Recent Developments of Flexible and Stretchable Electrochemical Biosensors
Abstract
:1. Introduction
2. Principles of Electrochemical Biosensors
2.1. Amperometric/Voltammetric Biosensors
2.2. Potentiometric Biosensors
2.3. Field-Effect Transistor (FET) Biosensors
2.4. Impedimetric Biosensors
3. Target Biofluids for the Wearable Electrochemical Biosensors
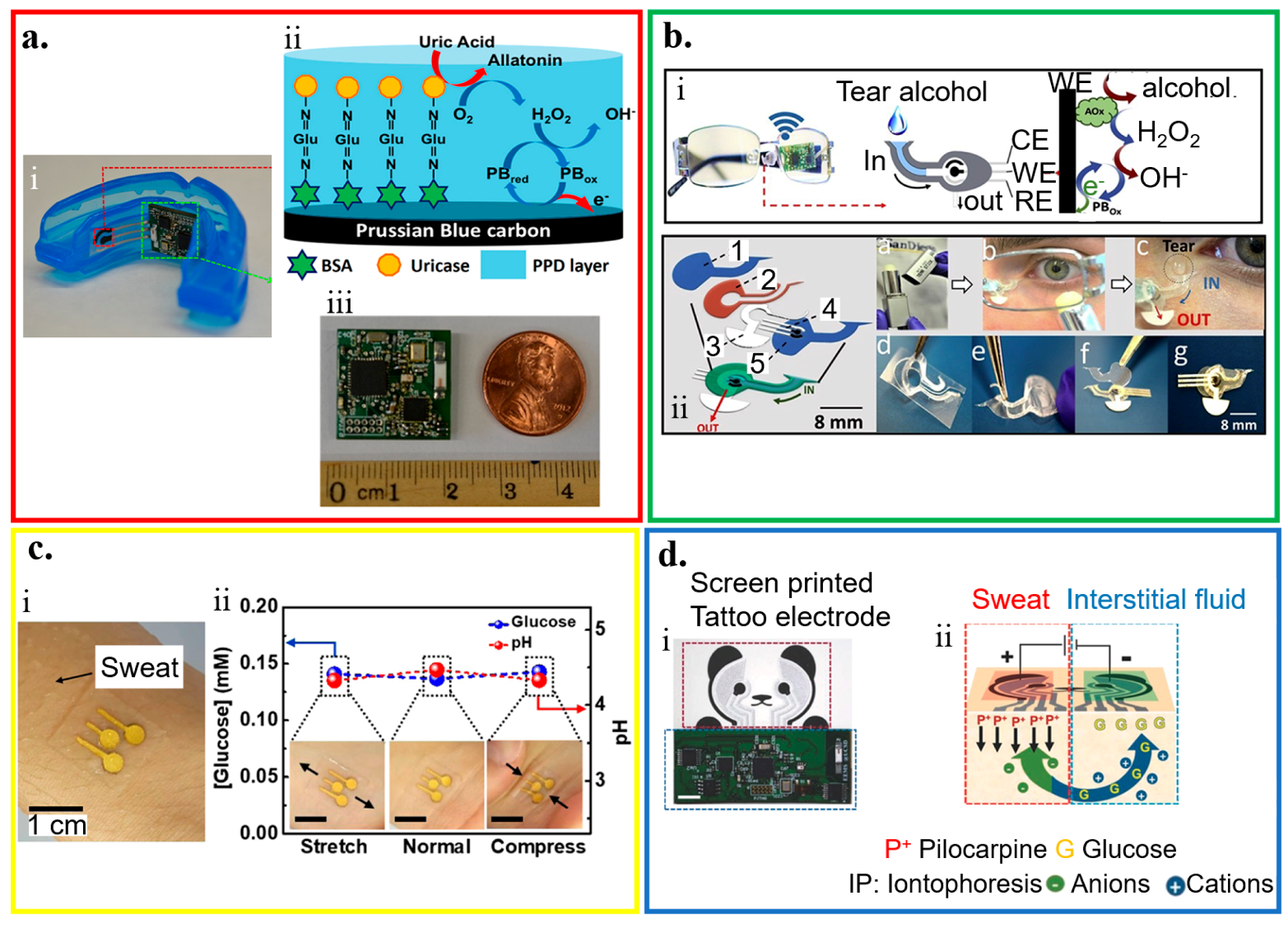
3.1. Saliva Analysis
3.2. Tear Analysis
3.3. Sweat Analysis
3.4. Interstitial Fluid (ISF) Analysis
4. Wearable Electrochemical Biosensors Based on Flexible and Stretchable Materials and Structures
4.1. Temple and Non-Template Fabrication Methods
4.2. Textile-Based Biosensors
4.3. Flexible Thin-Film Biosensors
4.3.1. Paper-Based Biosensors
4.3.2. Plastic-Based Biosensors
4.3.3. Temporary Tattoo-Based Biosensors
4.4. Stretchable Thin-Film Biosensors
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yi, N.; Cui, H.; Zhang, L.G.; Cheng, H. Integration of biological systems with electronic-mechanical assemblies. Acta Biomater. 2019, 95, 91–111. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yi, N. Dissolvable tattoo sensors: From science fiction to a viable technology. Phys. Scr. 2017, 92, 13001. [Google Scholar] [CrossRef]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-Integrated Wearable Systems: A Comprehensive Review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, Y.; Webb, R.C.; Krishnan, S.; Bian, Z.; Song, J.; Ning, X.; Crawford, K.; Kurniawan, J.; Bonifas, A.; et al. Flexible and Stretchable 3ω Sensors for Thermal Characterization of Human Skin. Adv. Funct. Mater. 2017, 27, 1701282. [Google Scholar] [CrossRef]
- Webb, R.C.; Pielak, R.M.; Bastien, P.; Ayers, J.; Niittynen, J.; Kurniawan, J.; Manco, M.; Lin, A.; Cho, N.H.; Malyrchuk, V.; et al. Thermal transport characteristics of human skin measured in vivo using ultrathin conformal arrays of thermal sensors and actuators. PLoS ONE 2015, 10, e0118131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Yang, T.; Li, X.; Zang, X.; Zhu, M.; Wang, K.; Wu, D.; Zhu, H. Wearable and highly sensitive graphene strain sensors for human motion monitoring. Adv. Funct. Mater. 2014, 24, 4666–4670. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, P.; Chou, J.B.; Xu, R.; Zhao, R.; Hart, A.J.; Kim, S.G. Extremely Elastic Wearable Carbon Nanotube Fiber Strain Sensor for Monitoring of Human Motion. ACS Nano 2015, 9, 5929–5936. [Google Scholar] [CrossRef]
- Chu, M.; Nguyen, T.; Pandey, V.; Zhou, Y.; Pham, H.N.; Bar-Yoseph, R.; Radom-Aizik, S.; Jain, R.; Cooper, D.M.; Khine, M. Respiration rate and volume measurements using wearable strain sensors. NPJ Digit. Med. 2019, 2, 8. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.E. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoringand Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef]
- Yang, L.; Yi, N.; Zhu, J.; Cheng, Z.; Yin, X.; Zhang, X.; Zhu, H.; Cheng, H. Novel gas sensing platform based on a stretchable laser-induced graphene pattern with self-heating capabilities. J. Mater. Chem. A 2020, in press. [Google Scholar] [CrossRef]
- Xiaoqi, Z.; Cheng, H. Special Topic: Flexible Electronics Manufacturing Flexible and stretchable metal oxide gas sensors for healthcare. Sci. China Technol. Sci. 2019, 62, 209–223. [Google Scholar]
- Jeong, J.W.; Kim, M.K.; Cheng, H.; Yeo, W.H.; Huang, X.; Liu, Y.; Zhang, Y.; Huang, Y.; Rogers, J.A. Capacitive epidermal electronics for electrically safe, long-term electrophysiological measurements. Adv. Healthc. Mater. 2014, 3, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.H.; Kim, Y.S.; Lee, J.; Ameen, A.; Shi, L.; Li, M.; Wang, S.; Ma, R.; Jin, S.H.; Kang, Z.; et al. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 2013, 25, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.J.S.; Lee, D.S.; Lee, J.W.; Lee, W.; Kwon, O.; Won, P.; Jung, S.-Y.; Cheng, H.; Jeong, J.-W.; Akce, A.; et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain–computer interface. Proc. Natl. Acad. Sci. USA 2015, 112, 3920–3925. [Google Scholar] [CrossRef] [Green Version]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Turner, A. Biosensors: Then and now. Trends Biotechnol. 2013, 31, 119–120. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Thvenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification (Technical Report). Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Gutbrod, S.R.; Bonifas, A.P.; Su, Y.; Sulkin, M.S.; Lu, N.; Chung, H.J.; Jang, K.I.; Liu, Z.; Ying, M.; et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 2014, 5, 3329. [Google Scholar] [CrossRef]
- Gutbrod, S.R.; Sulkin, M.S.; Rogers, J.A.; Efimov, I.R. Patient-specific flexible and stretchable devices for cardiac diagnostics and therapy. Prog. Biophys. Mol. Biol. 2014, 115, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Fahad, H.M.; Chen, K.; Emaminejad, S.; Gao, Y.; Tai, L.C.; Ota, H.; Wu, E.; et al. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.H. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lu, S.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Lu, B.; Wang, X.; Feng, X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef] [Green Version]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable Sensors for Biochemical Sweat Analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Ramnarayanan, A.; Cheng, H. Real time analysis of bioanalytes in healthcare, food, zoology and botany. Sensors 2018, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Albareda-Sirvent, M.; Merkoçi, A.; Alegret, S. Configurations used in the design of screen-printed enzymatic biosensors. A review. Sens. Actuators B Chem. 2000, 69, 153–163. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef]
