Study on the Mechanism of Solid-Phase Oxidant Action in Tribochemical Mechanical Polishing of SiC Single Crystal Substrate
Abstract
:1. Introduction
2. Experiment and Characterization
2.1. Polishing Test
2.2. Workpiece Surface Composition Testing
3. Analysis of Results
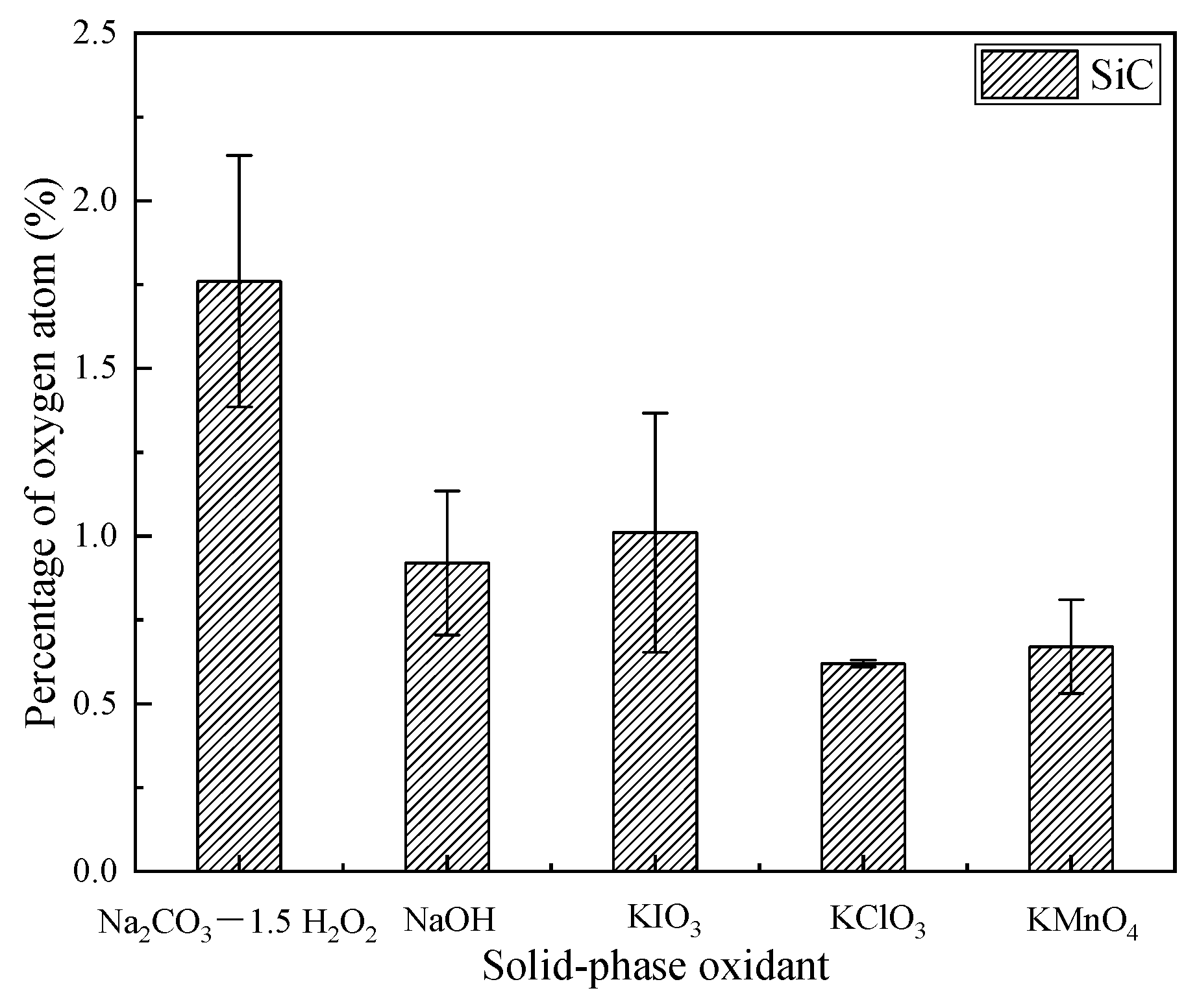
3.1. Elements and Content of SiC Surface after Polishing
