Fabrication and Study on Magnetic-Optical Properties of Ni-Doped ZnO Nanorod Arrays
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Design
2.2. Characterization Methods
3. Results and Discussions
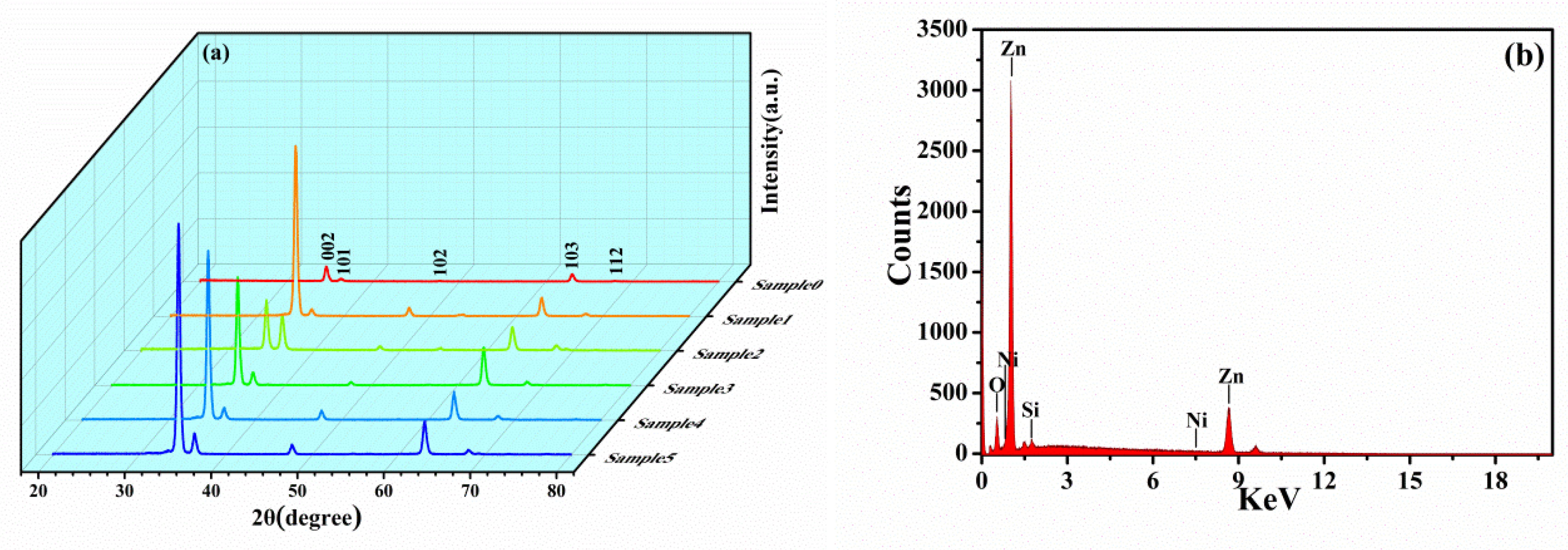
3.1. Phase Analysis
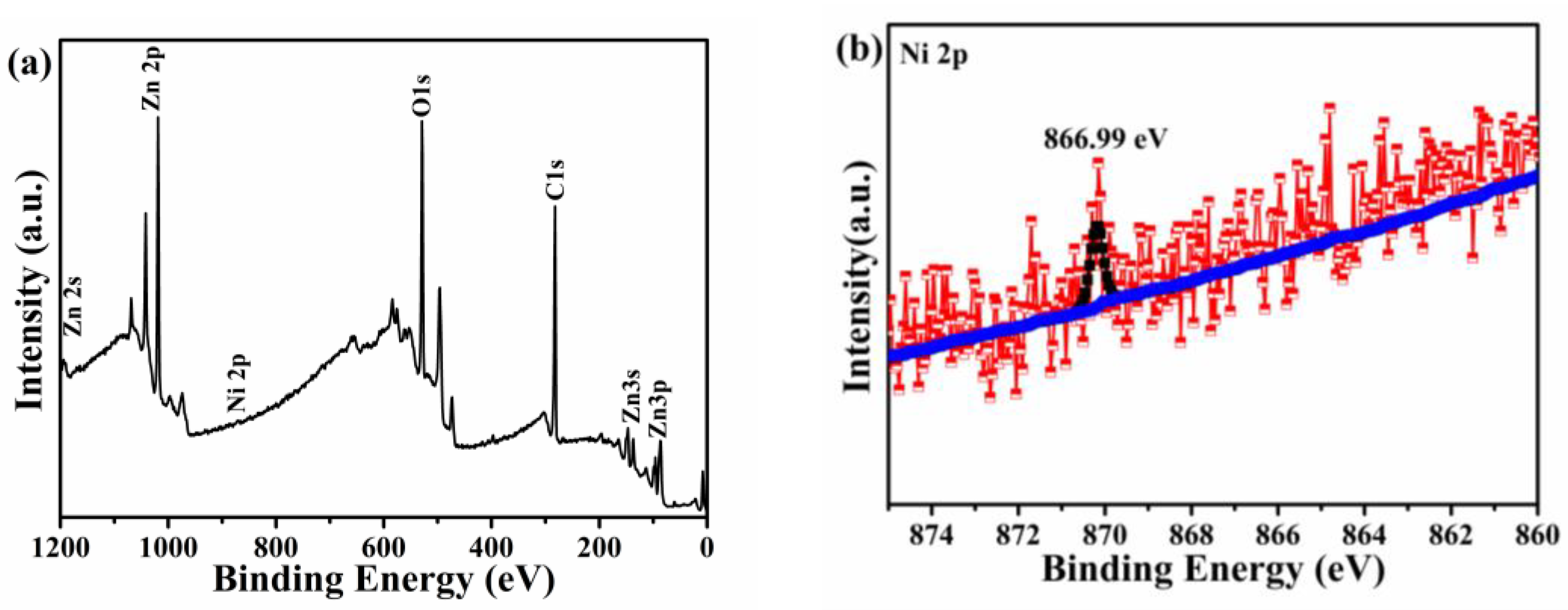
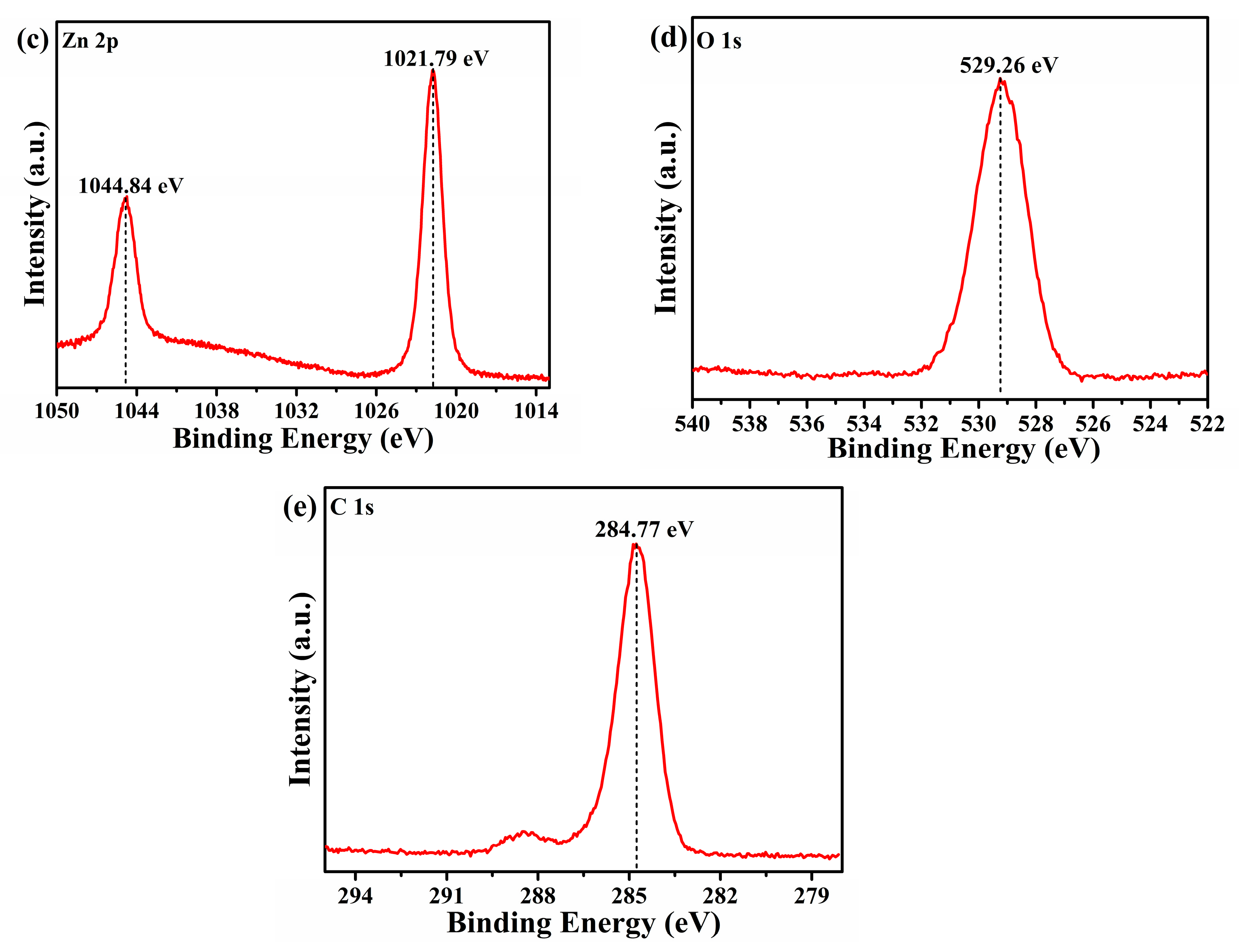
3.2. XPS Analysis
3.3. Morphology Analysis
3.4. Optical Performance Analysis
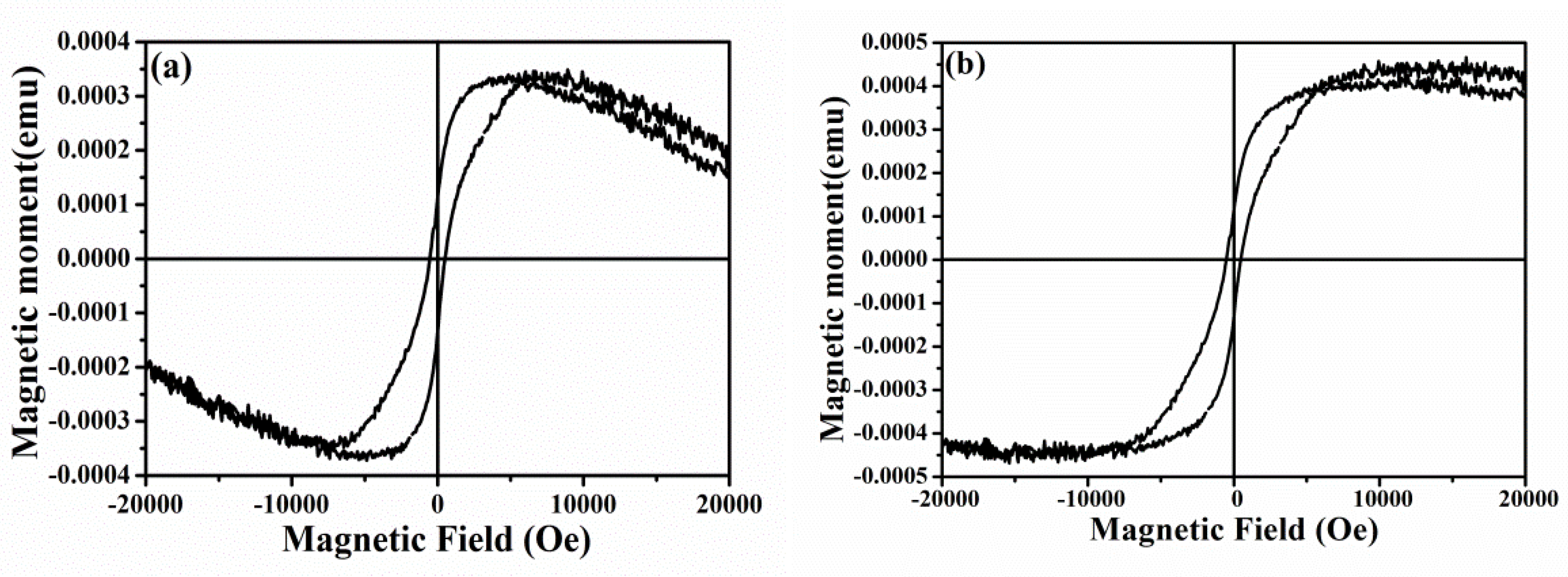
3.5. Magnetic Properties Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; Niu, S.; Han, D.; Liu, T.; Wang, G.; Li, Y. Progress in developing metal oxide nanomaterials for photoelectrochemical water splitting. Adv. Energy Mater. 2017, 7, 1700555. [Google Scholar] [CrossRef]
- Cai, L.; Ren, F.; Wang, M.; Cai, G.; Chen, Y.; Liu, Y.; Shen, S.; Guo, L. V ions implanted ZnO nanorod arrays for photoelectrochemical water splitting under visible light. Int. J. Hydrog. Energy 2015, 40, 1394–1401. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sensor. Actuat. Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Kamat, P.V. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar] [CrossRef]
- Dietl, T.; Ohno, H.; Matsukura, F.; Cibert, J.; Ferrand, D. Zener model description of ferromagnetism in zinc-blende magnetic semiconductors. Science 2000, 287, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.J.; Murakami, M.; Shono, T.J.; Hasegawa, T.Y.; Fukumura, T.T.; Kawasaki, M.S.; Ahmet, P.; Chikyow, T.H.; Koshihara, S.Y.; Koinuma, H.O. Room-temperature ferromagnetism in transparent transition metal-doped titanium dioxide. Science 2001, 291, 8542856. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gupta, A.; Rao, K.V.; Owens, F.J.; Sharma, R.; Ahuja, R.J.; Guillen, J.M.O.; Johansson, B.J.; Gehring, G.A. Ferromagnetism above room temperature in bulk and transparent thin films of Mn-doped ZnO. Nat. Mater. 2003, 2, 673–677. [Google Scholar] [CrossRef]