- Yang, Y.L.; Chuang, M.C.; Lou, S.L.; Wang, J. Thick-film textile-based amperometric sensors and biosensors. Analyst 2010, 135, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Huang, Z.; Xu, S. Materials and Structures toward Soft Electronics. Adv. Mater. 2018, 30, e1801368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.W.; Lee, C.H.; Cheng, H.; Jeong, J.W.; Kang, S.K.; Kim, J.H.; Shin, J.; Yang, J.; Liu, Z.; Ameer, G.A.; et al. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett. 2015, 15, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, G.; Zhang, Y.; Yue, M.; Chen, Y.; Cai, S.; Xie, T.; Feng, X. Direct Fabrication of Stretchable Electronics on a Polymer Substrate with Process-Integrated Programmable Rigidity. Adv. Funct. Mater. 2018, 28, 1804604. [Google Scholar] [CrossRef]
- Morris, D.; Coyle, S.; Wu, Y.; Lau, K.T.; Wallace, G.; Diamond, D. Bio-sensing textile based patch with integrated optical detection system for sweat monitoring. Sens. Actuators B Chem. 2009, 139, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Coyle, S.; Lau, K.T.; Moyna, N.; O’Gorman, D.; Diamond, D.; Di Francesco, F.; Costanzo, D.; Salvo, P.; Trivella, M.G.; De Rossi, D.E.; et al. BIOTEXBiosensing textiles for personalised healthcare management. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Benito-Lopez, F.; Coyle, S.; Byrne, R.; Smeaton, A.; O’Connor, N.E.; Diamond, D. Pump Less Wearable Microfluidic Device for Real Time pH Sweat Monitoring. Proc. Procedia Chem. 2009, 1, 1103–1106. [Google Scholar] [CrossRef] [Green Version]
- Bandodkar, A.J.; Jia, W.; Wang, J. Tattoo-Based Wearable Electrochemical Devices: A Review. Electroanalysis 2015, 27, 562–572. [Google Scholar] [CrossRef]
- Scilingo, E.P.; Lorussi, F.; Mazzoldi, A.; De Rossi, D. Strain-sensing fabrics for wearable kinaesthetic-like systems. IEEE Sens. J. 2003, 3, 460–467. [Google Scholar] [CrossRef]
- Steinberg, M.D.; Kassal, P.; Steinberg, I.M. System Architectures in Wearable Electrochemical Sensors. Electroanalysis 2016, 28, 1149–1169. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mettakoonpitak, J.; Boehle, K.; Nantaphol, S.; Teengam, P.; Adkins, J.A.; Srisa-Art, M.; Henry, C.S. Electrochemistry on Paper-based Analytical Devices: A Review. Electroanalysis 2016, 28, 1420–1436. [Google Scholar] [CrossRef]
- Moschou, D.; Tserepi, A. The lab-on-PCB approach: Tackling the μTAS commercial upscaling bottleneck. Lab Chip 2017, 17, 1388–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsubayashi, K.; Arakawa, T. Cavitas Sensors: Contact Lens Type Sensors & Mouthguard Sensors. Electroanalysis 2016, 28, 1170–1187. [Google Scholar]
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Song, Y.; Ye, Y.; Gong, C.; Shen, Y.; Wang, L.; Wang, L. A novel flexible electrochemical glucose sensor based on gold nanoparticles/polyaniline arrays/carbon cloth electrode. Sens. Actuators B Chem. 2017, 252, 1187–1193. [Google Scholar] [CrossRef]
- Yao, S.; Myers, A.; Malhotra, A.; Lin, F.; Bozkurt, A.; Muth, J.F.; Zhu, Y. A Wearable Hydration Sensor with Conformal Nanowire Electrodes. Adv. Healthc. Mater. 2017, 6, 55. [Google Scholar] [CrossRef]
- Kim, D.H.; Lu, N.; Ma, R.; Kim, Y.S.; Kim, R.H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Benson, J.; Fung, C.M.; Lloyd, J.S.; Deganello, D.; Smith, N.A.; Teng, K.S. Direct patterning of gold nanoparticles using flexographic printing for biosensing applications. Nanoscale Res. Lett. 2015, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Windmiller, J.R.; Wang, J. Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Rymansaib, Z.; Iravani, P.; Emslie, E.; Medvidović-Kosanović, M.; Sak-Bosnar, M.; Verdejo, R.; Marken, F. All-Polystyrene 3D-Printed Electrochemical Device with Embedded Carbon Nanofiber-Graphite-Polystyrene Composite Conductor. Electroanalysis 2016, 28, 1517–1523. [Google Scholar] [CrossRef] [Green Version]
- Weng, B.; Shepherd, R.L.; Crowley, K.; Killard, A.J.; Wallace, G.G. Printing conducting polymers. Analyst 2010, 135, 2779–2789. [Google Scholar] [CrossRef] [PubMed]
- Ruecha, N.; Chailapakul, O.; Suzuki, K.; Citterio, D. Fully Inkjet-Printed Paper-Based Potentiometric Ion-Sensing Devices. Anal. Chem. 2017, 89, 10608–10616. [Google Scholar] [CrossRef] [PubMed]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.M.; Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical detection for paper-based microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Bandodkar, A.J.; Valdés-Ramírez, G.; Parkhomovsky, S.; Martinez, A.G.; Wang, J. Electrochemical sensing based on printable temporary transfer tattoos. Chem. Commun. 2012, 48, 6794–6796. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; You, J.M.; Nuñez-Flores, R.; Wang, J. Highly Stretchable Fully-Printed CNT-Based Electrochemical Sensors and Biofuel Cells: Combining Intrinsic and Design-Induced Stretchability. Nano Lett. 2016, 16, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Liao, Y.; Lingley, A.R.; Afanasiev, A.; Lähdesmäki, I.; Otis, B.P.; Parviz, B.A. A contact lens with integrated telecommunication circuit and sensors for wireless and continuous tear glucose monitoring. J. Micromech. Microeng. 2012, 22, 7. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardimci, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. A biocompatible serine functionalized nanostructured zirconia based biosensing platform for non-invasive oral cancer detection. RSC Adv. 2016, 6, 77037–77046. [Google Scholar] [CrossRef]
- Nemiroski, A.; Christodouleas, D.C.; Hennek, J.W.; Kumar, A.A.; Maxwell, E.J.; Fernández-Abedul, M.T.; Whitesides, G.M. Universal mobile electrochemical detector designed for use in resource-limited applications. Proc. Natl. Acad. Sci. USA 2014, 111, 11984–11989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaisti, M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 2017, 98, 437–448. [Google Scholar] [CrossRef]
