Photonic Crystal Stimuli-Responsive Chromatic Sensors: A Short Review
Abstract
:1. Introduction
2. Natural Photonic Crystals and Structural Colors
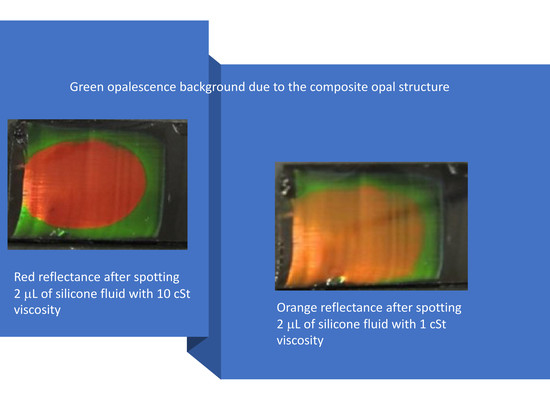
3. Photonic Crystals for Chemical Sensing
4. Photonic Crystals for Biological Sensing
5. Mechanochromic Sensors Based on Photonic Crystals
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1D | One-dimensional |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 3DIO | Three-dimensional inverse opal |
| BSW | Bloch surface waves |
| CAGR | Compound annual growth rate |
| CCA | Colloidal crystal array |
| LOD | Limit of detection |
| MS | Mechanochromic sensitivity |
| NP | Nanoparticle |
| PBG | Photonic band gap |
| PCF | Photonic crystal fiber |
| PFU | Plaque forming units |
| PhC | Photonic crystal |
| RH | Relative humidity |
| RI | Refractive index |
| RIU | Refractive index unit |
| SEM | Scanning electron microscopy |
| SPP | Surface plasmon polaritons |
| SPR | Surface plasmon resonance |
References
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef] [PubMed]
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bykov, V.P. Spontaneous Emission in a Periodic Structure. Sov. J. Exp. Theor. Phys. 1972, 35, 269–273. [Google Scholar]
- Bykov, V.P. Spontaneous emission from a medium with a band spectrum. Sov. J. Quantum Electr. 1975, 4, 861–871. [Google Scholar] [CrossRef]
- Ohtaka, K. Energy band of photons and low-energy photon diffraction. Phys. Rev. B 1979, 19, 5057–5067. [Google Scholar] [CrossRef]
- Joannopoulos, J.D.; Meade, R.D.; Winn, J.N.; Johnson, S.G. Photonic Crystals: Molding the Flow of Light; Princeton University Press: Princeton, NJ, USA, 1995. [Google Scholar]
- Noda, S.; Baba, T. Roadmap on Photonic Crystals; Kluwer Academic Publishers: Cambridge, MA, USA, 2003. [Google Scholar]
- Inoue, K.; Ohtaka, K. Photonic Crystals. Physics, Fabrication and Applications; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Yasumoto, H. Electromagnetic Theory and Applications for Photonic Crystals; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Lourtioz, J.-M.; Benisty, H.; Berger, V.; Gerard, J.-M.; Maystre, D.; Tchelnokov, A. Photonic Crystals. Towards Nanoscale Photonic Devices, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Sibilia, C.; Benson, T.M.; Marciniak, M.; Szoplik, T. Photonic Crystals: Physics and Technology; Springer: Milano, Italy, 2008. [Google Scholar]
- Sukhoivanov, I.A.; Guryev, I.V. Photonic Crystals. Physics and Practical Modeling; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Gong, Q.; Hu, X. Photonic Crystals: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Kumar, N.; Suthar, B. Advances in Photonic Crystals and Devices; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Sahand, S. Microresonators and Photonic Crystals for Quantum Optics and Sensing. Available online: http://hdl.handle.net/1903/8345 (accessed on 4 January 2020).
- Nazirizadeh, Y. Photonic Crystal Slabs for Low-Cost Biosensors. Available online: http://digbib.ubka.uni-karlsruhe.de/volltexte/1000019644 (accessed on 4 January 2020).
- Kraeh, C. Mid-Infrared Photonic Crystals for Gas Sensing. Available online: https://mediatum.ub.tum.de/1246163 (accessed on 4 January 2020).
- Baker, J.E. Development of a Two-Dimensional Photonic Crystal Biosensing Platform. Available online: https://urresearch.rochester.edu/fileDownloadForInstitutionalItem.action?itemId=29906&itemFileId=159367 (accessed on 4 January 2020).
- Piotrowska, A.K. Mechanochromic Photonic Crystals as Strain Sensors for Structural Applications. Available online: http://eprints-phd.biblio.unitn.it/2747/2/PhDthesis_AnnaPiotrowska_(13.11.2017).pdf (accessed on 4 January 2020).
- Sørensen, K.T. Photonic Crystal Slab Sensors in Microfluidics. Available online: https://backend.orbit.dtu.dk/ws/portalfiles/portal/146598268/Untitled.pdf (accessed on 4 January 2020).
