic transparent conductors. ACS Appl. Mater. Interfaces 2016, 8, 10937. [Google Scholar] [CrossRef]
- Mo, L.; Ran, J.; Yang, L.; Fang, Y.; Zhai, Q.; Li, L. Flexible transparent conductive films combining flexographic printed silver grids with CNT coating. Nanotechnology 2016, 27, 065202. [Google Scholar] [CrossRef]
- Park, H.; Chang, S.; Zhou, X.; Kong, J.; Palacios, T.; Gradečak, S. Flexible graphene electrode-based organic photovoltaics with record-high efficiency. Nano Lett. 2015, 14, 5148–5154. [Google Scholar] [CrossRef]
- Arco, L.G.D.; Zhang, Y.; Schlenker, C.W.; Ryu, K.; Thompson, M.E.; Zhou, C. Continuous, highly flexible, and transparent graphene films by chemical vapor deposition for organic photovoltaics. Acs Nano 2010, 4, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- He, X.; He, R.; Liu, A.; Chen, X.; Zhao, Z.; Feng, S.; Chen, N.; Zhang, M. A highly conductive, flexible transparent composite electrode based on the lamination of silver nanowires and polyvinyl alcohol. J. Mater. Chem. C 2014, 2, 9737–9745. [Google Scholar] [CrossRef]
- Selzer, F.; Weiß, N.; Bormann, L.; Sachse, C.; Gaponik, N.; Müller-Meskamp, L.; Eychmüller, A.; Leo, K. Highly conductive silver nanowire networks by organic matrix assisted low-temperature fusing. Org. Electron. 2014, 15, 3818–3824. [Google Scholar] [CrossRef]
- Li, L.; Yu, Z.; Weili, H.; Chang, C.H.; Chen, Q.; Pei, Q. Efficient flexible phosphorescent polymer light-emitting diodes based on silver nanowire-polymer composite electrode. Adv. Mater. 2011, 23, 5563–5567. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Suh, M.; Kim, D.; Lee, D.; Chang, H.; Lee, H.S.; Kim, Y.W.; Kim, T.Y.; Suh, K.S.; Jeon, D.Y. Improved operational stability of polymer light-emitting diodes based on silver nanowire electrode through pre-bias conditioning treatment. Adv. Funct. Mater. 2015, 24, 6465–6472. [Google Scholar] [CrossRef]
- Song, C.H.; Ok, K.H.; Lee, C.J.; Kim, Y.; Kwak, M.G.; Han, C.J.; Kim, N.; Ju, B.K.; Kim, J.W. Intense-pulsed-light irradiation of Ag nanowire-based transparent electrodes for use in flexible organic light emitting diodes. Org. Electron. 2015, 17, 208–215. [Google Scholar] [CrossRef]
- Song, T.B.; Chen, Y.; Chung, C.H.; Yang, Y.(M.); Bob, B.; Duan, H.S.; Li, G.; Tu, K.N.; Huang, Y.; Yang, Y. Nanoscale joule heating and electromigration enhanced ripening of silver nanowire contacts. ACS Nano 2014, 8, 2804–2811. [Google Scholar] [CrossRef]
- Miao, J.; Chen, S.; Liu, H.; Zhang, X. Low-temperature nanowelding adherent ultrathin silver nanowire sandwiched between polydopamine-functionalized graphene and conjugated polymer for highly stable and flexible transparent electrodes. Chem. Eng. J. 2018, 345, 260–270. [Google Scholar] [CrossRef]
- Yoon, S.S.; Khang, D.Y. Room-temperature chemical welding and sintering of metallic nanostructures by capillary condensation. Nano Lett. 2016, 16, 3550–3556. [Google Scholar] [CrossRef]
- Meng, Y.; Lou, K.; Qi, R.; Guo, Z.; Shin, B.; Liu, G.; Shan, F. Nature-inspired capillary-driven welding process for boosting metal-oxide nanofiber electronics. ACS Appl. Mater. Interfaces 2018, 10, 20703–20711. [Google Scholar] [CrossRef]
