Cord-Based Microfluidic Chips as A Platform for ELISA and Glucose Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microfluidic Analytical Device Fabrication
2.2.1. μCAD Platform Fabrication
2.2.2. Choice of Cord
2.2.3. Microfluidic Device Analysis
3. Results and Discussion
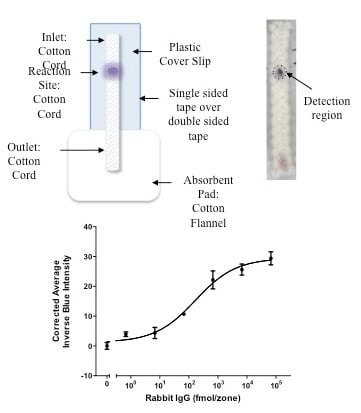
3.1. Detection of Biotinylated Goat Anti-Mouse IgG
3.2. Detection of Rabbit IgG Antibodies
3.3. Detection of Glucose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sensor Actuat. B-Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Avoundjian, A.; Jalali-Heravi, M.; Gomez, F.A. Use of chemometrics to optimize a glucose assay on a paper microfluidic platform. Anal. Bioanal. Chem. 2017, 409, 2697–2703. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.A. Paper microfluidics in bioanalysis. Bioanalysis 2014, 6, 2911–2914. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Nilghaz, A.; Chen, L.; Chou, K.C.; Lu, X. Modification of thread-based microfluidic device with polysiloxanes for the development of a sensitive and selective immunoassay. Sensor Actuat. B-Chem. 2018, 260, 1043–1051. [Google Scholar] [CrossRef]
- Agustini, D.; Bergamini, M.F.; Marcolino-Junior, L.H. Characterization and optimization of low cost microfluidic thread based electroanalytical device for micro flow injection analysis. Anal. Chim. Acta 2017, 951, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Cabot, J.M.; Breadmore, M.C.; Paull, B. Thread based electrofluidic platform for direct metabolite analysis in complex samples. Anal. Chim. Acta 2017, 1000, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, C.; Li, H.; Liu, M.; Wang, D.; Zhang, C. Bipolar electrochemiluminescence on thread: a new class of electroanalytical sensors. Biosens. Bioelectron. 2017, 94, 335–343. [Google Scholar] [CrossRef]
- Gerold, C.T.; Bakker, E.; Henry, C.S. Selectvie distinace-based K+ quantification on paper-based microfluidics. Anal. Chem. 2018, 90, 4894–4900. [Google Scholar] [CrossRef]
- Hamidon, N.N.; Hong, Y.; Salentijn, G.I.J.; Verpoorte, E. Water-based alkyl ketene dimer ink for user-friendly patterning in paper microfluidics. Anal. Chim. Acta 2018, 1000, 180–190. [Google Scholar] [CrossRef]
- Fu, E.; Downs, C. Progress in the development and integration of fluid flow control tools in paper microfluidics. Lab Chip 2017, 17, 614. [Google Scholar] [CrossRef]
- de Oliveira, R.A.G.; Camargo, F.; Pesquero, N.C.; Faria, R.C. A Simple method to produce 2D and 3D microfluidic paper-based analytical devices for clinical analysis. Anal. Chim. Acta 2017, 957, 40–46. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, S.; Fang, Z.; Li, R.; Koo, H.; Tingrui, P. Wearable microfluidics: fabric-based digital droplet flowmetry for perspiration analysis. Lab Chip 2017, 17, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Narahari, T.; Dendukuri, D.; Murthy, S.K. Electrically-actuated vales for woven fabric lateral flow devices. Anal. Chem. 2017, 89, 4671–4679. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.C.; Kenry, K.; Lim, C.T. Emergence of microfluidic wearable technologies. Lab Chip 2016, 16, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable vioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas III, S.W.; Sindi, H.; Whitesides, G.M. Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Bruzewicz, D.A.; Reches, M.; Whitesides, G.M. Low-cost printing of poly(dimethylsiloxane) barriers to define microchannels in paper. Anal. Chem. 2008, 80, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. FLASH: A rapid method for prototyping paper-based microfluidic devices. Lab Chip 2008, 8, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Renault, C.; Koehne, J.; Ricco, A.J.; Crooks, R.M. Three-dimensional wax pattering of paper fluidic devices. Langmuir 2014, 30, 7030–7036. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.-J.; Xu, J.-R.; Wang, Y.-H.; Zheng, G.-X. Paper-based three-dimensional microfluidic device for monitoring of heavy metals with a camera cell phone. Anal. Bioanal. Chem. 2014, 406, 2799–2807. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Wong, W.S.; Liu, L.; Dewar, R.; Klapperich, C.M. A fully integrated paper fluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab Chip 2016, 16, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Shin, J.H.; Park, H.K.; Choi, S. A low-cost, monometallic, surface-enhanced Raman scattering-functionalized paper platform for spot-on bioassays. Sensor Actuat. B-Chem. 2016, 222, 1112–1118. [Google Scholar] [CrossRef]