- Safdari, M.J. Design, Analysis, and Optimization of Photonic Crystal Sensors. Available online: https://spectrum.library.concordia.ca/984009/1/Safdari_MSc_F2018.pdf (accessed on 4 January 2020).
- Borta, P. Diamond Photonic Crystals for New Bio-Sensors. Available online: https://tel.archives-ouvertes.fr/tel-02014478/file/72354_BORTA_2019_archivage.pdf (accessed on 4 January 2020).
- Portosi, V.; Laneve, D.; Falconi, M.C.; Prudenzano, F. Advances on Photonic Crystal Fiber Sensors and Applications. Sensors 2019, 19, 1892. [Google Scholar] [CrossRef] [Green Version]
- De, M.; Gangopadhyay, T.K.; Singh, V.K. Prospects of Photonic Crystal Fiber as Physical Sensor: An Overview. Sensors 2019, 19, 464. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, K.-Q. Photonic Crystal Structures with Tunable Structure Color as Colorimetric Sensors. Sensors 2013, 13, 4192. [Google Scholar] [CrossRef] [Green Version]
- Chiappini, A.; Armellini, C.; Piccolo, V.; Zur, L.; Ristic, D.; Jovanovic, D.J.; Vaccari, A.; Zonta, D.; Righini, G.C.; Ferrari, M. Colloidal crystals based portable chromatic sensor for butanol isomers and water mixtures detection. Opt. Mater. 2019, 90, 152–158. [Google Scholar] [CrossRef]
- Painam, B.; Kaler, R.S.; Kumar, M. Photonic Crystal Waveguide Biochemical Sensor for the Approximation of Chemical Components Concentrations. Plasmonics 2017, 12, 899–904. [Google Scholar] [CrossRef]
- Wellenzohn, M.; Melnik, E.; Muellner, P.; O’Faolain, L.; Hainberger, R. Design of a Photonic Crystal Defect Waveguide Biosensor Operating in Aqueous Solutions at 1.34 µm. Proceedings 2018, 2, 1026. [Google Scholar] [CrossRef] [Green Version]
- Sakib, M.N.; Hossain, M.B.; Al-tabatabaie, K.F.; Mehedi, I.M.; Hasan, M.T.; Hossain, M.A.; Amiri, I.S. High performance dual core D-shape PCF-SPR sensor modeling employing gold coat. Results Phys. 2019, 15, 102788. [Google Scholar] [CrossRef]
- Otipka, P.; Vlček, J.; Lesňák, M.; Sobota, J. Design of MO-SPR sensor element with photonic crystal. Photonics Nanostruct. Fund. Appl. 2018, 31, 77–80. [Google Scholar] [CrossRef]
- Sinibaldi, A.; Occhicone, A.; Munzert, P.; Danz, N.; Sonntag, F.; Michelotti, F. Label-Free Monitoring of Human IgG/Anti-IgG Recognition Using Bloch Surface Waves on 1D Photonic Crystals. Biosensors 2018, 8, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.; Fan, T.; Lou, S.; Zhang, D. Biomimetic optical materials: Integration of nature’s design for manipulation of light. Prog. Mater. Sci. 2013, 58, 825–873. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, Q.; Shi, L.; Zhang, X.; Zhang, K.-Q. Bio-inspired sensors based on photonic structures of Morpho butterfly wings: A review. J. Mater. Chem. C 2016, 4, 1752–1763. [Google Scholar] [CrossRef]
- Ghazzal, M.N.; Deparis, O.; Errachid, A.; Kebaili, H.; Simonis, P.; Eloy, P.; Vigneron, J.P.; De Coninck, J.; Gaigneaux, E.M. Porosity control and surface sensitivity of titania/silica mesoporous multilayer coatings: Applications to optical Bragg resonance tuning and molecular sensing. J. Mater. Chem. 2012, 22, 25302–25310. [Google Scholar] [CrossRef]
- Colusso, E.; Perotto, G.; Wang, Y.; Sturaro, M.; Omenetto, F.; Martucci, A. Bioinspired stimuli-responsive multilayer film made of silk–titanate nanocomposites. J. Mater. Chem. C 2017, 5, 3924–3931. [Google Scholar] [CrossRef]
- Lova, P.; Bastianini, C.; Giusto, P.; Patrini, M.; Rizzo, P.; Guerra, G.; Iodice, M.; Soci, C.; Comoretto, D. Label-Free Vapor Selectivity in Poly(p-Phenylene Oxide) Photonic Crystal Sensors. ACS Appl. Mater. Interfaces 2016, 8, 31941–31950. [Google Scholar] [CrossRef] [Green Version]
- Lova, P.; Manfredi, G.; Bastianini, C.; Mennucci, C.; Buatier de Mongeot, F.; Servida, A.; Comoretto, D. Flory–Huggins Photonic Sensors for the Optical Assessment of Molecular Diffusion Coefficients in Polymers. ACS Appl. Mater. Interfaces 2019, 11, 16872–16880. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Smith, N.L.; Zhang, J.-T.; Asher, S.A. Two-Dimensional Photonic Crystal Chemical and Biomolecular Sensors. Anal. Chem. 2015, 87, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Meng, Z.; Qi, F.; Xue, M.; Wang, F.; Chen, W.; Yan, Z. Two-dimensional inverse opal hydrogel for pH sensing. Analyst 2014, 139, 6192–6196. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, K.; Wang, J.; Hu, Y.; Shen, L.; Zhu, J. Full-color photonic hydrogels for pH and ionic strength sensing. Eur. Polym. J. 2016, 83, 60–66. [Google Scholar] [CrossRef]