- Dietl, T. Dilute magnetic semiconductors: functional ferromagnets. Nat. Mater. 2003, 2, 646. [Google Scholar] [CrossRef]
- Chiba, D.; Yamanouchi, M.; Matsukura, F.; Ohno, H. Electrical manipulation of magnetization reversal in a ferromagnetic semiconductor. Science 2003, 301, 943. [Google Scholar] [CrossRef]
- Park, Y.D.; Hanbicki, A.T.; Erwin, S.C.; Hellberg, C.S.; Sullivan, J.M.; Mattson, J.E.; Ambrose, T.F.; Wilson, A.; Spanos, G.; Jonker, B.T. A group-IV ferromagnetic semiconductor: MnxGe1-x. Science 2002, 295, 651. [Google Scholar] [CrossRef]
- Ohno, H. Making nonmagnetic semiconductors ferromagnetic. Science 1998, 281, 951. [Google Scholar] [CrossRef] [PubMed]
- Basri, S.H.; AbdMajid, W.H.; Talik, N.A.; Sarjidan, M.A.M. Tailoring electronics structure, electrical and magnetic properties of synthesized transition metal (Ni)-doped ZnO thin film. J. Alloy. Compd. 2018, 769, 640–648. [Google Scholar] [CrossRef]
- Ozgür, M.; Hofstetter, D.; Morkoç, H. ZnO devices and applications: A review of current status and future prospects. Proc. IEEE 2010, 98, 1255–1268. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: from basics towards applications. Phys. Stat. Solidi 2007, 244, 3027–30733. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Ueda, K.; Kamiya, T.; Hirano, M.; Hosono, H. Thin-film transistor fabricated in single-crystalline transparent oxide semiconductor. Science 2003, 300, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Yu, M.H.; Zhou, W.L. Fabrication of Mn-doped ZnO diluted magnetic semiconductor nanostructures by chemical vapor deposition. Appl. Phys. 2006, 99, 08M119. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, D.; Lin, F.; Shi, W.; Ma, X. Fe-doped ZnO magnetic semiconductor by mechanical alloying. J. Alloy. Compd. 2007, 436, 30–33. [Google Scholar] [CrossRef]