- He, H.; Chang, D.C.; Lee, Y.K. Nonlinear current response of micro electroporation and resealing dynamics for human cancer cells. Bioelectrochemistry 2008, 72, 161–168. [Google Scholar] [CrossRef]
- De Guzman, K.; Morrin, A. Screen-printed Tattoo Sensor towards the Non-invasive Assessment of the Skin Barrier. Electroanalysis 2017, 29, 188–196. [Google Scholar] [CrossRef]
- Flanagan, R.J.; Perrett, D.; Whelpton, R. Royal Society of Chemistry (Great Britain) Electrochemical detection in HPLC analysis of drugs and poisons. RSC Chromatogr. Monogr. 2005, Chapter 2. 6–16. [Google Scholar]
- Helm, I.; Jalukse, L.; Leito, I. Measurement uncertainty estimation in amperometric sensors: A tutorial review. Sensors 2010, 10, 4430–4455. [Google Scholar] [CrossRef] [Green Version]
- Ghoreishizadeh, S.S.; Taurino, I.; De Micheli, G.; Carrara, S.; Georgiou, P. A Differential Electrochemical Readout ASIC with Heterogeneous Integration of Bio-Nano Sensors for Amperometric Sensing. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 1148–1159. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, K.; Xia, X. Highly ordered platinum-nanotubule arrays for amperometric glucose sensing. Adv. Funct. Mater. 2005, 15, 803–809. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Bobrova, O.A.; Lukachova, L.V.; Karyakina, E.E. Potentiometric biosensors based on polyaniline semiconductor films. Sens. Actuators B Chem. 1996, 33, 34–38. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Z.; Yan, B.; Zheng, Z.; Bao, Q.L.; Wu, T.; Li, C.M.; Shen, Z.X.; Zhang, J.X.; Gong, H.; et al. Multifunctional CuO nanowire devices: P-type field effect transistors and CO gas sensors. Nanotechnology 2009, 20, 085203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Li, X.; Kraatz, H.B. Impedimetric immobilized DNA-based sensor for simultaneous detection of Pb2+, Ag+, and Hg2+. Anal. Chem. 2011, 83, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Covington, A.K. Terminology and conventions for microelectronic ion-selective field effect transistor devices in electrochemistry (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Julian, R.; Mercier, P.; Wang, J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar]
- Ciui, B.; Tertis, M.; Feurdean, C.N.; Ilea, A.; Sandulescu, R.; Wang, J.; Cristea, C. Cavitas electrochemical sensor toward detection of N-epsilon (carboxymethyl)lysine in oral cavity. Sens. Actuators B Chem. 2019, 281, 399–407. [Google Scholar] [CrossRef]
- Rabti, A.; Argoubi, W.; Raouafi, N. Enzymatic sensing of glucose in artificial saliva using a flat electrode consisting of a nanocomposite prepared from reduced graphene oxide, chitosan, nafion and glucose oxidase. Microchim. Acta 2016, 183, 1227–1233. [Google Scholar] [CrossRef]
- Zhang, W.; Du, Y.; Wang, M.L. On-chip highly sensitive saliva glucose sensing using multilayer films composed of single-walled carbon nanotubes, gold nanoparticles, and glucose oxidase. Sens. Bio-Sens. Res. 2015, 4, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Sheng, Y.; Sun, Y.; Feng, J.; Wang, S.; Zhang, J.; Xu, J.; Jiang, D. A glucose oxidase-coupled DNAzyme sensor for glucose detection in tears and saliva. Biosens. Bioelectron. 2015, 70, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Lähdesmäki, I.; Parviz, B.A. A contact lens with an integrated lactate sensor. Sens. Actuators B Chem. 2012, 162, 128–134. [Google Scholar] [CrossRef]
- Yao, H.; Marcheselli, C.; Afanasiev, A.; Lähdesmäki, I.; Parviz, B.A. A soft hydrogel contact lens with an encapsulated sensor for tear glucose monitoring. Proc. IEEE Int. Conf. Micro Electro Mech. Syst. 2012, 769–772. [Google Scholar]
- Romeo, A.; Moya, A.; Leung, T.S.; Gabriel, G.; Villa, R.; Sánchez, S. Inkjet printed flexible non-enzymatic glucose sensor for tear fluid analysis. Appl. Mater. Today 2018, 10, 133–141. [Google Scholar] [CrossRef]
- Oh, S.Y.; Hong, S.Y.; Jeong, Y.R.; Yun, J.; Park, H.; Jin, S.W.; Lee, G.; Oh, J.H.; Lee, H.; Lee, S.S.; et al. Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; De Araujo, W.R.; Samek, I.A.; Bandodkar, A.J.; Jia, W.; Brunetti, B.; Paixão, T.R.L.C.; Wang, J. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 2015, 51, 41–45. [Google Scholar] [CrossRef]
- Parrilla, M.; Ferré, J.; Guinovart, T.; Andrade, F.J. Wearable Potentiometric Sensors Based on Commercial Carbon Fibres for Monitoring Sodium in Sweat. Electroanalysis 2016, 28, 1267–1275. [Google Scholar] [CrossRef]
- Lin, Y.; Bariya, M.; Nyein, H.Y.Y.; Kivimäki, L.; Uusitalo, S.; Jansson, E.; Ji, W.; Yuan, Z.; Happonen, T.; Liedert, C.; et al. Porous Enzymatic Membrane for Nanotextured Glucose Sweat Sensors with High Stability toward Reliable Noninvasive Health Monitoring. Adv. Funct. Mater. 2019, 29, 1902521. [Google Scholar] [CrossRef]
- Tur-García, E.L.; Davis, F.; Collyer, S.D.; Holmes, J.L.; Barr, H.; Higson, S.P.J. Novel flexible enzyme laminate-based sensor for analysis of lactate in sweat. Sens. Actuators B Chem. 2017, 242, 502–510. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci. 2018, 5, 1800880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sempionatto, J.R.; Brazaca, L.C.; García-Carmona, L.; Bolat, G.; Campbell, A.S.; Martin, A.; Tang, G.; Shah, R.; Mishra, R.K.; Kim, J.; et al. Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 2019, 137, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Timchalk, C.; Lin, Y. Carbon Nanotube-Based Electrochemical Sensor for Assay of Salivary Cholinesterase Enzyme Activity: An Exposure Biomarker of Organophosphate Pesticides and Nerve Agents. Environ. Sci. Technol. 2008, 42, 2688–2693. [Google Scholar] [CrossRef]
- Jalbert, I. Diet, nutraceuticals and the tear film. Exp. Eye Res. 2013, 117, 138–146. [Google Scholar] [CrossRef]
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–3296. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.T.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B.P. A 3-μW CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE J. Solid-State Circuits 2012, 47, 335–344. [Google Scholar] [CrossRef]