- Li, G.; Xiao, F.; Liao, S.; Chen, Q.; Zhou, J.; Wu, Z.; Yu, R. Label-free 2D colloidal photonic crystal hydrogel biosensor for urea and urease inhibitor. Sens. Actuat. B Chem. 2018, 277, 591–597. [Google Scholar] [CrossRef]
- Qi, F.; Lan, Y.; Meng, Z.; Yan, C.; Li, S.; Xue, M.; Wang, Y.; Qiu, L.; He, X.; Liu, X. Acetylcholinesterase-functionalized two-dimensional photonic crystals for the detection of organophosphates. RSC Adv. 2018, 8, 29385–29391. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.; Xue, M.; Qiu, L.; Meng, Z. Clinical Evaluation of a Photonic Crystal Sensor for Glucose Monitoring in Urine. Chem. Sel. 2019, 4, 6547–6551. [Google Scholar] [CrossRef]
- Xing, R.; Du, Y.; Zhao, X.; Zhang, X. Gas Sensor Based on 3-D WO3 Inverse Opal: Design and Applications. Sensors 2017, 17, 710. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Dong Xu, L.; Zhang, X.; Chen, J.; Sun, X.; Xu, H.; Zhang, T.; Bai, X.; Zhang, S.; Song, H. Three-dimensional ordered ZnO–Fe3O4 inverse opal gas sensor toward trace concentration acetone detection. Sens. Actuator B Chem. 2017, 252, 367–374. [Google Scholar] [CrossRef]
- Lee, C.S.; Dai, Z.; Kim, D.H.; Li, H.Y.; Jo, Y.M.; Kim, B.Y.; Byun, H.G.; Hwang, I.; Lee, J.H. Highly discriminative and sensitive detection of volatile organic compounds for monitoring indoor air quality using pure and Au-loaded 2D In2O3 inverse opal thin films. Sens. Actuator B Chem. 2018, 273, 1–8. [Google Scholar] [CrossRef]
- Yu, B.; Cong, H.; Yang, Z.; Yang, S.; Wang, Y.; Zhai, F.; Wang, Y. Preparation of Humidity-Sensitive Poly(Ethylene Glycol) Inverse Opal Micropatterns Using Colloidal Lithography. Materials 2017, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, A.; Armellini, C.; Carpentiero, A.; Minati, L.; Righini, G.C.; Ferrari, M. Solvent sensitive polymer composite structures. Opt. Mater. 2014, 36, 130–134. [Google Scholar] [CrossRef]
- Kuo, W.K.; Weng, H.P.; Hsu, J.J.; Yu, H.H. Photonic Crystal-Based Sensors for Detecting Alcohol Concentration. Appl. Sci. 2016, 6, 67. [Google Scholar] [CrossRef]
- Huang, C.; Cheng, Y.; Gao, Z.; Zhang, H.; Wei, J. Portable label-free inverse opal photonic hydrogel particles serve as facile pesticides colorimetric monitoring. Sens. Actuator B Chem. 2018, 273, 1705–1712. [Google Scholar] [CrossRef]
- Barry, R.A.; Wiltzius, P. Humidity-Sensing Inverse Opal Hydrogels. Langmuir 2006, 22, 1369–1374. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Heitzman, C.E.; Frei, W.R.; Johnson, H.T.; Braun, P.V. Transformation of Hydrogel-Based Inverse Opal Photonic Sensors from FCC to L11 during Swelling. J. Phys. Chem. B 2006, 110, 19300–19306. [Google Scholar] [CrossRef]
- Nishijima, Y.; Ueno, K.; Juodkazis, S.; Mizeikis, V.; Misawa, H.; Tanimura, T.; Maeda, K. Inverse silica opal photonic crystals for optical sensing applications. Opt. Express 2007, 15, 12979–12988. [Google Scholar] [CrossRef]
- Baratto, C.; Faglia, G.; Ferroni, M.; Calestani, G.; Sutti, A.; Dionigi, C.; Sberveglieri, G. Inverse opal gas sensors: Zn(II)-doped tin dioxide systems for low temperature detection of pollutant gases. Sens. Act. B 2008, 130, 567–573. [Google Scholar] [CrossRef]
- Shin, J.; Braun, P.V.; Lee, W. Fast response photonic crystal pH sensor based on templated photo-polymerized hydrogel inverse opal. Sens. Act. B 2010, 150, 183–190. [Google Scholar] [CrossRef]
- Shin, J.; Han, S.G.; Lee, W. Inverse opal pH sensors with various protic monomers copolymerized with polyhydroxyethylmethacrylate hydrogel. Anal Chim. Acta. 2012, 752, 87–93. [Google Scholar] [CrossRef]
- You, X.; Pikul, J.H.; King, W.P.; Pak, J.J. Zinc oxide inverse opal enzymatic biosensor. Appl. Phys. Lett. 2013, 102, 253103. [Google Scholar] [CrossRef]
- Kim, S.; Han, S.G.; Koh, Y.G.; Lee, H.; Lee, W. Colorimetric Humidity Sensor Using Inverse Opal Photonic Gel in Hydrophilic Ionic Liquid. Sensors 2018, 18, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komori, Y.; Murakami, H.; Kimura, T.; Onodera, T. Fabrication of inverse opal photonic crystals with mesopores using binary colloidal co-assembly method for signal enhancement in formaldehyde detection. Sens. Mater. 2019, 31, 645–659. [Google Scholar] [CrossRef]