- Zhang, Q.; Di, Y.; Huard, C.M.; Guo, L.J.; Wei, J.; Guo, J. High-stable and stretchable graphene-polymer processed silver nanowires hybrid electrodes for flexible displays. J. Mater. Chem. C 2015, 3, 1528–1536. [Google Scholar] [CrossRef]
- Seo, K.W.; Lee, J.H.; Cho, N.G.; Kang, S.J.; Kim, H.K.; Na, S.I.; Koo, H.W.; Kim, T.W. Simple brush painted Ag nanowire network on graphene sheets for flexible organic solar cells. J. Vac. Sci. Technol. A 2014, 32, 061201. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.; Ahn, Y.; Jeong, Y.; Lee, D.Y.; Lee, Y. Highly stable and flexible silver nanowire-graphene hybrid transparent conducting electrodes for emerging optoelectronic devices. Nanoscale 2013, 5, 7750–7755. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Wang, B.; Wei, B.; Yang, L. Enhanced photolithography with Al film insertion for large-scale patterning of CVD graphene. Opt. Mater. Express 2018, 8, 2403–2414. [Google Scholar] [CrossRef]
- De, S.; Coleman, J.N. Are there fundamental limitations on the sheet resistance and transmittance of thin graphene films? Acs Nano 2010, 4, 2713–2720. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Zhang, Q.K.; Yu, R.M.; Lu, C.Z. A new transparent conductor: Silver nanowire film buried at the surface of a transparent polymer. Adv. Mater. 2010, 22, 4484–4488. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, W.I.; Kim, K.H.; Yang, D.J.; Heo, S.; Yun, D.J. Nanoscale chemical and electrical stabilities of graphene-covered silver nanowire networks for transparent conducting electrodes. Sci. Rep. 2016, 6, 33074. [Google Scholar] [CrossRef]