- Chu, M.X.; Miyajima, K.; Takahashi, D.; Arakawa, T.; Sano, K.; Sawada, S.I.; Kudo, H.; Iwasaki, Y.; Akiyoshi, K.; Mochizuki, M.; et al. Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta 2011, 83, 960–965. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.S.; Kim, K.; Ji, S.; Kim, Y.T.; Park, J.; Na, K.; Bae, K.H.; Kim, H.K.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Fogh-Andersen, N.; Altura, B.M.; Altura, B.T.; Siggaard-Andersen, O. Composition of interstitial fluid. Clin. Chem. 1995, 41, 1522–1525. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Guy, R.H.; Glikfeld, P.; LaCourse, W.R.; Leung, L.; Tamada, J.; Potts, R.O.; Azimi, N. Reverse Iontophoresis: Noninvasive Glucose Monitoring in Vivo in Humans. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 1869–1873. [Google Scholar]
- Leboulanger, B.; Guy, R.H.; Delgado-Charro, M.B. Reverse iontophoresis for non-invasive transdermal monitoring. Physiol. Meas. 2004, 25, R35–R50. [Google Scholar] [CrossRef] [PubMed]
- Edelman, S.V. Watching your glucose with the glucowatch. Diabetes Technol. Ther. 2001, 3, 283–284. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef]
- Yu, H.; Li, D.; Roberts, R.C.; Xu, K.; Tien, N.C. An interstitial fluid transdermal extraction system for continuous glucose monitoring. J. Microelectromec. Syst. 2012, 21, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Hwang, D.; Yu, Z.; Takei, K.; Park, J.; Chen, T.; Ma, B.; Javey, A. User-interactive electronic skin for instantaneous pressure visualization. Nat. Mater. 2013, 12, 899–904. [Google Scholar] [CrossRef]
- Hondred, J.A.; Stromberg, L.R.; Mosher, C.L.; Claussen, J.C. High-Resolution Graphene Films for Electrochemical Sensing via Inkjet Maskless Lithography. ACS Nano 2017, 11, 9836–9845. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Chen, C.H.; Hwang, B.J.; Luo, J.D.; Chiou, C.C.; Tian, Y.C.; Lin, C.Y.; Cheng, C.H.; Lai, C.S. Spin-coated Au-nanohole arrays engineered by nanosphere lithography for a Staphylococcus aureus 16S rRNA electrochemical sensor. Biosens. Bioelectron. 2016, 77, 1086–1094. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, S.Y.; Cheong, W.H.; Jang, J.; Park, Y.G.; Na, K.; Kim, Y.T.; Heo, J.H.; Lee, C.Y.; et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018, 4, eaap9841. [Google Scholar] [CrossRef] [Green Version]
- Eshkeiti, A.; Reddy, A.S.G.; Emamian, S.; Narakathu, B.B.; Joyce, M.; Joyce, M.; Fleming, P.D.; Bazuin, B.J.; Atashbar, M.Z. Screen printing of multilayered hybrid printed circuit boards on different substrates. IEEE Trans. Compon. Packag. Manuf. Technol. 2015, 5, 415–421. [Google Scholar] [CrossRef]
- Fung, C.M.; Lloyd, J.S.; Samavat, S.; Deganello, D.; Teng, K.S. Facile fabrication of electrochemical ZnO nanowire glucose biosensor using roll to roll printing technique. Sens. Actuators B Chem. 2017, 247, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Bariya, M.; Shahpar, Z.; Park, H.; Sun, J.; Jung, Y.; Gao, W.; Nyein, H.Y.Y.; Liaw, T.S.; Tai, L.C.; Ngo, Q.P.; et al. Roll-to-Roll Gravure Printed Electrochemical Sensors for Wearable and Medical Devices. ACS Nano 2018, 12, 6978–6987. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Fu, J.Z.; Gao, Q.; Qiu, J.J. Developments of 3D Printing Microfluidics and Applications in Chemistry and Biology: A Review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Moya, A.; Gabriel, G.; Villa, R.; Javier del Campo, F. Inkjet-printed electrochemical sensors. Curr. Opin. Electrochem. 2017, 3, 29–39. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Hart, J.P. Screen-printed electrochemical sensors for monitoring metal pollutants. TrAC-Trends Anal. Chem. 2003, 22, 456–469. [Google Scholar] [CrossRef]
- Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Screen-printed biosensors in microbiology; A review. Talanta 2010, 82, 1629–1636. [Google Scholar] [CrossRef]
- Chuang, M.C.; Windmiller, J.R.; Santhosh, P.; Ramírez, G.V.; Galik, M.; Chou, T.Y.; Wang, J. Textile-based Electrochemical Sensing: Effect of Fabric Substrate and Detection of Nitroaromatic Explosives. Electroanalysis 2010, 22, 2511–2518. [Google Scholar] [CrossRef]
- Overgaard, M.H.; Sahlgren, N.M.; Hvidsten, R.; Kühnel, M.; Dalby, K.N.; Vosch, T.; Laursen, B.W.; Nørgaard, K. Facile Synthesis of Mildly Oxidized Graphite Inks for Screen-Printing of Highly Conductive Electrodes. Adv. Eng. Mater. 2019, 21, 1801304. [Google Scholar] [CrossRef]
- Kim, S.; Sojoudi, H.; Zhao, H.; Mariappan, D.; McKinley, G.H.; Gleason, K.K.; Hart, A.J. Ultrathin high-resolution flexographic printing using nanoporous stamps. Sci. Adv. 2016, 2, e1601660. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Park, H.; Park, J.A.; Noh, J.; Choi, Y.; Jung, M.; Jung, K.; Pyo, M.; Chen, K.; Javey, A.; et al. Fully printed flexible and disposable wireless cyclic voltammetry tag. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, P.H.; Takei, K.; Wang, C.; Ju, Y.; Kim, J.; Yu, Z.; Takahashi, T.; Cho, G.; Javey, A. Fully printed, high performance carbon nanotube thin-film transistors on flexible substrates. Nano Lett. 2013, 13, 3864–3869. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Koo, H.; Sun, J.; Noh, J.; Kwon, K.S.; Yeom, C.; Choi, Y.; Chen, K.; Javey, A.; Cho, G. A fully roll-to-roll gravure-printed carbon nanotube-based active matrix for multi-touch sensors. Sci. Rep. 2015, 5, 17707. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.; Chen, K.; Kiriya, D.; Yu, Z.; Cho, G.; Javey, A. Large-area compliant tactile sensors using printed carbon nanotube active-matrix backplanes. Adv. Mater. 2015, 27, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced Materials for Printed Wearable Electrochemical Devices: A Review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Suganuma, K. Introduction to Printed Electronics; Springer: New York, NY, USA, 2014; Volume 74, ISBN 978-1-4614-9624-3. [Google Scholar]