- Tilton, H.B. Scotopic luminosity function and color-mixture data. J. Opt. Soc. Am. 1977, 67, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Zou, C.; Su, Y.; Li, M.; Yang, Z.; Ge, M.; Zhang, Y. One-step synthesis of 2D C3N4-tin oxide gas sensors for enhanced acetone vapor detection. Sens. Actuators B 2017, 253, 641–651. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Wang, W.; Xue, Y.; Sun, Y.; Li, P.; Lian, K.; Zhang, W.; Chen, L.; Shi, J.; et al. Synthesis and gas sensing properties of NiO/SnO2 herarchical structures toward ppb-level acetone detection. Mater. Res. Bull. 2018, 102, 294–303. [Google Scholar] [CrossRef]
- Park, S. Acetone gas detection using TiO2 nanoparticles functionalized In2O3 nanowires for diagnosis of diabetes. J. Alloy Compd. 2017, 696, 655–662. [Google Scholar] [CrossRef]
- Li, X.; Lu, D.; Shao, C.; Lu, G.; Li, X.; Liu, Y. Hollow CuFe2O4/-Fe2O3 composite with ultrathin porous shell for acetone detection at ppb levels. Sens. Actuators B 2018, 258, 436–446. [Google Scholar] [CrossRef]
- Asgari, M.; Saboor, F.H.; Mortazavi, Y.; Khodadadi, A.K. SnO2 decorated SiO2 chemical sensors: Enhanced sensing performance toward ethanol and acetone. Mater. Sci. Semicond. Proccess. 2018, 68, 87–96. [Google Scholar] [CrossRef]
- Ma, R.-J.; Li, G.-D.; Zou, X.; Gao, R.; Chen, H.; Zhao, X. Bimetallic Pt-Au nanocatalysts decorated In2O3 nests composed of ultrathin nanosheets for type 1 diabetes diagnosis. Sens. Actuators B 2018, 270, 247–255. [Google Scholar] [CrossRef]
- Hao, X.; Wang, B.; Ma, C.; Liu, F.; Yang, X.; Liu, T.; Liang, X.; Yang, C.; Zhu, H.; Lu, G. Mixed -potential type sensor based on stabilized zirconia and Co1-xZnxFe2O4 sensing electrode for detection of acetone. Sens. Actuators B 2018, 255, 1173–1181. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jang, J.-S.; Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Kim, I.-D. Hierarchically interconnected porosity control of catalyst-loaded WO3 nanofiber scaffold: Superior acetone sensing layers for exhaled breath analysis. Sens. Actuators B 2018, 259, 616–625. [Google Scholar] [CrossRef]
- Kollbek, K.; Szkudlarek, A.; Klejna, S.; Rydosz, A. Electronic sensitization of CuO thin films by Cr-doping for enhanced gas sensor response at low detection limit. Mater. Res. Express 2018, 5, 126406:1–126406:14. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, Z.; Ding, D.; Lu, W.; Xue, Q. Multi-shelled ZnCO2O4 yolk-shell spheres for high-performance acetone gas sensor. Appl. Surf. Sci. 2018, 443, 114–121. [Google Scholar] [CrossRef]
- Li, Y.; Hua, Z.; Zeng, Y.; Qiu, Z.; Tian, X.; Wang, M.; Li, E. Modified impregnation synthesis of Ru-loaded WO3 nanoparticles for acetone sensing. Sens. Actuators B 2018, 265, 249–256. [Google Scholar] [CrossRef]
- Wang, X.-F.; Ma, W.; Jiang, F.; Cao, E.-S.; Sun, K.-M.; Cheng, L.; Song, X.-Z. Prussian Blue analogue derived prorous NiFe2O4 nanocubes for low-concentration acetone sensing at low working temperature. Chem. Eng. J. 2018, 338, 504–512. [Google Scholar] [CrossRef]
- Wongrat, E.; Chanlek, N.; Chueaiarrom, C.; Thupthimchun, W.; Samransuksamer, B.; Choopun, S. Acetone gas sensors based on ZnO nanostructures dectorated with Pt and Nb. Ceram. Int. 2017, 43, S557–S566. [Google Scholar] [CrossRef]
- Wang, X.; Qin, H.; Pei, J.; Chen, Y.; Li, L.; Xie, J.; Hu, J. Sensing performances to low concentration acetone for palladium doped LaFeO3 sensors. J. Rare Earths 2016, 34, 704–710. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.; Lin, Y.; Sai, L.; Chang, Q.; Shi, W.; Chen, Q. Fully gravure-printed WO3/Pt-decorated rGO nanosheets composite film for detection of acetone. Sens. Actuators B 2018, 255, 1482–1490. [Google Scholar] [CrossRef]
- Tomer, V.K.; Singh, K.; Kaur, H.; Shorie, M.; Sabherwal, P. Rapid acetone detection using indium loaded WO3/SnO2 nanohybrid sensor. Sens. Actuators B 2017, 253, 703–713. [Google Scholar] [CrossRef]