- Ueland, M.; Blanes, L.; Taudte, R.V.; Stuart, B.H.; Cole, N.; Willis, P.; Roux, C.; Doble, P. Capillary-driven microfluidic paper-pased analytical devices for lab on a chip screening of explosive residues in soil. J. Chromatogr. A 2016, 1436, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Q.; Guo, S.M.; Zuo, P.; Ye, B.C. A cost-effective Z-folding controlled liquid handling microfluidic paper analysis device for pathogen detection via ATP quantification. Biosens. Bioelectron. 2015, 63, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, P.; Ye, B.C. A low-cost and simple paper-based microfluidic device for simultaneous multiplex determination of different types of chemical contaminants in food. Biosens. Bioelectron. 2015, 68, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Song, S. Facile and precise flow control for a paper-based microfluidic device through varying paper permeability. Lab Chip 2015, 15, 3405–33412. [Google Scholar] [CrossRef] [PubMed]
- Arrastia, M.; Avoundjian, A.; Ehrlich, P.S.; Eropkin, M.; Levine, L.; Gomez, F.A. Development of a microfluidic-based assay on a novel nitrocellulose platform. Electrophoresis 2015, 36, 884–888. [Google Scholar] [CrossRef]
- Safevieh, R.; Zhou, G.Z.; Juncker, D. Microfluidics made of yarns and knots: from fundamental properties to simple networks and operations. Lab Chip 2011, 11, 2618–2624. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Shen, W. Thread as a versatile material for low-cost microfluidic diagnostics. ACS Appl. Mater. Interface 2010, 2, 1–6. [Google Scholar] [CrossRef]
- Reches, M.; Mirica, K.A.; Dasgupta, R.; Dickey, M.D.; Butte, M.J.; Whitesides, G.M. Thread as a matrix for biomedical assays. ACS Appl. Mater. Interface 2010, 2, 1722–1728. [Google Scholar] [CrossRef]
- Zhou, G.; Mao, X.; Juncker, D. Immunochromatographic assay on thread. Anal. Chem. 2012, 84, 7736–7743. [Google Scholar] [CrossRef] [PubMed]
- Nilghaz, A.; Ballerini, D.R.; Shen, W. Exploration of microfluidic devices based on multi-filament threads and textiles: A review. Biomicrofliudics 2013, 7, 051501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glavan, A.C.; Ainla, A.; Hamedi, M.M.; Fernández-Abedul, M.T.; Whitesides, G.M. Electroanalytical devices with pins and threads. Lab Chip 2016, 16, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Rama, E.C.; Costa-García, A.; Fernández-Abedul, M.T. Pin-based electrochemical glucose sensor with multiplexing possibilities. Biosens. Bioelectron. 2017, 88, 34–40. [Google Scholar] [CrossRef]
- Gaines, M.; Gonzalez-Guerrero, M.J.; Uchida, K.; Gomez, F.A. A microfluidic glucose sensor incorporating a novel thread-based electrode system. Electrophoresis 2018, 39, 2131–2135. [Google Scholar] [CrossRef]
- Choudhary, T.; Rajamanickam, G.P.; Dendukuri, D. Woven electrochemical fabric-based test sensors (WEFTS): A new class of multiplexed electrochemical sensors. Lab Chip 2015, 15, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Martinez, A.W.; Gong, J.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-based ELISA. Angew. Chem. Int. Ed. 2010, 49, 4771–4774. [Google Scholar] [CrossRef]
- Gonzalez, A.; Gaines, M.; Gallegos, L.Y.; Guevara, R.; Gomez, F.A. Enzyme-linked immunosorbent assays (ELISA) based on thread, paper, and fabric. Electrophoresis 2018, 39, 476–484. [Google Scholar] [CrossRef]
- Diabetes: Blood Sugar Level Ranges. Available online: https://www.diabetes.co.uk/diabetes_care/blood-sugar-level-ranges.html (accessed on 1 October 2018).
- Healthline: Urine Glucose Test. Available online: https://www.healthline.com/health/glucose-test-urine (accessed on 2 October 2018).



| Known Glucose Concentration (mM) | Detected Concentration (mM) | Percent Difference |
|---|---|---|
| 0.5 | 0.46 ± 0.05 | 9.4 |
| 1.0 | 0.92 ± 0.08 | 8.3 |
| 4.5 | 4.53 ± 0.03 | 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elomaa, J.; Gallegos, L.; Gomez, F.A. Cord-Based Microfluidic Chips as A Platform for ELISA and Glucose Assays. Micromachines 2019, 10, 614. https://doi.org/10.3390/mi10090614
Elomaa J, Gallegos L, Gomez FA. Cord-Based Microfluidic Chips as A Platform for ELISA and Glucose Assays. Micromachines. 2019; 10(9):614. https://doi.org/10.3390/mi10090614
Chicago/Turabian StyleElomaa, Jenny, Laura Gallegos, and Frank A. Gomez. 2019. "Cord-Based Microfluidic Chips as A Platform for ELISA and Glucose Assays" Micromachines 10, no. 9: 614. https://doi.org/10.3390/mi10090614