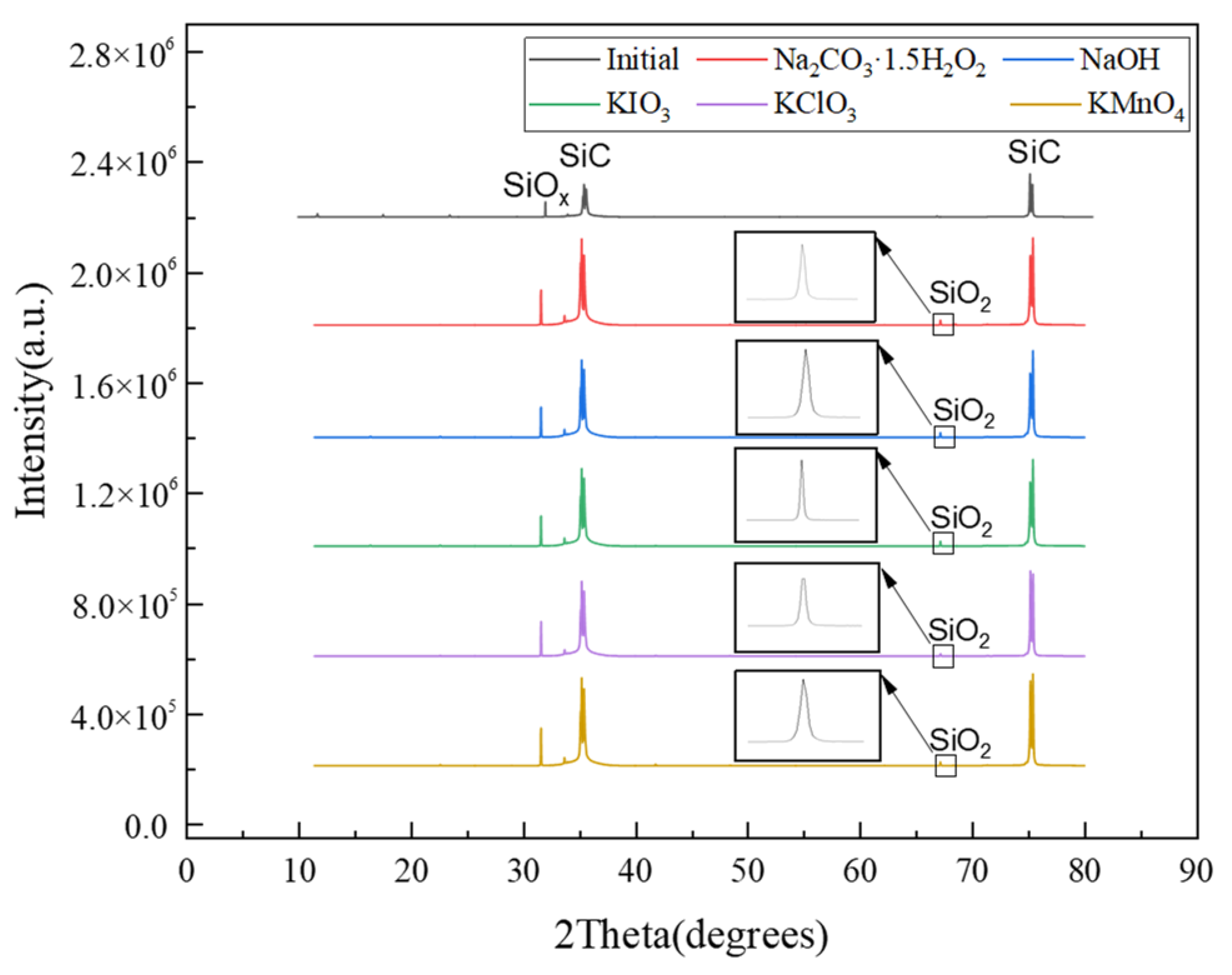
3.2. Physical Phase Analysis of SiC Surface after Polishing
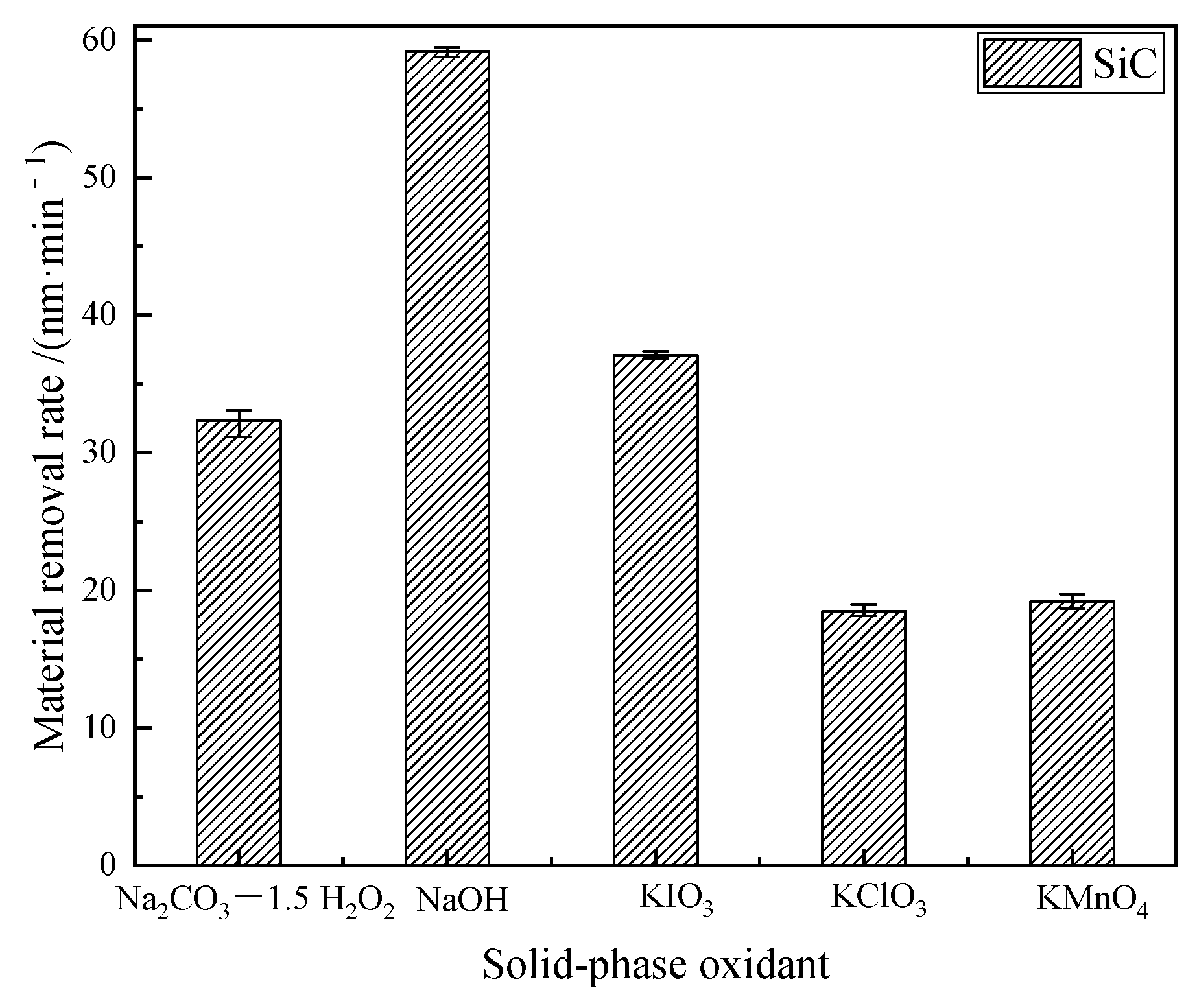
3.3. Material Removal Rate after Polishing
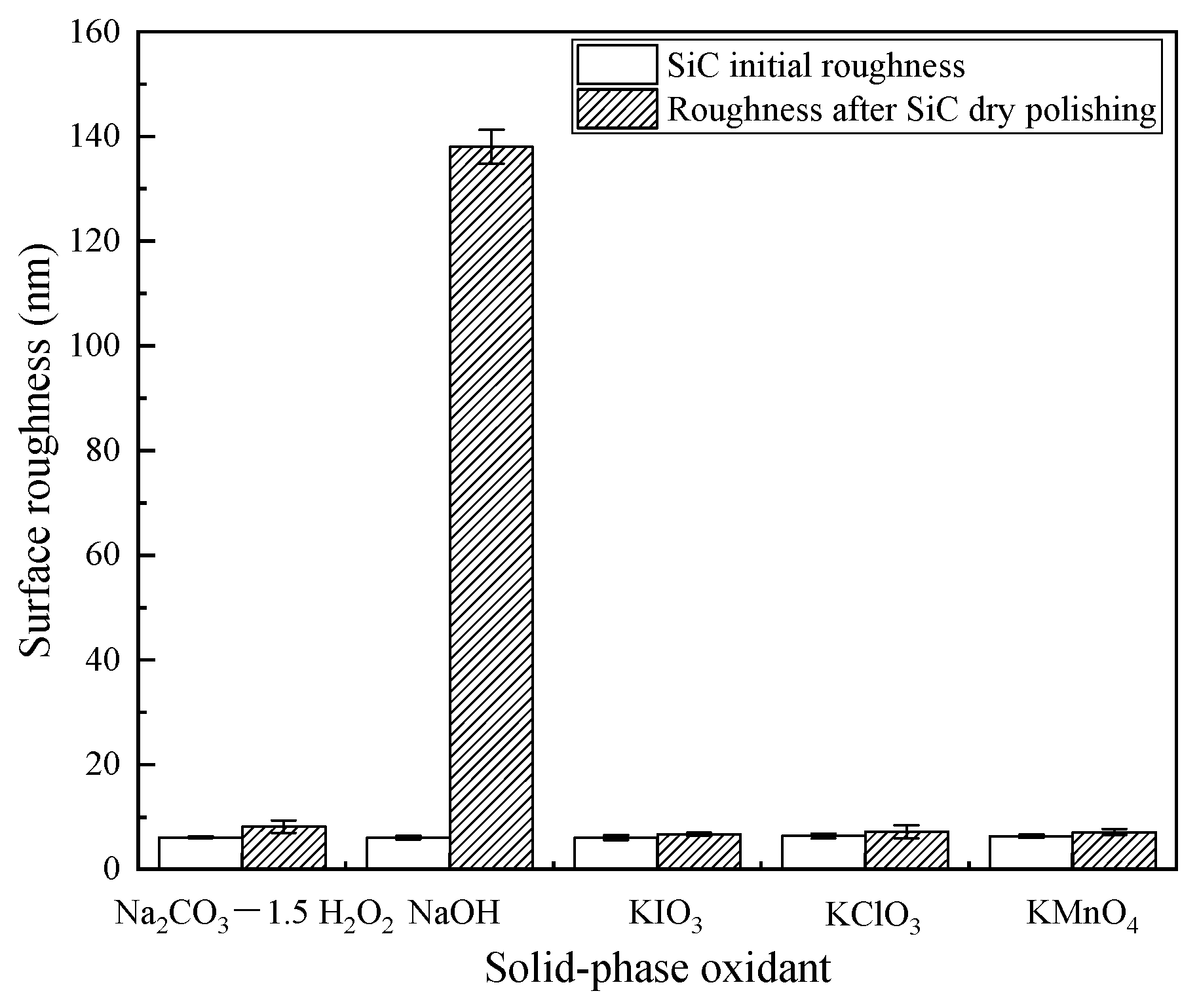
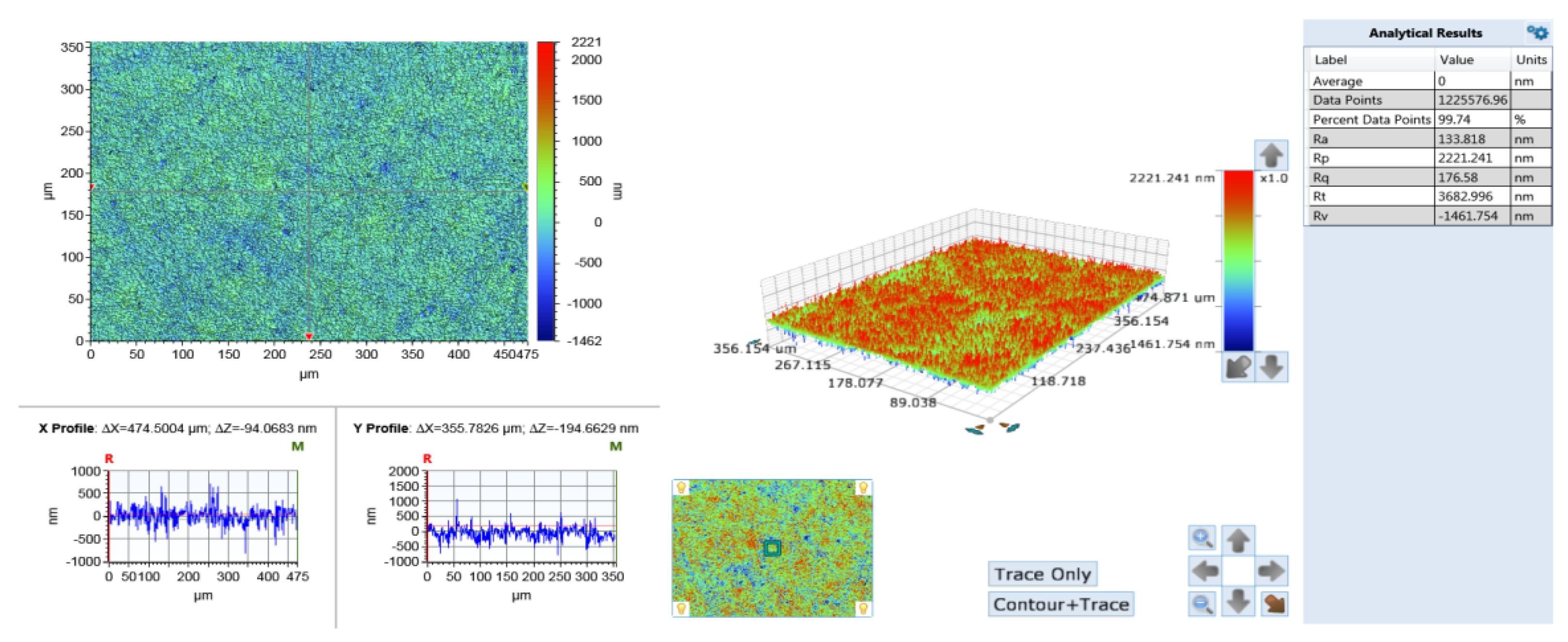
3.4. Surface Roughness and Surface Morphology after Polishing
4. Discussion