- Bonifacio, L.D.; Puzzo, D.P.; Breslav, S.; Willey, B.M.; McGeer, A.; Ozin, G.A. Photonic Sensors: Towards the Photonic Nose: A Novel Platform for Molecule and Bacteria Identification. Adv. Mater. 2010, 22, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yan, C.; Meng, Z.; Li, S.; Xu, J.; Hu, X.; Xue, M. Acetylcholinesterase-functionalized two-dimensional photonic crystal for the sensing of G-series nerve agents. Anal. Bioanal. Chem. 2019, 411, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, J.; Liu, J.; Zhao, W. The Visual Sensor of Inverse Opal hydrogel for the Colorimetric Detection of Glucose. J. Mater. Chem. B 2019, 7, 3576–3581. [Google Scholar] [CrossRef]
- Choi, E.; Choi, Y.; Nejad, Y.H.; Shin, K.; Parka, J. Label-free specific detection of immunoglobulin G antibody using nanoporous hydrogel photonic crystals. Sens. Actuator B Chem. 2013, 180, 107–113. [Google Scholar] [CrossRef]
- Lee, W.S.; Kang, T.; Kim, S.H.; Jeong, J. An Antibody-Immobilized Silica Inverse Opal Nanostructure for Label-Free Optical Biosensors. Sensors 2018, 18, 307. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gu, H.; Chen, Z.; Shang, L.; Zhao, Z.; Gu, Z.; Zhao, Y. Enzimatyc Inverse Opal Hydrogel Particles for Biocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 12914–12918. [Google Scholar] [CrossRef]
- Chiappini, A.; Pasquardini, L.; Nodehi, S.; Armellini, C.; Bazzanella, N.; Lunelli, L.; Pelli, S.; Ferrari, M.; Pietralunga, S.M. Fluorescent Aptamer Immobilization on Inverse Colloidal Crystals. Sensors 2018, 18, 4326. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Park, S.; Han, S.W.; Lim, T.G.; Koh, W.-G. Preparation of photolithographically patterned inverse opal hydrogel microstructures and its application to protein patterning. Biosens. Bioelectron 2012, 35, 243–250. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Butt, H.; Volpatti, L.R.; Pavlichenko, I.ì.; Humar, M.; Kwok, S.J.J.; Koo, H.; Kim, K.S.; Naydenova, I.; Khademhosseini, A.; et al. Photonic hydrogel sensors. Biotechnol. Adv. 2016, 34, 250–271. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.S.; Choi, S.-W.; Xia, Y. Inverse opal scaffolds for applications in regenerative medicine. Soft Matter 2013, 9, 9747–9754. [Google Scholar] [CrossRef]
- Zhu, C.; Qiu, J.; Pongkitwitoon, S.; Thomopoulos, S.; Xia, Y. Inverse Opal Scaffolds with Gradations in Mineral Content for Spatial Control of Osteogenesis. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.-R.; Zhang, N.; Ye, Z.; Huang, N.-P. Microtopography based on inverse opal structures regulates the behavior of bone marrow-derived mesenchymal stem cells. Polym. Adv. Technol. 2019, 30, 1182–1188. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Nie, F. Non-dye cell viability monitoring by using pH-responsive inverse opal hydrogels. J. Mater. Chem. B 2018, 6, 1055–1065. [Google Scholar] [CrossRef]
- Zhang, B.; Cheng, Y.; Wang, H.; Ye, B.; Shang, L.; Zhao, Y.; Gu, Z. Multifunctional inverse opal particles for drug delivery and monitoring. Nanoscale 2015, 7, 10590–10594. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Li, F.; Sun, R.; Wang, W.; Wen, Y.; Wen, Y.; Song, Y.; Zhang, X. Voltage-Responsive Controlled Release Film with Cargo Release Self-Monitoring Property Based on Hydrophobicity Switching. ACS Appl. Mater. Interfaces 2017, 9, 10992–10999. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, S.; Yao, Y.; Xu, S.; Dong, Q.; Chen, P.; Wang, Z.; Zhang, M.; Zhu, M.; Xu, G.; et al. Dynamic Optics with Transparency and Color Changes under Ambient Conditions. Polymers 2019, 11, 103. [Google Scholar] [CrossRef] [Green Version]
- Correa-Duarte, M.A.; Salgueiriño-Maceira, V.; Rinaldi, A.; Sieradzki, K.; Giersig, M.; Liz-Marzán, L.M. Optical strain detectors based on gold/elastomer nanoparticulated films. Gold Bull. 2008, 40, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Dumanli, A.G.; Savin, T. Recent advances in the biomimicry of structural colours. Chem. Soc. Rev. 2016, 45, 6698–6724. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.P.; Walish, J.J.; Urbas, A.M.; Thomas, E.L. Mechanochromic Photonic Gels. Adv. Mater. 2013, 25, 3934–3947. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Q.; Zheng, X. Flexible mechanochromic photonic crystals: Routes to visual sensors and their mechanical properties. J. Mater. Chem. C 2018, 6, 3182–3199. [Google Scholar] [CrossRef]