- Wang, Y.; Qing, X.; Zhou, Q.; Zhang, Y.; Liu, Q.; Liu, K.; Wang, W.; Li, M.; Lu, Z.; Chen, Y.; et al. The woven fiber organic electrochemical transistors based on polypyrrole nanowires/reduced graphene oxide composites for glucose sensing. Biosens. Bioelectron. 2017, 95, 138–145. [Google Scholar] [CrossRef]
- Wu, S.; Liu, P.; Zhang, Y.; Zhang, H.; Qin, X. Flexible and conductive nanofiber-structured single yarn sensor for smart wearable devices. Sens. Actuators B Chem. 2017, 252, 697–705. [Google Scholar] [CrossRef]
- Parrilla, M.; Cánovas, R.; Jeerapan, I.; Andrade, F.J.; Wang, J. A textile-based stretchable multi-ion potentiometric sensor. Adv. Healthc. Mater. 2016, 5, 996–1001. [Google Scholar] [CrossRef]
- Malzahn, K.; Windmiller, J.R.; Valdés-Ramírez, G.; Schöning, M.J.; Wang, J. Wearable electrochemical sensors for in situ analysis in marine environments. Analyst 2011, 136, 2912–2917. [Google Scholar] [CrossRef]
- Malon, R.S.P.; Chua, K.Y.; Wicaksono, D.H.B.; Córcoles, E.P. Cotton fabric-based electrochemical device for lactate measurement in saliva. Analyst 2014, 139, 3009–3016. [Google Scholar] [CrossRef]
- Modali, A.; Vanjari, S.R.K.; Dendukuri, D. Wearable Woven Electrochemical Biosensor Patch for Non-invasive Diagnostics. Electroanalysis 2016, 28, 1276–1282. [Google Scholar] [CrossRef]
- Liu, X.; Lillehoj, P.B. Embroidered electrochemical sensors for biomolecular detection. Lab Chip 2016, 16, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Mohammadifar, M.; Choi, S. A single-use, self-powered, paper-based sensor patch for detection of exercise-induced hypoglycemia. Micromachines 2017, 8, 265. [Google Scholar]
- Cinti, S.; Fiore, L.; Massoud, R.; Cortese, C.; Moscone, D.; Palleschi, G.; Arduini, F. Low-cost and reagent-free paper-based device to detect chloride ions in serum and sweat. Talanta 2018, 179, 186–192. [Google Scholar] [CrossRef]
- Cho, E.; Mohammadifar, M.; Choi, S. A self-powered sensor patch for glucose monitoring in sweat. IEEE Int. Conf. Micro Electro Mech. Syst. 2017, 366–369. [Google Scholar]
- Bujes-Garrido, J.; Arcos-Martínez, M.J. Development of a wearable electrochemical sensor for voltammetric determination of chloride ions. Sens. Actuators B Chem. 2017, 240, 224–228. [Google Scholar] [CrossRef]
- Santana-Jiménez, L.; Márquez-Lucero, A.; Osuna, V.; Estrada-Moreno, I.; Dominguez, R.B. Naked-Eye detection of glucose in saliva with bienzymatic paper-based sensor. Sensors 2018, 18, 1071. [Google Scholar]
- Weltin, A.; Kieninger, J.; Enderle, B.; Gellner, A.K.; Fritsch, B.; Urban, G.A. Polymer-based, flexible glutamate and lactate microsensors for in vivo applications. Biosens. Bioelectron. 2014, 61, 192–199. [Google Scholar] [CrossRef]
- Yang, X.; Fu, T.; Kota, P.; Tjia, M.; Nguyen, C.; Chiao, J.-C. Lactate Sensors on Flexible Substrates. Biosensors 2016, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.W.; Yan, F. Flexible organic electrochemical transistors for highly selective enzyme biosensors and used for saliva testing. Adv. Mater. 2015, 27, 676–681. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, T.; Song, X.; Ran, Q.; Yu, C.; Yang, J.; Feng, H.; Yu, L.; Wei, D. Flexible electrochemical biosensors based on graphene nanowalls for the real-time measurement of lactate. Nanotechnology 2017, 28, 315501. [Google Scholar] [CrossRef] [PubMed]
- Munje, R.D.; Muthukumar, S.; Prasad, S. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuators B Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Minami, T.; Sato, T.; Minamiki, T.; Fukuda, K.; Kumaki, D.; Tokito, S. A novel OFET-based biosensor for the selective and sensitive detection of lactate levels. Biosens. Bioelectron. 2015, 74, 45–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallón, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Abrar, M.A.; Dong, Y.; Lee, P.K.; Kim, W.S. Bendable Electro-chemical Lactate Sensor Printed with Silver Nano-particles. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bandodkar, A.J.; Hung, V.W.S.; Jia, W.; Valdés-Ramírez, G.; Windmiller, J.R.; Martinez, A.G.; Ramírez, J.; Chan, G.; Kerman, K.; Wang, J. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 2013, 138, 123–128. [Google Scholar] [CrossRef]
- Guinovart, T.; Bandodkar, A.J.; Windmiller, J.R.; Andrade, F.J.; Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 2013, 138, 7031–7038. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 1, 1011–1019. [Google Scholar] [CrossRef]
- Yin, S.; Jin, Z.; Miyake, T. Wearable high-powered biofuel cells using enzyme/carbon nanotube composite fibers on textile cloth. Biosens. Bioelectron. 2019, 141, 111471. [Google Scholar] [CrossRef]
- Choudhary, T.; Rajamanickam, G.P.; Dendukuri, D. Woven electrochemical fabric-based test sensors (WEFTS): A new class of multiplexed electrochemical sensors. Lab Chip 2015, 15, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ran, R.; Yang, Z.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.H. An efficient flexible electrochemical glucose sensor based on carbon nanotubes/carbonized silk fabrics decorated with Pt microspheres. Sens. Actuators B Chem. 2018, 256, 63–70. [Google Scholar] [CrossRef]
- Economou, A.; Kokkinos, C.; Prodromidis, M. Flexible plastic, paper and textile lab-on-a chip platforms for electrochemical biosensing. Lab Chip 2018, 18, 1812–1830. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TrAC-Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Mak, W.C.; Beni, V.; Turner, A.P.F. Lateral-flow technology: From visual to instrumental. TrAC-Trends Anal. Chem. 2016, 79, 297–305. [Google Scholar] [CrossRef]
- Ruiz-Vega, G.; Kitsara, M.; Pellitero, M.A.; Baldrich, E.; del Campo, F.J. Electrochemical Lateral Flow Devices: Towards Rapid Immunomagnetic Assays. ChemElectroChem 2017, 4, 880–889. [Google Scholar] [CrossRef]
