Metallization of Organically Modified Ceramics for Microfluidic Electrochemical Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Ormocomp Microchannels
2.2. Metallization and Bonding of Ormocomp Microchannels
2.3. Contact Angle Goniometry
2.4. Adhesion Tests
2.5. Characterization of the Electrical Conductivity
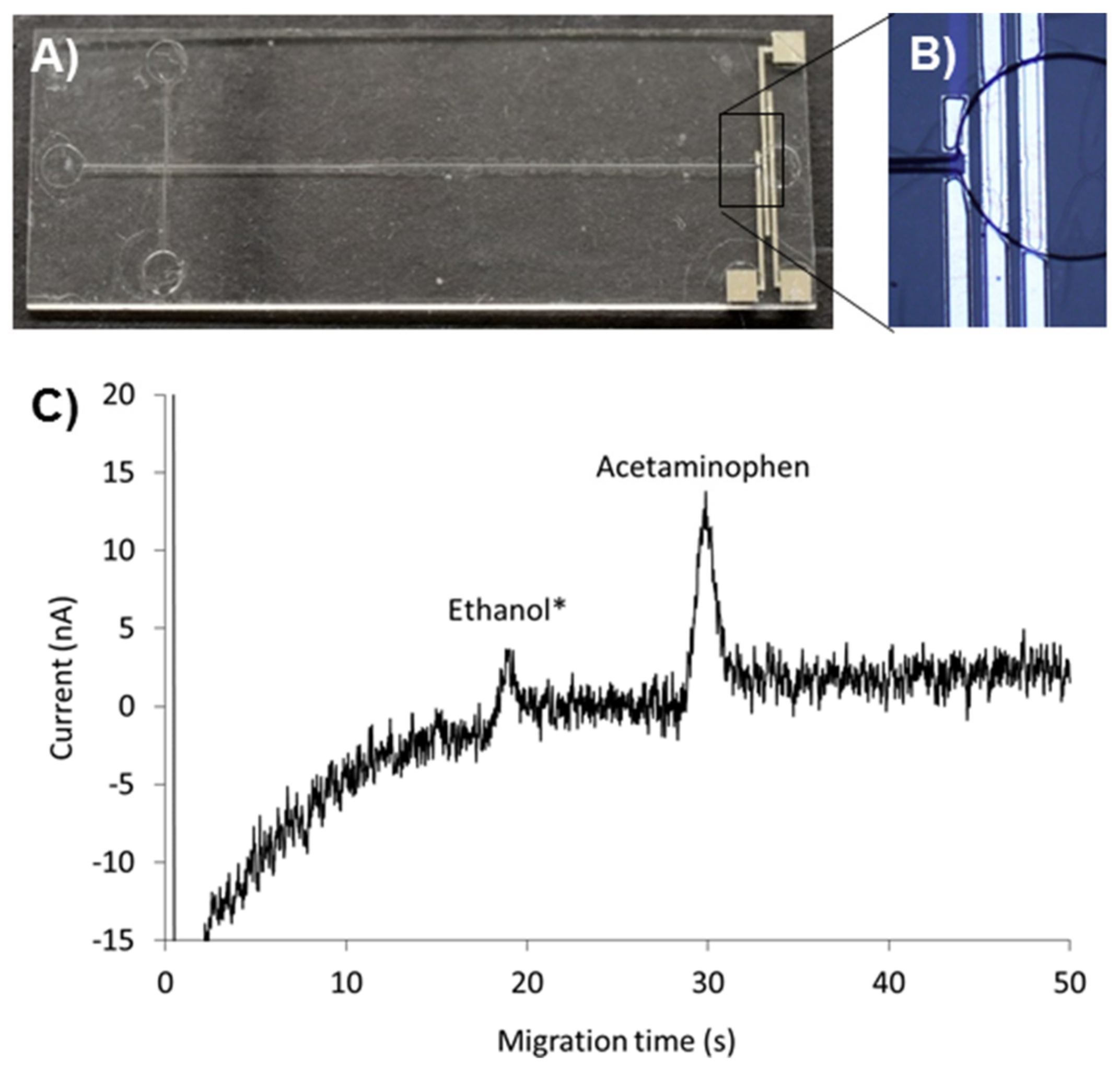
2.6. Microchip Electrophoresis with On-chip Amperometric Detection
3. Results
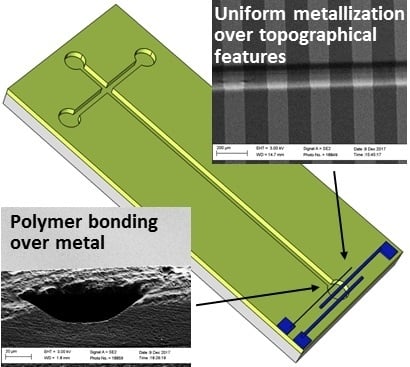
3.1. General Notes on Ormocomp Metallization Possibilities
3.2. Step Coverage and Polymer Bonding over Metal
3.3. Electrical Conductivity
3.4. Amperometric Detection of Acetaminophen
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Houbertz, R.; Fröhlich, L.; Popall, M.; Streppel, U.; Dannberg, P.; Bräuer, A.; Serbin, J.; Chichkov, B.N. Inorganic-organic hybrid polymers for information technology: from planar technology to 3D nanostructures. Adv. Eng. Mater. 2003, 5, 551–555. [Google Scholar] [CrossRef]
- Haas, K.H.; Amberg-Schwab, S.; Rose, K. Functionalized coating materials based on inorganic-organic polymers. Thin Solid Films 1999, 351, 198–203. [Google Scholar] [CrossRef]
- Singh, A.; Scotti, G.; Sikanen, T.; Jokinen, V.; Franssila, S. Laser direct writing of thick hybrid polymers for microfluidic chips. Micromachines 2014, 5, 472–485. [Google Scholar] [CrossRef]
- Aura, S.; Sikanen, T.; Kotiaho, T.; Franssila, S. Novel hybrid material for microfluidic devices. Sensors Actuat. B 2008, 132, 397–403. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Sikanen, T.; Aura, S.; Heikkilä, L.; Kotiaho, T.; Franssila, S.; Kostiainen, R. Hybrid ceramic polymers: New nonbiofouling and optically transparent materials for microfluidics. Anal. Chem. 2010, 82, 3874–3882. [Google Scholar] [CrossRef] [PubMed]
- Sikanen, T.; Aura, S.; Franssila, S.; Kotiaho, T.; Kostiainen, R. Microchip capillary electrophoresis-electrospray ionization-mass spectrometry of intact proteins using uncoated Ormocomp microchips. Anal. Chim. Acta 2012, 711, 69–76. [Google Scholar] [CrossRef]
- Schlie, S.; Ngezahay, A.; Ovsianikov, A.; Fabian, T.; Kolb, H.A.; Haferkamp, H.; Chichkov, B.N. Three-dimensional cell growth on structures fabricated from ORMOCER® by two-photon polymerization technique. J. Biomater. Appl. 2007, 22, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Bonabi, A.; Cito, S.; Tammela, P.; Jokinen, V.; Sikanen, T. Fabrication of concave micromirrors for single cell imaging via controlled over-exposure of organically modified ceramics in single step lithography. Biomicrofluidics. 2017, 11, 034118. [Google Scholar] [CrossRef]
- Girschikofsky, M.; Rosenberger, M.; Förthner, M.; Rommel, M.; Frey, L.; Hellmann, R. Waveguide bragg gratings in Ormocer® s for temperature sensing. Sensors 2017, 17, 2459. [Google Scholar] [CrossRef]