- Dommelen, R.; Fanzio, P.; Sasso, L. Surface self-assembly of colloidal crystals for micro- and nano-patterning. Adv. Colloid Interface Sci. 2018, 251, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; O’Brien, K.; Zhang, Y.H.; Yu, H.; Jiang, H. Tunable optical gratings based on buckled nanoscale thin films on transparent elastomeric substrates. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Singamaneni, S.; Tsukruk, V.V. Buckling instabilities in periodic composite polymeric materials. Soft Matter 2010, 6, 5681–5692. [Google Scholar] [CrossRef]
- Piccolo, V.; Chiappini, A.; Armellini, C.; Vaccari, A.; Ferrari, M.; Zonta, D. Colloidal Photonic Crystals and their application in sensoristic field. In Proceedings of the 105° Congresso Nazionale SIF, L’Aquila, Italy, 23–27 September 2019. [Google Scholar]
- Minati, L.; Chiappini, A.; Armellini, C.; Carpentiero, A.; Maniglio, D.; Vaccari, A.; Zur, L.; Lukowiak, A.; Ferrari, M.; Speranza, G. Gold nanoparticles 1D array as mechanochromic strain sensor. Mater. Chem. Phys. 2017, 192, 94–99. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Anisotropic hydrogel based on bilayers: Color, strength, toughness, and fatigue resistance. Soft Matter 2012, 8, 8008–8016. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.; Sato, M.; Kajita, H.; Okuda, N.; Tanaka, S.; Hisamoto, H. Printed two-dimensional photonic crystals for single-step label-free biosensing of insulin under wet conditions. Lab Chip 2012, 12, 1995–1999. [Google Scholar] [CrossRef]
- Endo, T.; Kajita, H.; Kawaguchi, Y.; Himi, T.K.T. Label-free optical detection of C-reactive protein by nanoimprint lithography-based 2D-photonic crystal film. Biotechnol. J. 2016, 11, 831–837. [Google Scholar] [CrossRef]
- Guo, H.; Tang, J.; Qian, K.; Tsoukalas, D.; Zhao, M.; Yang, J.; Zhang, B.; Chou, X.; Liu, J.; Xue, C.; et al. Vectorial strain gauge method using single flexible orthogonal polydimethylsiloxane gratings. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Piccolo, V.; Chiappini, A.; Armellini, C.; Barozzi, M.; Lukowiak, A.; Sazio, P.J.A.; Vaccari, A.; Ferrari, M.; Zonta, D. 2D Optical Gratings Based on Hexagonal Voids on Transparent Elastomeric Substrate. Micromachines 2018, 9, 345. [Google Scholar] [CrossRef] [Green Version]
- Piccolo, V.; Chiappini, A.; Armellini, C.; Mazzola, M.; Lukowiak, A.; Seddon, A.; Ferrari, M.; Zonta, D. Quasi-hemispherical voids micropatterned PDMS as strain sensor. Opt. Mater. 2018, 86, 408–413. [Google Scholar] [CrossRef]
- Zhao, P.; Li, B.; Tang, Z.; Gao, Y.; Tian, H.; Chen, H. Stretchable photonic crystals with periodic cylinder shaped air holes for improving mechanochromic performance. Smart Mater. Struct. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Fudouzi, H.; Sawada, T. Photonic rubber sheets with tunable color by elastic deformation. Langmuir 2006, 22, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Fudouzi, H.; Tsuchiyaa, K.; Todoroki, S. Smart photonic coating for civil engineering field: For a future inspection technology on concrete bridge. Proc. SPIE 2017, 10168. [Google Scholar] [CrossRef]
- Chiappini, A.; Chiasera, A.; Berneschi, S.; Armellini, C.; Carpentiero, A.; Mazzola, M.; Moser, E.; Varas, S.; Righini, G.C.; Ferrari, M. Sol–gel-derived photonic structures: Fabrication, assessment, and application. J. Sol Gel Sci. Technol. 2011, 60, 408–425. [Google Scholar] [CrossRef]
- Yang, D.; Ye, S.; Ge, J. From Metastable Colloidal Crystalline Arrays to Fast Responsive Mechanochromic Photonic Gels: An Organic Gel for Deformation-Based Display Panels. Adv. Funct. Mater. 2014, 24, 3197–3205. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, S.Y.; Ellerthorp, L.; Fen, G.; Lin, G.; Wu, G.; Yin, J.; Yang, S. Elastoplastic Inverse Opals as Power-Free Mechanochromic Sensors for Force Recording. Adv. Funct. Mater. 2015, 25, 6041–6049. [Google Scholar] [CrossRef]
- Snapp, P.; Kang, P.; Leem, J.; Nam, S. Colloidal Photonic Crystal Strain Sensor Integrated with Deformable Graphene. Phototransducer. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Pitruzzello, G.; Krauss, T.F. Photonic crystal resonances for sensing and imaging. J. Opt. 2018, 20, 1–23. [Google Scholar] [CrossRef]
- Available online: https://www.alliedmarketresearch.com/photonics-sensor-market (accessed on 4 January 2020).