- Wang, C.C.; Hennek, J.W.; Ainla, A.; Kumar, A.A.; Lan, W.J.; Im, J.; Smith, B.S.; Zhao, M.; Whitesides, G.M. A Paper-Based Pop-up Electrochemical Device for Analysis of Beta-Hydroxybutyrate. Anal. Chem. 2016, 88, 6326–6333. [Google Scholar] [CrossRef] [Green Version]
- Apilux, A.; Dungchai, W.; Siangproh, W.; Praphairaksit, N.; Henry, C.S.; Chailapakul, O. Lab-on-paper with dual electrochemical/colorimetric detection for simultaneous determination of gold and iron. Anal. Chem. 2010, 82, 1727–1732. [Google Scholar] [CrossRef]
- Colozza, N.; Kehe, K.; Dionisi, G.; Popp, T.; Tsoutsoulopoulos, A.; Steinritz, D.; Moscone, D.; Arduini, F. A wearable origami-like paper-based electrochemical biosensor for sulfur mustard detection. Biosens. Bioelectron. 2019, 129, 15–23. [Google Scholar] [CrossRef]
- Fosdick, S.E.; Anderson, M.J.; Renault, C.; Degregory, P.R.; Loussaert, J.A.; Crooks, R.M. Wire, mesh, and fiber electrodes for paper-based electroanalytical devices. Anal. Chem. 2014, 86, 3659–3666. [Google Scholar] [CrossRef]
- Dossi, N.; Toniolo, R.; Impellizzieri, F.; Bontempelli, G. Doped pencil leads for drawing modified electrodes on paper-based electrochemical devices. J. Electroanal. Chem. 2014, 722, 90–94. [Google Scholar] [CrossRef]
- Tortorich, R.P.; Shamkhalichenar, H.; Choi, J.W. Inkjet-printed and paper-based electrochemical sensors. Appl. Sci. 2018, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Määttänen, A.; Vanamo, U.; Ihalainen, P.; Pulkkinen, P.; Tenhu, H.; Bobacka, J.; Peltonen, J. A low-cost paper-based inkjet-printed platform for electrochemical analyses. Sens. Actuators B Chem. 2013, 177, 153–162. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. A Disposable Planar Paper-Based Potentiometric Ion-Sensing Platform. Angew. Chem.-Int. Ed. 2016, 55, 7544–7547. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Goswami, D.; Cuellar, H.E.; Castro, B.; Kuang, S.; Martinez, R.V. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 2018, 117, 696–705. [Google Scholar] [CrossRef] [PubMed]
- McLister, A.; McHugh, J.; Cundell, J.; Davis, J. New Developments in Smart Bandage Technologies for Wound Diagnostics. Adv. Mater. 2016, 28, 5732–5737. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bao, N.; Yuan, W.; Si, N.; Bai, H.; Li, H.; Zhang, Q. Simultaneous determination of lead, arsenic, and mercury in cosmetics using a plastic based disposable electrochemical sensor. Microchem. J. 2019, 148, 240–247. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Lu, W.; Yuan, Q.; Zheng, Y.; Yao, B. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta 2019, 198, 86–92. [Google Scholar] [CrossRef]
- Avuthu, S.G.R.; Wabeke, J.T.; Narakathu, B.B.; Maddipatla, D.; Arachchilage, J.S.; Obare, S.O.; Atashbar, M.Z. A Screen Printed Phenanthroline-Based Flexible Electrochemical Sensor for Selective Detection of Toxic Heavy Metal Ions. Proc. IEEE Sens. J. 2016, 16, 8678–8684. [Google Scholar] [CrossRef]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 84, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, K.; Jiang, N.; Liu, X.; Kan, V.; Barry, N.; Maes, P.; Yetisen, A.; Paradiso, J. The dermal abyss: Interfacing with the skin by tattooing biosensors. In Proceedings of the International Symposium on Wearable Computers, Maui, HI, USA, 13–15 September 2017. [Google Scholar]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schöning, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Barfidokht, A.; Karajic, A.; Sempionatto, J.R.; Wang, J.; Wang, J. Wearable potentiometric tattoo biosensor for on-body detection of G-type nerve agents simulants. Sens. Actuators B Chem. 2018, 273, 966–972. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, W.; Shuang, S.; Nair, M.; Li, C.Z. Intelligent tattoos, patches, and other wearable biosensors. In Medical Biosensors for Point of Care (POC) Applications; Woodhead Publishing: Cambridge, UK, 2017; pp. 133–150. ISBN 9780081000786. [Google Scholar]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Iguchi, S.; Kudo, H.; Saito, T.; Ogawa, M.; Saito, H.; Otsuka, K.; Funakubo, A.; Mitsubayashi, K. A flexible and wearable biosensor for tear glucose measurement. Biomed. Microdevices 2007, 9, 603–609. [Google Scholar] [CrossRef]
- Lim, H.R.; Kim, H.S.; Qazi, R.; Kwon, Y.T.; Jeong, J.W.; Yeo, W.H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2019, 1901924. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, H.; Su, Y.; Xu, S.; Cheng, H.; Fan, J.A.; Hwang, K.C.; Rogers, J.A.; Huang, Y. Mechanics of ultra-stretchable self-similar serpentine interconnects. Acta Mater. 2013, 61, 7816–7827. [Google Scholar] [CrossRef]
- Jang, K.I.; Han, S.Y.; Xu, S.; Mathewson, K.E.; Zhang, Y.; Jeong, J.W.; Kim, G.T.; Webb, R.C.; Lee, J.W.; Dawidczyk, T.J.; et al. Rugged and breathable forms of stretchable electronics with adherent composite substrates for transcutaneous monitoring. Nat. Commun. 2014, 5, 4779. [Google Scholar] [CrossRef]
- Ying, M.; Bonifas, A.P.; Lu, N.; Su, Y.; Li, R.; Cheng, H.; Ameen, A.; Huang, Y.; Rogers, J.A. Silicon nanomembranes for fingertip electronics. Nanotechnology 2012, 23, 344004. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Cheng, H.; Shin, W.J.; Fan, J.A.; Liu, Z.; Lu, C.J.; Kong, G.W.; Chen, K.; Patnaik, D.; et al. Materials and designs for wireless epidermal sensors of hydration and strain. Adv. Funct. Mater. 2014, 24, 3846–3854. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Chen, X.; Bao, Z.; Someya, T. Materials and structural designs of stretchable conductors. Chem. Soc. Rev. 2019, 48, 2946–2966. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.H.; Liu, Y.L.; Chen, J.J.; Cai, S.L.; Xu, J.Q.; Huang, W.H. Conductive Polymer-Coated Carbon Nanotubes to Construct Stretchable and Transparent Electrochemical Sensors. Anal. Chem. 2017, 89, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ni, B.; Yuan, Q.; Wang, Y.; Zhang, Q.; Wang, X. Finely Composition-Tunable Synthesis of Ultrafine Wavy PtRu Nanowires as Effective Electrochemical Sensors for Dopamine Detection. Langmuir 2017, 33, 8070–8075. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Nuñez-Flores, R.; Jia, W.; Wang, J. All-printed stretchable electrochemical devices. Adv. Mater. 2015, 27, 3060–3065. [Google Scholar] [CrossRef]