- Kalra, S.; Singh, A.; Gupta, M.; Chadha, V. Ormocer: An aesthetic direct restorative material; An in vitro study comparing the marginal sealing ability of organically modified ceramics and a hybrid composite using an ormocer-based bonding agent and a conventional fifth-generation bonding agent. Contemp. Clin. Dent. 2012, 3, 48–53. [Google Scholar] [CrossRef]
- Smy, T.; Dew, S.; Joshi, R. Modeling 3D effects of substrate topography on step coverage and film morphology of thin metal films. J. Thin Solid Films 2002, 415, 32–45. [Google Scholar] [CrossRef]
- Popall, M.; Dabek, A.; Robertsson, M.E.; Valizadeh, S.; Hagel, O.J.; Buestrich, R.; Nagel, R.; Cergel, L.; Lambert, D.; Schaub, M. ORMOCER® S-Inorganic-organic hybrid materials for e/o-interconnection-technology. J. Mol. Cryst. Liq. Crys. 2000, 354, 123–142. [Google Scholar] [CrossRef]
- Robertsson, M.; Haglund, J.; Johansson, C.; Gibaud, P.; Lambert, D.; Popall, M.; Fröhlich, L.; Tolvgärd, A.; Alpin, A.; Linde, L.; et al. Large area patterning of high density interconnects by novel UV-excimer lithography and photo patternable ORMOCER™-dielectrica. Proc. of the Int. Microelectron. Packag. Soc. 2001, 2001, 276–281. [Google Scholar]
- Johansson, C.; Uhlig, S.; Tageman, O.; Alping, A.; Haglund, J.; Robertsson, M.; Popall, M.; Fröhlich, L. Microwave circuits in multilayer inorganic-organic polymer thin film technology on laminate substrates. IEEE Trans. Adv. Packag. 2003, 26, 81–89. [Google Scholar] [CrossRef]
- Gittard, S.D.; Narayan, R.J.; Jin, C.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Stafslien, S.; Chisholm, B. Pulsed laser deposition of antimicrobial silver coating on Ormocer® microneedles. Biofabrication 2009, 1, 41001. [Google Scholar] [CrossRef]
- Nordström, M.; Johansson, A.; Nogueron, E.S.; Clausen, B.; Calleja, M.; Boisen, A. Investigation of the bond strength between the photo-sensitive polymer SU-8 and gold. J. Microelectron. Eng. 2005, 78–79, 152–157. [Google Scholar] [CrossRef]
- Marelli, M.; Divitini, G.; Collini, C.; Ravagnan, L.; Corbelli, G.; Ghisleri, C.; Gianfelice, A.; Lenardi, C.; Milani, P.; Lorenzelli, L. Flexible and biocompatible microelectrode arrays fabricated by supersonic cluster beam deposition on SU-8. J. Micromech. Microeng. 2011, 21, 045013. [Google Scholar] [CrossRef]
- Tatikonda, A.; Jokinen, V.P.; Evard, H.; Franssila, S. Sacrificial layer technique for releasing metallized multilayer SU-8 devices. Micromachines 2018, 9, 673. [Google Scholar] [CrossRef]
- Ge, J.; Turunen, M.P.K.; Kusevic, M.; Kivilahti, J.K. Effects of surface treatment on the adhesion of copper to a hybrid polymer material. J. Mater. Res. 2003, 18, 2697–2707. [Google Scholar] [CrossRef] [Green Version]