- Available online: https://www.alliedmarketresearch.com/biophotonics-market (accessed on 4 January 2020).
- Guozhen, S.; Zhiyong, F. Flexible Electronics: From Materials to Devices; World Scientific: Singapore, 2016. [Google Scholar]
- Garner, S.M. Flexible Glass—Enabling Thin, Lightweight, and Flexible Electronics; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Huang, Y.-A.; Yin, Z.; Wan, X. Modeling and Application of Flexible Electronics Packaging; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Li, L.; Lin, H.; Qiao, S.; Huang, Y.-Z.; Li, J.-Y.; Michon, J.; Gu, T.; Alosno-Ramos, C.; Vivien, L.; Yadav, A.; et al. Monolithically integrated stretchable photonics. Light Sci. Appl. 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, M.; Kurashina, Y.; Onoe, H. Eye-recognizable and repeatable biochemical flexible sensors using low angle-dependent photonic colloidal crystal hydrogel microbeads. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Iwase, E.; Onoe, H. Microfluidically Patterned Dome-Shaped Photonic Colloidal Crystals Exhibiting Structural Colors with Low Angle Dependency. Adv. Opt. Mater. 2017, 5. [Google Scholar] [CrossRef]













| PhC | PhC Structure and Material | External Stimulus or Analyte Detected | Sensor’s Response | Ref. |
|---|---|---|---|---|
| 1D | Mesoporous layers of silica and titania | Hydrophobic and hydrophilic molecules | ∆λB = 47 nm for DHDP in THF | [35] |
| 1D | Hybrid silk-titanate layers | Humidity (RH) | ∆λB = 40 nm sensitivity (∆λ /∆RH%) = 0.28 | [36] |
| 1D | All-polymer Bragg multilayers (PPO, CA) | Volatile organic compounds | Chromatic discrimination of benzene, ODCB, CT, toluene | [37] |
| 1D | All-polymer Bragg multilayers (PS, CA) | Pollutants | LOD: MeOH (29 mg/L); EtOH (12 mg/L); 1 POH (12 mg/L); 2 POH (6 mg/L); BuOH (64 mg/L) | [38] |
| 2D | Composite Hydrogel (PS, poly HEMA-AA) | pH | ∆λB = 130 nm when pH goes from 2 to 8 | [40] |
| 2D | Composite hydrogel (Fe3O4 NPs embedded in PAM-PAA) | pH and ionic strength | ∆λB = 202 nm when pH goes from 2.02 to 7.03; ∆λB = 181 nm when NaCl varies from 0.02 M to 1 M | [41] |
| 2D | Composite Hydrogel (PS, AM) | Urea and urease inhibitor | ∆λB = 28 nm when urea concentration varies from 1 to10 mM | [42] |
| 2D | Composite Hydrogel (PS, PAM-AA) | Organophosphate pesticide | LOD: 7.7 × 10−12 mmol/L for Dipterex | [43] |
| 3D | WO3 inverse opal | Acetone | LOD: 0.2 ppm | [45] |
| 3D | ZnO-Fe3O4 inverse opal | Acetone | LOD: 0.1 ppm | [46] |
| 3D | PEG inverse opal | Humidity | ∆λB = 40 nm when RH% goes from 30 to 90 | [48] |
| 3D | Composite direct opal based on PS and PDMS | Organic solvents | Chromatic discrimination of EtOH, MeOH, Silicone fluids | [49] |
| 3D | Composite direct opal based on PS and PDMS | Butanol isomers | Chromatic discrimination of TerB, NB, 2B | [27] |
| 3D | Titanium oxide inverse opal | Ethanol solutions | Sensitivity = 9090 nm/RIU | [50] |
| 3D | MIP-hydrogel particles | MPA | Detection down to 1×10−6 M | [51] |
| Hue Sensation (Name of Color) | Wavelength Range (nm) | Range Width (nm) |
|---|---|---|
| Violet | 388–429 | 41 |
| Indigo | 429–458 | 29 |