- Cánovas, R.; Parrilla, M.; Mercier, P.; Andrade, F.J.; Wang, J. Balloon-Embedded Sensors Withstanding Extreme Multiaxial Stretching and Global Bending Mechanical Stress: Towards Environmental and Security Monitoring. Adv. Mater. Technol. 2016, 1, 1600061. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566. [Google Scholar] [CrossRef]
- Lim, Y.; Heo, J.-I.; Shin, H. Fabrication and application of a stacked carbon electrode set including a suspended mesh made of nanowires and a substrate-bound planar electrode toward for an electrochemical/biosensor platform. Sens. Actuators B Chem. 2014, 192, 796–803. [Google Scholar] [CrossRef]
- Mohan, A.M.V.; Kim, N.H.; Gu, Y.; Bandodkar, A.J.; You, J.M.; Kumar, R.; Kurniawan, J.F.; Xu, S.; Wang, J. Merging of Thin- and Thick-Film Fabrication Technologies: Toward Soft Stretchable “Island–Bridge” Devices. Adv. Mater. Technol. 2017, 2, 1600284. [Google Scholar] [CrossRef]
- Filiatrault, H.L.; Porteous, G.C.; Carmichael, R.S.; Davidson, G.J.E.; Carmichael, T.B. Stretchable light-emitting electrochemical cells using an elastomeric emissive material. Adv. Mater. 2012, 24, 2673–2678. [Google Scholar] [CrossRef]
- Kim, J.; Banks, A.; Cheng, H.; Xie, Z.; Xu, S.; Jang, K.-I.; Lee, J.W.; Liu, Z.; Gutruf, P.; Huang, X.; et al. Epidermal electronics with advanced capabilities in near-field communication. Small 2015, 11, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cheng, H. Recent Development of Flexible and Stretchable Antennas for Bio-Integrated Electronics. Sensors 2018, 18, 4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Avila, R.; Huang, Y.; Rogers, J.A. Flexible and Stretchable Antennas for Biointegrated Electronics. Adv. Mater. 2019, 1902767. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.J.; Tamada, J.A.; Potts, R.O.; Eastman, R.C.; Pitzer, K.; Ackerman, N.R.; Fermi, S.J. The GlucoWatch® biographer: A frequent, automatic and noninvasive glucose monitor. Ann. Med. 2000, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Barry Keenan, D.; Mastrototaro, J.J.; Weinzimer, S.A.; Steil, G.M. Interstitial fluid glucose time-lag correction for real-time continuous glucose monitoring. Biomed. Signal Process. Control 2013, 8, 81–89. [Google Scholar] [CrossRef]
- Pu, Z.; Zou, C.; Wang, R.; Lai, X.; Yu, H.; Xu, K.; Li, D. A continuous glucose monitoring device by graphene modified electrochemical sensor in microfluidic system. Biomicrofluidics 2016, 10, 011910. [Google Scholar] [CrossRef] [Green Version]
- Harris, K.D.; Elias, A.L.; Chung, H.J. Flexible electronics under strain: A review of mechanical characterization and durability enhancement strategies. J. Mater. Sci. 2016, 51, 2771–2805. [Google Scholar] [CrossRef]
- Chung, H.; Grubbs, R.H. Rapidly cross-linkable DOPA containing terpolymer adhesives and PEG-based cross-linkers for biomedical applications. Macromolecules 2012, 45, 9666–9673. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Yu, Z.; Cheng, H. Tunable Adhesion for Bio-Integrated Devices. Micromachines 2018, 9, 529. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.H.; Zhang, S.; Hjort, K.; Hilborn, J.; Wu, Z. PDMS-Based Elastomer Tuned Soft, Stretchable, and Sticky for Epidermal Electronics. Adv. Mater. 2016, 28, 5830–5836. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Tao, H.; Kim, D.H.; Cheng, H.; Song, J.K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.S.; et al. A physically transient form of silicon electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.W.; Park, G.; Cheng, H.; Song, J.K.; Kang, S.K.; Yin, L.; Kim, J.H.; Omenetto, F.G.; Huang, Y.; Lee, K.M.; et al. 25th anniversary article: Materials for high-performance biodegradable semiconductor devices. Adv. Mater. 2014, 26, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cheng, H.; Mao, S.; Haasch, R.; Liu, Y.; Xie, X.; Hwang, S.W.; Jain, H.; Kang, S.K.; Su, Y.; et al. Dissolvable metals for transient electronics. Adv. Funct. Mater. 2014, 24, 645–658. [Google Scholar] [CrossRef]
- Kang, S.K.; Hwang, S.W.; Cheng, H.; Yu, S.; Kim, B.H.; Kim, J.H.; Huang, Y.; Rogers, J.A. Dissolution behaviors and applications of silicon oxides and nitrides in transient electronics. Adv. Funct. Mater. 2014, 24, 4427–4434. [Google Scholar] [CrossRef]
- Hwang, S.W.; Park, G.; Edwards, C.; Corbin, E.A.; Kang, S.K.; Cheng, H.; Song, J.K.; Kim, J.H.; Yu, S.; Ng, J.; et al. Dissolution chemistry and biocompatibility of single-crystalline silicon nanomembranes and associated materials for transient electronics. ACS Nano 2014, 8, 5843–5851. [Google Scholar] [CrossRef]
- Brenckle, M.A.; Cheng, H.; Hwang, S.; Tao, H.; Paquette, M.; Kaplan, D.L.; Rogers, J.A.; Huang, Y.; Omenetto, F.G. Modulated degradation of transient electronic devices through multilayer silk fibroin pockets. ACS Appl. Mater. Interfaces 2015, 7, 19870–19875. [Google Scholar] [CrossRef]
- Yi, N.; Cheng, Z.; Yang, L.; Edelman, G.; Xue, C.; Ma, Y.; Zhu, H.; Cheng, H. Fully Water-Soluble, High-Performance Transient Sensors on a Versatile Galactomannan Substrate Derived from the Endosperm. ACS Appl. Mater. Interfaces 2018, 10, 36664–36674. [Google Scholar] [CrossRef]
- Cheng, H. Inorganic dissolvable electronics: Materials and devices for biomedicine and environment. J. Mater. Res. 2016, 31, 2549–2570. [Google Scholar] [CrossRef]