- Hopcroft, M.; Kim, G.; Takashima, K.; Higo, Y.; Moore, D.; Brugger, J. Micromechanical testing of SU-8 cantilevers. J. FatiG. Fract. Eng. Mater. Struct. 2005, 28, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Köllensperger, P.A.; Karl, W.J.; Ahmad, M.M.; Pike, W.T.; Green, M. Patterning of platinum (Pt) thin films by chemical wet etching in Aqua Regia. J. Micromech. Microeng. 2012, 22, 067001. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Hassler, B.L.; Worden, R.M.; Mason, A.J. Amperometric Electrochemical Microsystem for a Miniaturized Protein Biosensor Array. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 161–168. [Google Scholar] [CrossRef]
- Hsu, L.-S.; Tung, S.-W.; Kuo, C.-H.; Yang, Y.-J. Developing Barbed Microtip-Based Electrode Arrays for Biopotential Measurement. Sensors 2014, 14, 12370–12386. [Google Scholar] [CrossRef] [Green Version]
- Gandikota, S.; Voss, S.; Tao, R.; Duboust, A.; Cong, D.; Chen, L.Y.; Ramaswami, S.; Carl, D. Adhesion studies of CVD copper metallization. Microelectron. Eng. 2000, 50, 547–553. [Google Scholar] [CrossRef]
- Serway, R.A.; Jewett, J.W. Principles of Physics, 2nd ed.; Saunders College Publishing: New York, NY, USA, 1998. [Google Scholar]




| Metal | Evaporation Parameters | ||
|---|---|---|---|
| Current (mA) | Pressure (×10−10 bar) | Rate (Å/s) | |
| Ti | 60 | 5.0 | 0.5 |
| Au | 40 | 0.9 | 1.1 |
| Ag | 32 | 2.0 | 2.3 |
| Al | 15 | 1.0 | 1.2 |
| Metal | Sputtering Parameters | ||
| DC power (W) | Argon (sccm) | Time (s) | |
| Cr | 200 | 50 | 105 |
| Pt | 500 | 70 | 480 |
| Metal (nm) | Etching Conditions | Bonding Conditions | ||
|---|---|---|---|---|
| Etchant | Time | O2 Plasma | Temperature | |
| Pt (220) | aqua regia (55 °C) | 3–4 min | Yes | at 70–95 °C |
| Au (30) | aqua regia (RT) | 10 s | Yes | at 70–95 °C |
| Ag (30) | 0.8% NH4OH + 15% H2O2 in H2O | 10 s | Yes (before resist removal) | at 70–95 °C |
| Al (30) | AZ351B developer | 30 min | No | at RT |
| Ti (5) | 0.8% NH4OH + 15% H2O2 in H2O | 1–2 min | adhesion metals | |
| Cr (17) | 17% Ce(NH4)2(NO3)6 + 4% HClO4 in H2O | 7–10 s | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonabi, A.; Tähkä, S.; Ollikainen, E.; Jokinen, V.; Sikanen, T. Metallization of Organically Modified Ceramics for Microfluidic Electrochemical Assays. Micromachines 2019, 10, 605. https://doi.org/10.3390/mi10090605
Bonabi A, Tähkä S, Ollikainen E, Jokinen V, Sikanen T. Metallization of Organically Modified Ceramics for Microfluidic Electrochemical Assays. Micromachines. 2019; 10(9):605. https://doi.org/10.3390/mi10090605
Chicago/Turabian StyleBonabi, Ashkan, Sari Tähkä, Elisa Ollikainen, Ville Jokinen, and Tiina Sikanen. 2019. "Metallization of Organically Modified Ceramics for Microfluidic Electrochemical Assays" Micromachines 10, no. 9: 605. https://doi.org/10.3390/mi10090605