- Javed, I.; Tariq, J.; Yu, R.H. Effect of Co doping on morphology, optical and magnetic properties of ZnO 1-D nanostructures. J. Mater. Sci.: Matet. Electron. 2013, 24, 4393–4398. [Google Scholar]
- Vassieres, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003, 15, 464. [Google Scholar] [CrossRef]
- Williams, G.; Hunt, M.; Boehm, B.; May, A.; Taverne, M.; Ho, D.; Giblin, S.; Read, D.; Allenspach, R.; Ladak, S. Two-photon lithography for 3D magnetic nanostructure fabrication. Nano Research 2018, 11, 845–854. [Google Scholar] [CrossRef]
- Parkin, S.S.P.; Hayashi, M.; Thomas, L. Magnetic domain-wall racetrack memory. Science 2008, 320, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Da Col, S.; Jamet, S.; Rougemaille, N.; Locatelli, A.; Mentes, T.O.; Burgos, B.S.; Afid, R.; Darques, M.; Cagnon, L.; Toussaint, J.C.; et al. Observation of bloch-point domainwalls in cylindrical magnetic nanowires. Phys. Rev. B 2014, 89, 180405. [Google Scholar] [CrossRef]
- Ivanov, Y.P.; Chuvilin, A.; Vivas, L.G.; Kosel, J.; Chubykalo-Fesenko, O.; Vázquez, M. Single crystalline cylindrical nanowires-toward dense 3D arrays of magnetic vortices. Sci. Rep. 2016, 6, 23844. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.Q.; Luo, X.H.; Xu, X.Y.; Li, L.; Chen, J.P.; Wang, R.M.; Yu, D.P. Synthesis, characterization and magnetic property measurements of Zn1-xMnxO nanoparticles via vapor phase growth. Chin. Phys. Lett. 2003, 20, 2058–2060. [Google Scholar]
- RoyV, A.L.; Djurii, A.B.; Liu, H.; Zhang, X.X.; Leung, Y.H.; Xie, M.H.; Gao, J.; Lui, H.F.; Surya, C. Magnetic properties of Mn doped ZnO tetrapod structures. Appl. Phys. Lett. 2004, 84, 756–758. [Google Scholar] [Green Version]