| Blue | 458–481 | 23 |
| Cyan | 481–499 | 18 |
| Turquoise | 499–513 | 14 |
| Green | 513–528 | 15 |
| Lime | 528–546 | 18 |
| Chartreuse | 546–561 | 15 |
| Yellow | 561–575 | 14 |
| Lemon | 575–587 | 12 |
| Ocher | 587–599 | 12 |
| Orange | 599–610 | 11 |
| Tangerine | 610–622 | 12 |
| Ruby | 622–636 | 14 |
| Red | 636–782 | 146 |
| Material | Temperature (°C) | Acetone (ppm) | LOD (ppm) | Reference |
|---|---|---|---|---|
| C3N4-SnO2 | 380 | 20 | 0.087 | [63] |
| NiO/SnO2 | 300 | 50 | 0.01 | [64] |
| WO3 3DIO * | 370 | 5 | 0.1 | [45] |
| TiO2/In2O3 | 250 | 10 | 0.1 | [65] |
| CuFe2O4/α-Fe2O3 | 275 | 70 | 0.1 | [66] |
| ZnO–Fe3O2 3DIO * | 475 | 50 | 0.15 | [46] |
| GO-SnO2-TiO2 | 200 | 5 | 0.25 | [67] |
| Pt0.3Au0.7–In2O3 | 160 | 50 | 0.3 | [68] |
| Co1−xZnx Fe2O4 | 650 | 50 | 0.3 | [69] |
| WO3 NFs | 350 | 5 | 0.4 | [70] |
| Cr-doped CuO | 450 | 3.2 | 0.4 | [71] |
| SnO2/SiO2 | 270 | 300 | 0.5 | [67] |
| ZnCo2O4 | 200 | 500 | 0.5 | [72] |
| Ru/WO3 | 300 | 1.5 | 0.5 | [73] |
| NiFe2O4 | 160 | 200 | 0.52 | [74] |
| ZnO:Pt | 400 | 1000 | 1 | [75] |
| ZnO:Nb | 400 | 1000 | 1 | [76] |
| Pd/LaFeO3 | 200 | 1 | 1 | [76] |
| WO3/Pt-GNs ** | 200 | 10 | 1 | [77] |
| In/WO3-SnO2 | 200 | 50 | 1 | [78] |
| PhC | PhC Structure and Material | External Stimulus or Analyte Detected | Sensor’s Response | Ref. |
|---|---|---|---|---|
| 1D | Mesoporous multilayer Bragg stack (SiO2-TiO2 NPs) | Bacteria | Chromatic discrimination of ATCC 27853, ATCC 25922, ATCC 29213, ATCC 12228 | [79] |
| 2D | Composite hydrogel (PS, PAM-AA) | Glucose | Detection range from 0.4 to 53.3 mmol/L | [44] |
| 2D | Composite hydrogel (PS, HEMA) | Sarin | LOD: 6.7 × 10−14 mmol/L | [80] |
| 3D | hydrogel inverse opal (3-APBA) | Glucose | ΔλB = 139 nm for glucose concentration of 5 mmol | [81] |
| 3D | hydrogel inverse opal (PEG-DA) | Immunoglobulin G antibody | ΔλB = 50 nm for a concentration of 10 mg/mL | [82] |
| 3D | Inverse opal (functionalized Si) | Influenza viruses | Selectivity of H1N1 subtype; detection in the range 103–105 PFU | [83] |
| 3D | Hollow hydrogel particles (AAm) | Enzyme | Chromatic detection of enzyme activity | [84] |
| 3D | Inverse silica opal (functionalized) | Tumor necrosis factor (TNF) | Detection of TNF-alpha via fluorescence quenching | [85] |
| 3D | Inverse opal hydrogel (PHEMA) | Streptavidin | LOD: 1.0 nM | [86] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappini, A.; Tran, L.T.N.; Trejo-García, P.M.; Zur, L.; Lukowiak, A.; Ferrari, M.; Righini, G.C. Photonic Crystal Stimuli-Responsive Chromatic Sensors: A Short Review. Micromachines 2020, 11, 290. https://doi.org/10.3390/mi11030290
Chiappini A, Tran LTN, Trejo-García PM, Zur L, Lukowiak A, Ferrari M, Righini GC. Photonic Crystal Stimuli-Responsive Chromatic Sensors: A Short Review. Micromachines. 2020; 11(3):290. https://doi.org/10.3390/mi11030290
Chicago/Turabian StyleChiappini, Andrea, Lam Thi Ngoc Tran, Pablo Marco Trejo-García, Lidia Zur, Anna Lukowiak, Maurizio Ferrari, and Giancarlo C. Righini. 2020. "Photonic Crystal Stimuli-Responsive Chromatic Sensors: A Short Review" Micromachines 11, no. 3: 290. https://doi.org/10.3390/mi11030290