- Cheng, H.; Vepachedu, V. Recent development of transient electronics. Theor. Appl. Mech. Lett. 2016, 6, 21–31. [Google Scholar] [CrossRef] [Green Version]








| Detection Mode | Transducer | Analytes |
|---|---|---|
| Amperometric/voltammetric sensors | Carbon, metal, chemically modified electrodes | Alcohols, glucose, phenols, lactate |
| Potentiometric sensors | Ion-selective, carbon, metal, glass electrodes | K+, Cl-, Ca2+, Na+ |
| Field-effect transistors | Ion-sensitive/enzyme field-effect transistor | K+, H+ |
| Impedimetric sensors | Interdigitated/metal electrodes | K+, helicobacter pylori |
| Biofluid | Platform | Recognition Element | Analyte | Technique | Response Time | Linearity Range | LOD | Detection Sensitivity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Saliva | PET | Uricase enzyme | Uric acid | Amperometry | NR (real-time) | 0–1.0 mM | NR | 2.32 μA/mM in artificial saliva 1.08 µA/mM in undiluted human saliva | [76] |
| PB | LOx | Lactate | Amperometry | NR (real-time) | 0.1–1.0 mM | NR | 0.202 µA/mM in undiluted human saliva | [77] | |
| Foil | Carbon | (carboxymethyl)lysine | Amperometry | NR (real-time) | 0.5–10 μg/mL | 0.8 μM | NR | [78] | |
| polyester | GOx | Glucose | Amperometry | NR (real-time) | 0–1.0 mM | 5 μM | 41.7 μA·mM−1·cm−2 | [79] | |
| PETG | GOx | Glucose | Amperometry | NR (real-time) | 0.1–1.0 mM | NR | NR | [80] | |
| Tear | PET | GOx | Glucose | Amperometry | NR | 10 μM–100 mM | 5 μM | NR | [81] |
| Amperometry | 35 s | 0–100 μM | 50 μM | 53 μA·mM−1·cm−2 | [82] | ||||
| Amperometry | NR (real-time) | 0.1–0.6mM | NR | NR | [83] | ||||
| Polyethylene | CuO | Glucose | Amperometry | NR (real-time) | 0–0.7 mM | 2.99 μM | 850 μA·mM−1·cm−2 | [84] | |
| Sweat | PET | Carbon ink | Glucose | Amperometry | NR (real-time) | 0.05 to 0.3 mM | NR | 10.89 μA·mM−1·cm−2 | [85] |
| Tattoo | Carbon ink | Zinc | Amperometry | NR (real-time) | 20–100 mM | 0.05 μg/mL | 23.8 μA mL/μg | [86] | |
| Carbon fibres | CNT ink | Na+ | Potentiometry | NR | 10−6 M to 10−1 M | 4.02 × 10−7 M | 0.19 mV/decade | [87] | |
| PET | GOx | Glucose | Amperometry | NR | NR | 10 × 10−6 M | 41.8 nA·µm−1·cm−2 | [88] | |
| Polycarbonate | LOx | Lactate | Amperometry | NR | NR | NR | 0.2 mM | [89] | |
| Tattoo | LOx | Lactate | Amperometry | NR (real-time) | 1–20 mM | NR | 0.1031 μA·mm−2·mM−1 | [60] | |
| ETH 129, PANI | Ca2+, pH | Potentiometry | NR (real-time) | NR | NR | 32.2 mV/decade, 62.5 mV/decade | [90] | ||
| PDMS | GOx | Glucose | Amperometry | NR (real-time) | 0–1 mM | NR | NR | [91] | |
| Interstitial fluid | Tattoo | GOx | Glucose | Amperometry | NR | 0–0.16 mM | NR | NR | [92] |
| Method | Template Printing | Non-Template Printing | |||
|---|---|---|---|---|---|
| Screen Printing | Gravure Printing | Flexography Printing | Inkjet Printing | 3D Printing | |
| Ink viscosity (cP) | 500–5000 | 100–1000 | 50–500 | 10–20 | >300 k |
| Line width (μm) | 50–100 | 10–100 | 45–100 | 2.3–50 | 1–100 |
| Line thickness (μm) | 3–250 | 1 | <1 | 1–10 | 1–100 |
| Speed (m/s) | 70 | 1000 | ~500 | ~1 | <1 |
| Substrate | Recognition Element | Analytes | Technique | Response Time | Limit of Detection | Flexible/Stretchable | Intimate Contact | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Fabrics/Textiles | Woven fiber | GOx | Glucose | FET | 0.5 s | NR | Yes/Yes | No | [127] |
| Textiles | PANI/PAN | Ammonia | Amperometry | 9 s | 10 ppm | Yes/Yes | [128] | ||
| Carbon ink | TNT | Amperometry | NR | NR | Yes/NR | [118] | |||
| Ionselective membranes | Na+, K+ | potentiometry | NR (real-time) | 10−4.9 M, 10−4.9 M | Yes/Yes | [129] | |||
| Underwater garments | Tyrosinase | Phenols | Amperometry | NR | 0.25 μM | Yes/NR | [130] | ||
| Cotton | LOx | Lactate | Amperometry | NR | 0.3 mM | Yes/NR | [131] | ||
| Silk | LOx | Lactate | Amperometry | 5 s | NR | Yes/NR | [132] | ||
| Fabrics | GOx | Glucose | Amperometry | NR | NR | Yes/NR | [133] | ||
| Paper | GOx | Glucose | Amperometry | NR | NR | Yes/No | Yes | [134] | |
| Ag | Chloride | Amperometry | 30–120 s | 1.5 mM | Yes/No | [135] | |||
| GOx | Glucose | Amperometry | NR | NR | Yes/No | [136] | |||
| Ag | Chloride | Voltammetry | NR | NR | Yes/No | [137] | |||
| Bienzymatic GOx-HRP | Glucose | Amperometry | NR | 0.37 mg/dL | Yes/No | [138] | |||
| Plastic | Polyimide | Glutamate and LOx | Glutamate, lactate | Amperometry | A few seconds | 220 nM, 2 mM | Yes/No | Yes | [139] |
| Polyimide | LOx | Lactate | Amperometry | NR | NR | Yes/No | [140] | ||
| PET | Enzymes | Uric acid, cholesterol, glucose | FET | NR | 3 × 10−9 M, 30 × 10−9 M, 100 × 10−9 M | Yes/No | [141] | ||
| LOx | Lactate | Amperometry | NR (real-time) | 1.0 μM | Yes/No | [142] | |||
| Polyamide | GOx | Glucose | Amperometry | NR | 0.1 mg/dL | Yes/No | [143] | ||
| PEN | LOx | Lactate | FET | NR (real-time) | 66 nM | Yes/No | [144] | ||
| Polyurethane | GOx | Glucose | Amperometry | NR (real-time) | 0.010 mM | Yes/No | [145] | ||
| LOx | Lactate | Amperometry | 50 s | NR | Yes/No | [146] | |||
| Tattoo | Carbon ink | pH | Potentiometry | 10 s | NR | Yes/No | Yes | [147] | |
| Ionselective membranes | Ammonium | Potentiometry | 5 s | 10−4.9 M | Yes/No | [148] | |||
| Silver ink | Water content (hydration) | Impedimetry | NR | NR | Yes/No | [67] | |||
| Alcohol oxidase | Ethanol | Potentiometry | 30 s | NR | Yes/No | [149] | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Cheng, H. Recent Developments of Flexible and Stretchable Electrochemical Biosensors. Micromachines 2020, 11, 243. https://doi.org/10.3390/mi11030243
Yang X, Cheng H. Recent Developments of Flexible and Stretchable Electrochemical Biosensors. Micromachines. 2020; 11(3):243. https://doi.org/10.3390/mi11030243
Chicago/Turabian StyleYang, Xudong, and Huanyu Cheng. 2020. "Recent Developments of Flexible and Stretchable Electrochemical Biosensors" Micromachines 11, no. 3: 243. https://doi.org/10.3390/mi11030243