- Norton, D.P.; Pearton, S.J.; Hebard, A.F.; Theodoropoulou, N.; Boatner, L.A.; Wilson, R.G. Ferromagnetism in Mn-implanted ZnO: Sn single crystals. Appl. Phys. Lett. 2003, 82, 239–241. [Google Scholar] [CrossRef]
- Ip, K.; Frazier, R.M.; Heo, Y.W.; Norton, D.P.; Abernathy, C.R.; Pearton, S.J.; Zavada, J.M.; Kelly, J.; Rairigh, R.; Hebard, A.F.; et al. Ferromagnetism in Mn- and Co-implanted ZnO nanorods. J. Vac. Sci. Technol. 2003, 21, 1476. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.W.; An, S.J.; Yi, G.C.; Jung, C.U.; Lee, S.I.; Cho, S. Ferromagnetic properties of Zn1-xMnxO epitaxial thin films. Appl. Phys. Lett. 2002, 80, 4561–4563. [Google Scholar] [CrossRef]
- Chang, Y.Q.; Wang, D.B.; Luo, X.H.; Xu, X.Y.; Chen, X.H.; Li, L.; Chen, C.P.; Wang, R.M.; Xu, J.; Yu, D.P. Synthesis, optical and magnetic properties of diluted magnetic semiconductorzn1-xMnxO nanowires via vapor phase growth. Appl. Phys. Lett. 2003, 83, 4020. [Google Scholar] [CrossRef]
- Chang, Y.Q.; Xu, X.Y.; Luo, X.H.; Long, Y.; Ye, R.C. Magnetic properties of diluted magnetic semiconductor Zn1-xMnxO nanowires. Chin. Phys. Lett. 2005, 22, 991–994. [Google Scholar]
- Wang, D.D.; Chen, Q.; Xing, G.Z.; Yi, J.B.; Bakaul, S.R.; Ding, J.; Wang, J.L.; Wu, T. Robust room-temperature ferromagnetism with giant anisotropy in Nd-doped ZnO nanowire arrays. Nano Lett. 2012, 12, 3994–4000. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Saito, H.; Zheng, W.J.; Fukumura, T. Magneto-optical properties of ZnO-based diluted magnetic semiconductors. J. Appl. Phys. 2001, 89, 7284. [Google Scholar] [CrossRef]
- Wei, Z.R.; Liu, C.; Li, J.; Lin, L.; Zheng, Y.B.; Ge, S.Y.; Zhang, H.W.; Dou, J.H. Hydrothermal synthesis of Zn1-xCoxO diluted magnetic semiconductors. J. Synth. Cryst. 2006, 35. [Google Scholar]