- Ok, K.H.; Kim, J.; Park, S.R.; Kim, Y.; Lee, C.J.; Hong, S.J.; Kwak, M.G.; Kim, N.; Han, C.J.; Kim, J.W. Ultra-thin and smooth transparent electrode for flexible and leakage-free organic light-emitting diodes. Sci. Rep. 2015, 5, 9464. [Google Scholar] [CrossRef]

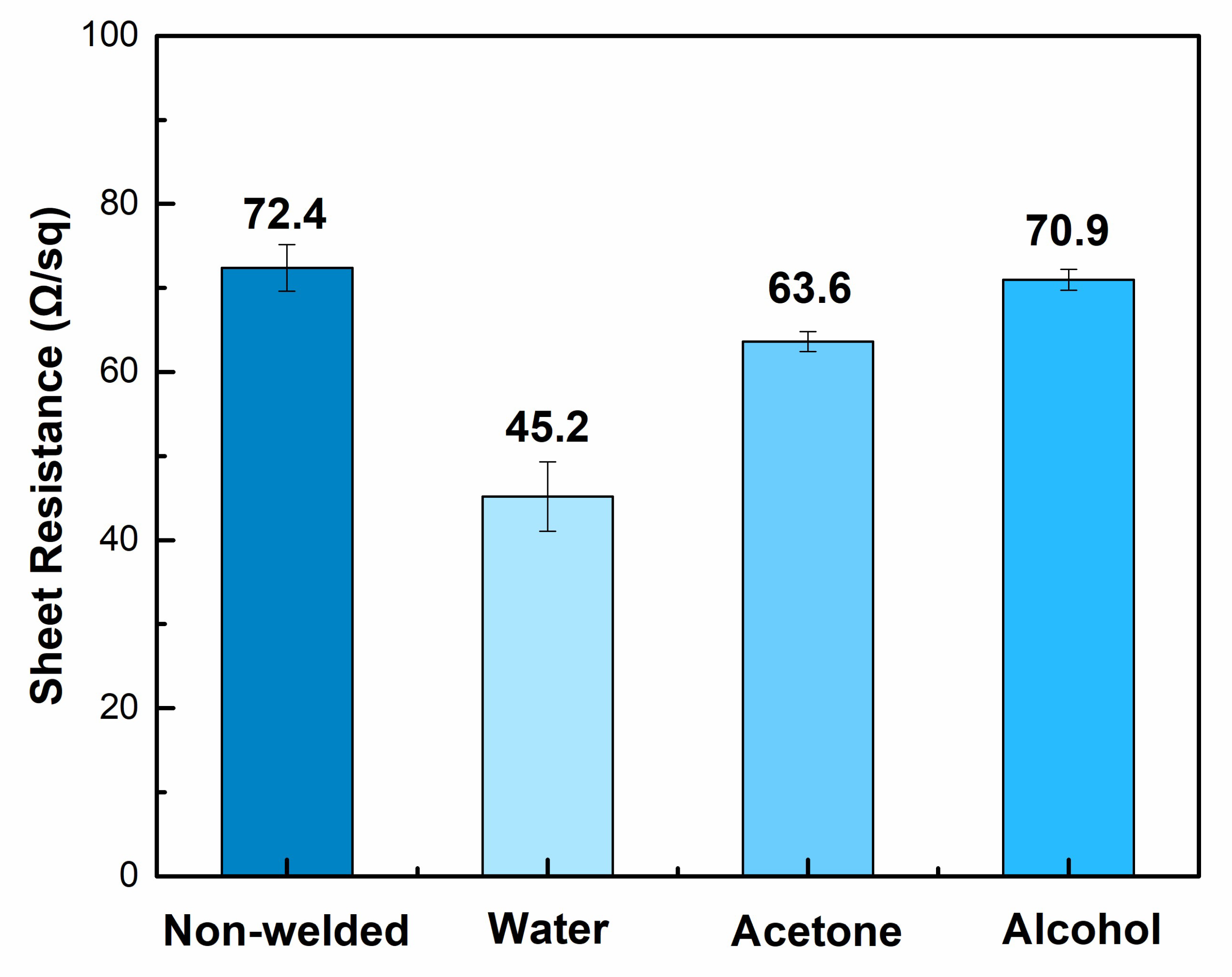



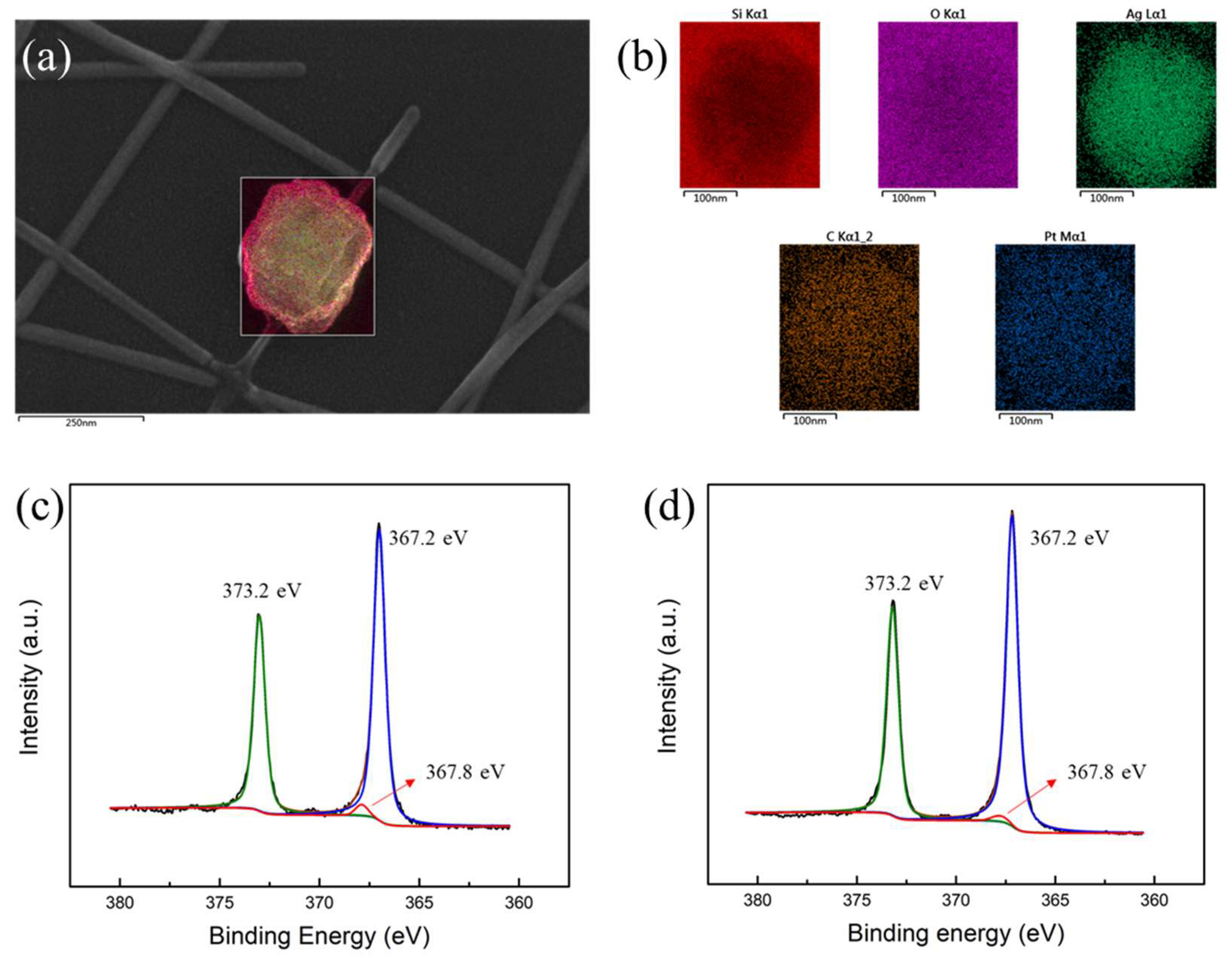
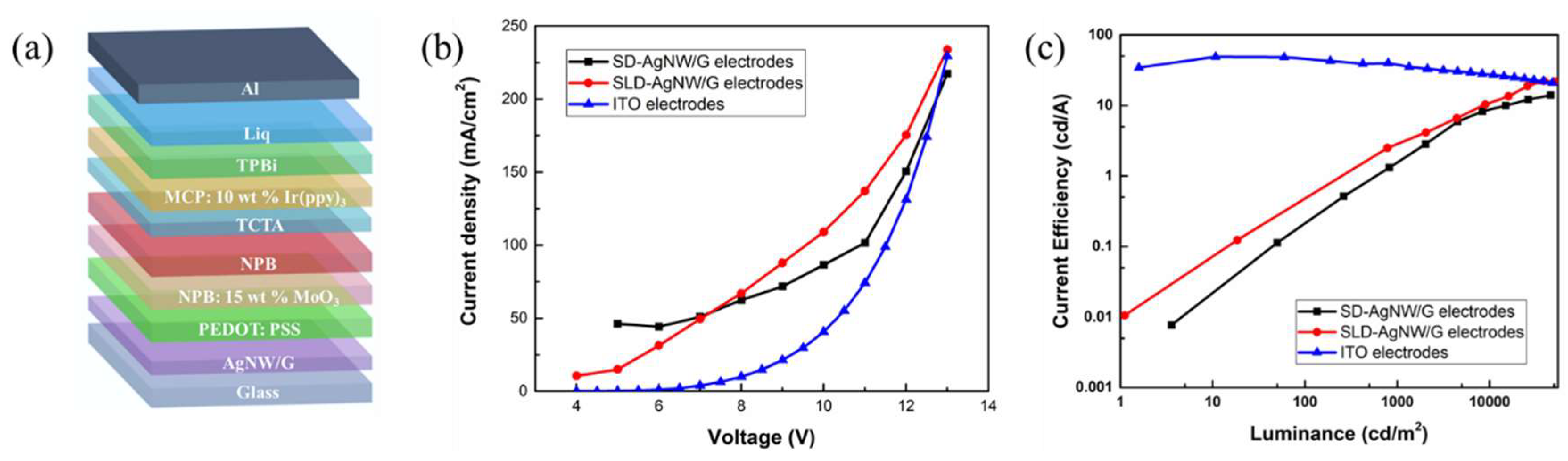


| Electrode Composition | Sheet Resistance (ohm/sq) | Transmission (%) | FoM |
|---|---|---|---|
| SD-AgNW | 302.9 | 98.4 | 59.4 |
| LD-AgNW | 38.6 | 80.6 | 42.9 |
| SLD-AgNW | 71.5 | 94.5 | 91.9 |
| Welded SLD-AgNW | 45.2 | 94.5 | 145.2 |
| Welded SLD-AgNW/graphene (SLD-AgNW/G) | 26.4 | 91.5 | 157.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, Y.; Wang, B.; Hu, H.; Wei, B.; Yang, L. High Brightness Organic Light-Emitting Diodes with Capillary-Welded Hybrid Diameter Silver Nanowire/Graphene Layers as Electrodes. Micromachines 2019, 10, 517. https://doi.org/10.3390/mi10080517
Zhang J, Li Y, Wang B, Hu H, Wei B, Yang L. High Brightness Organic Light-Emitting Diodes with Capillary-Welded Hybrid Diameter Silver Nanowire/Graphene Layers as Electrodes. Micromachines. 2019; 10(8):517. https://doi.org/10.3390/mi10080517
Chicago/Turabian StyleZhang, Jianhua, Yiru Li, Bo Wang, Huaying Hu, Bin Wei, and Lianqiao Yang. 2019. "High Brightness Organic Light-Emitting Diodes with Capillary-Welded Hybrid Diameter Silver Nanowire/Graphene Layers as Electrodes" Micromachines 10, no. 8: 517. https://doi.org/10.3390/mi10080517