- Miao, H.Y.; Li, H.Q.; Tan, G.Q.; An, B.J.; Wei, Y.X. Hydrothermal synthesis of Zn1-xCrxO diluted magnetic semiconductors. J. Inorg. Mater. 2008, 23. [Google Scholar] [CrossRef]
- Zhou, S.M.; Yuan, H.L.; Liu, L.S.; Chen, X.L.; Lou, S.Y.; Hao, Y.M.; Yuan, R.J.; Li, N. Magnetic properties of Ni-doped ZnO nano combs by CVD approach. Nanoscale Res. Lett. 2010, 5, 1284–1288. [Google Scholar]
- Liu, Y.M.; Wang, T.; Sun, X.; Fang, Q.Q.; Lv, Q.R.; Song, X.P.; Sun, Z.Q. Structural and photoluminescent properties of Ni doped ZnO nanorod arrays prepared by hydrothermal method. Appl. Surf. Sci. 2011, 257, 6540–6545. [Google Scholar] [CrossRef]
- Samanta, A.; Goswami, M.N.; Mahapatra, P.K. Magnetic and electric properties of Ni-doped ZnO nanoparticles exhibit diluted magnetic semiconductor in nature. J. Alloy. Compd. 2018, 730, 399–407. [Google Scholar] [CrossRef]
- Pal, B.; Sarkar, D.; Giri, P.K. Structural, optical, and magnetic properties of Ni doped ZnO nanoparticles: Correlation of magnetic moment with defect density. Appl. Surf. Sci. 2015, 356, 804–811. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.S.; Jilani, A.; Yahia, I.S.; Al-Ghamdi, A.A. Enhanced the photocatalytic activity of Ni-doped ZnO thin films: Morphological, optical and XPS analysis. Superlattice. Microst. 2016, 94, 108–118. [Google Scholar] [CrossRef]
- SankarGanesh, R.; Durgadevi, E.; Navaneethan, M.; Patil, V.L.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.S.; Hayakawa, Y. Controlled synthesis of Ni-doped ZnO hexagonal microdiscs and their gas sensing properties at low temperature. Chem. Phys. Lett. 2017, 689, 92–99. [Google Scholar]
- Wang, W.; Zhang, F.C.; Zhou, Q.; Wang, X.Y.; Zhang, S.L. Fabrication and optical property of ZnO nanorod arrays by hydrothermal method. Ferroelectrics 2018, 540. in print. [Google Scholar]
- Ohtomo, A.; Kawasaki, M.; Koida, T.; Masabuchi, K.; Koinuma, H.; Sakurai, Y.; Yoshida, Y.; Yasuda, T.; Segawa, Y. MgxZn1-xO as a II–VI widegap semiconductor alloy. Appl. Phys. Lett. 1998, 72, 2466. [Google Scholar] [CrossRef]
- Meng, Y.C.; Zhou, Z.H.; Wu, Y.P.; Wang, D.; Huang, Y. Preparation and growth mechanism of ZnO nanorods by low temperature hydrothermal method. J.Xiamen Univ. (Nat. Sci. Edition) 2012, 51, 60–65. [Google Scholar]
- Zhang, H.; Yang, D.R.; Ji, Y.J.; Ma, X.Y.; Xu, J.; Que, D.L. Low temperature synthesis of flowerlike ZnO nanostructures by cetyltrimethyl-ammonium bromide-assisted hydrothermal process. J. Phys. Chem. B 2004, 108, 3955–3958. [Google Scholar] [CrossRef]
- Yan, X.L.; Hu, D.; Li, H.S.; Li, L.X.; Chong, X.Y.; Wang, Y.D. Nanostructure and optical properties of M doped ZnO (M¼Ni, Mn) thin films prepared by solegel process. Phys. B 2011, 406, 3956–3962. [Google Scholar] [CrossRef]
- Hu, P.; Han, N.; Zhang, D.; Ho, J.C.; Chen, Y. Highly formaldehyde-sensitive, transition-metal doped ZnO nanorods prepared by plasma-enhanced chemical vapor deposition. Sensor. Actuat. B 2012, 69, 74–80. [Google Scholar] [CrossRef]
- Singh, A.K.; Thool, G.S.; Bangal, P.R.; Madhavendra, S.S.; Singh, S.P. Low temperature Mn doped ZnO nanorod array: Synthesis and its photoluminescence behavior. Ind. Eng. Chem. Res. 2014, 53, 9383–9390. [Google Scholar] [CrossRef]
- Hang, L.M. Fabrication and properties of rod-like ZnO-based diluted magnetic semiconductors. Ph.D. Thesis, Xi’an Institute of Optical Precision Machinery, Xi’an, China, 2012. [Google Scholar]
- Yuan, M.X. Preparation, doping and characterization of Zinc oxide nanorod arrays. Master’s Thesis, Jilin University, Changchun, China, 2009. [Google Scholar]
- He, J.H.; Lao, C.S.; Chen, L.J.; Davidovic, D.; Wang, Z.L. Large-scale Ni-doped ZnO nanowire arrays and electrical and optical properties. J. Am. Chem. Soc. 2005, 127, 16376–16377. [Google Scholar] [CrossRef]
- Xu, K.; Liu, C.Z.; Chen, R.; Fang, X.X.; Wu, X.L.; Liu, J. Structural and room temperature ferromagnetic properties of Ni doped ZnO nanoparticles via low-temperature hydrothermal method. Phys. B 2016, 502, 155–159. [Google Scholar] [CrossRef]
- Lee, J.J.; Xing, G.Z.; Yi, J.B.; Chen, T.; Ionescu, M.; Li, S. Tailoring the coercivity in ferromagnetic ZnO thin films by 3d and 4f elements codoping. Appl. Phys. Lett. 2014, 104, 012405. [Google Scholar] [CrossRef]
- Ponnusamy, R.; Selvaraj, S.C.; Ramachandran, M.; Murugan, P.; Nambissan, P.M.G.; Sivasubramanian, D. Diverse spectroscopic studies and first-principles investigations of the zinc vacancy mediated ferromagnetism in Mn-doped ZnO nanoparticles. Cryst. Growth Des. 2016, 16, 3656–3668. [Google Scholar] [CrossRef]
- Cheng, C.W.; Xu, G.Y.; Zhang, H.Q.; Luo, Y. Hydrothermal synthesis Ni-doped ZnO nanorods with room-temperature ferromagnetism. Mater. Lett. 2008, 62, 1617–1620. [Google Scholar] [CrossRef]
- Gao, F.; Tan, L.X.; Wu, Z.H.; Liu, X.Y. Microstructural and optical properties of ZnO/(Ni) thin films prepared by DC magnetron sputtering. J. Alloy. Compd. 2009, 484, 489–493. [Google Scholar] [CrossRef]
- Wu, D.W.; Yang, M.; Huang, Z.B.; Yin, G.F.; Liao, X.M.; Kang, Y.Q.; Chen, X.F.; Wang, H. Preparation and properties of Ni-doped ZnO rod arrays from aqueous solution. J. Colloid Interface Sci. 2009, 330, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.M.; Wang, P.; Li, S.; Zhang, B.; Gong, H.C.; Du, Z.L. Ferromagnetism from Co-doped ZnO nanocantilevers above room temperature. Chin. Phys. Lett. 2008, 25, 4446. [Google Scholar]
- El-Hilo, M.; Dakhel, A.A.; Ali-Mohamed, A.Y. Room temperature ferromagnetism in nanocrystalline Ni-doped ZnO synthesized by co-precipitation. J. Magn. Magn. Mater. 2009, 321, 2279–2283. [Google Scholar] [CrossRef]
- Xing, G.Z.; Wang, D.D.; Yi, J.B.; Yang, L.L.; Gao, M.; He, M.; Yang, J.H.; Ding, J.; Sum, T.C.; Wu, T. Correlated d0 ferromagnetism and photoluminescence in undoped ZnO nanowires. Appl. Phys. Lett. 2010, 96, 112511. [Google Scholar] [CrossRef]


| Factors | OH−/Zn2+ | Zn2+ Concentration (mol/L) | Temperature (°C) | Ni2+ Doped Amount (%) | Time (h) |
|---|---|---|---|---|---|
| Sample 0 | 10 | 0.125 | 100 | 0 | 3 |
| Sample 1 | 10 | 0.125 | 100 | 1 | 3 |
| Sample 2 | 10 | 0.125 | 100 | 2 | 3 |
| Sample 3 | 10 | 0.125 | 100 | 3 | 3 |
| Sample 4 | 10 | 0.125 | 100 | 4 | 3 |
| Sample 5 | 10 | 0.125 | 100 | 5 | 3 |
| Factors | O Atom% | Zn Atom% | Ni Atom% | O/Zn Ratio |
|---|---|---|---|---|
| Content | 20.97% | 50.57% | 0.14% | 0.414 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Hui, S.; Zhang, F.; Wang, X.; Zhang, S.; Yan, J.; Zhang, W. Fabrication and Study on Magnetic-Optical Properties of Ni-Doped ZnO Nanorod Arrays. Micromachines 2019, 10, 622. https://doi.org/10.3390/mi10090622
Wang W, Hui S, Zhang F, Wang X, Zhang S, Yan J, Zhang W. Fabrication and Study on Magnetic-Optical Properties of Ni-Doped ZnO Nanorod Arrays. Micromachines. 2019; 10(9):622. https://doi.org/10.3390/mi10090622
Chicago/Turabian StyleWang, Wei, Shoulong Hui, Fuchun Zhang, Xiaoyang Wang, Shuili Zhang, Junfeng Yan, and Weihu Zhang. 2019. "Fabrication and Study on Magnetic-Optical Properties of Ni-Doped ZnO Nanorod Arrays" Micromachines 10, no. 9: 622. https://doi.org/10.3390/mi10090622