4.1. Solid-Phase Oxidant Tribochemical Reaction Oxygen Generation Mechanism
4.2. Mechanism of Tribochemical Oxidation Reaction on the Surface of SiC
- (1)
- Solid-phase oxidant NaOH
- (2)
- Other solid-phase oxidants
- (3)
- Tribochemical hydration reaction on SiC surface
4.3. Material Removal Mechanism of Solid-Phase Oxidant
4.3.1. Solid-Phase Oxidant Sodium Hydroxide (NaOH)
- (1)
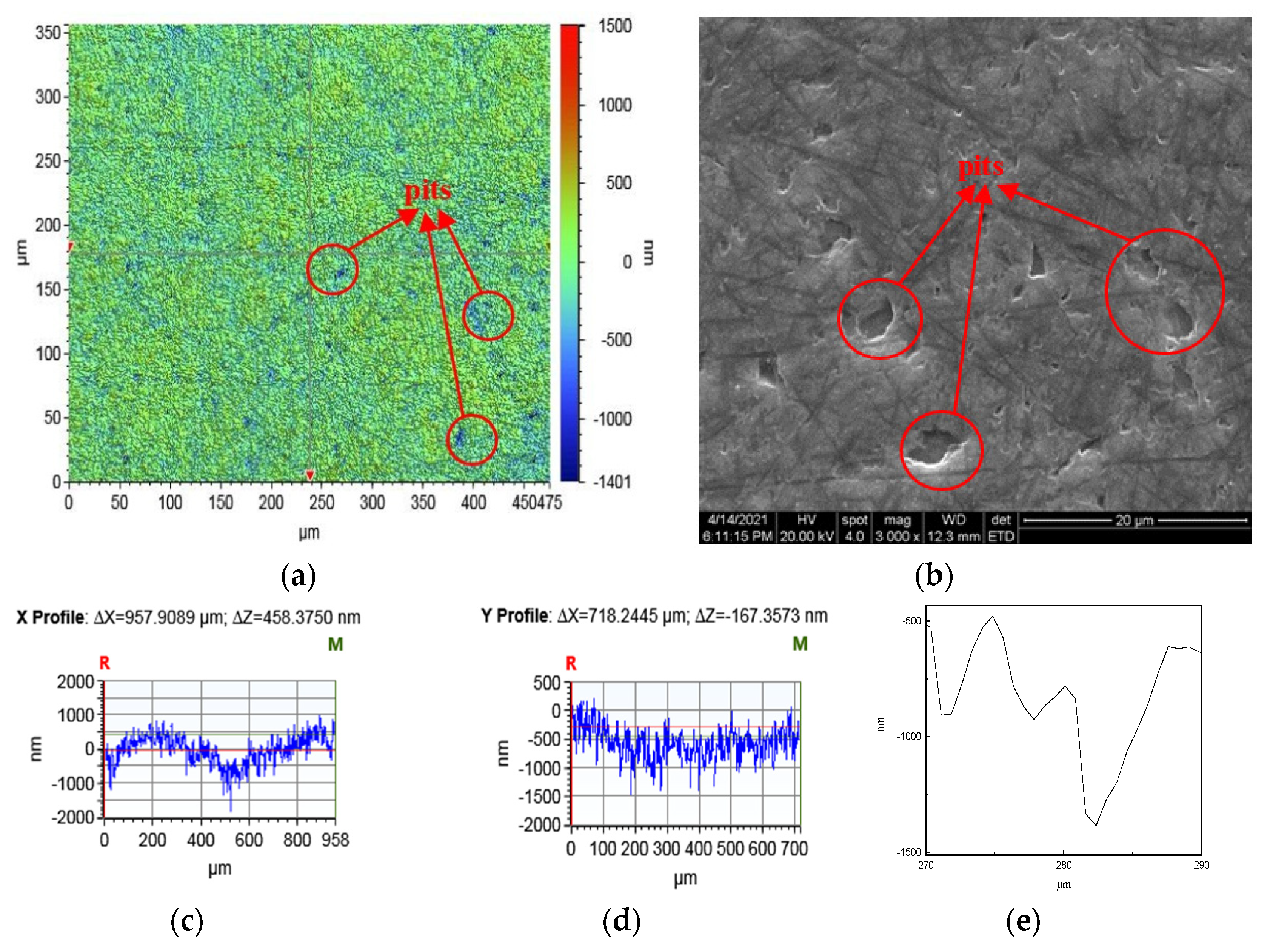
- The surface phases of SiC after polishing all contain oxygen, and the surface compounds are SiO2 and silicon oxides. On the one hand, the solid-phase oxidant NaOH reacts with CO2 in the air under the action of friction to form Na2CO3 and H2O, see Equation (6).On the other hand, the SiC reacts with H2O in the air to form SiO2 on its surface, see Equations (18)–(20).The CO and CO2 generated by the reaction escape into the air, and the generated C is removed by friction; the SiO2 generated is attached to the SiC surface. Figure 8 and Figure 9 show the surface morphology of SiC after dry polishing with NaOH.
- (2)
- Under the action of friction, the suspended silicon bonds of SiC also undergo a tribochemical reaction with NaOH, see Equation (16). The reaction produces soluble Na2SiO3, which is removed from the SiC surface. As can be seen from the SEM inspection of the enlarged Figure 7c and Figure 9b, after polishing, more small pits appear on the SiC surface with a smoother edge, not just brittle fracture removal. It can be shown that the Na2SiO3 produced by the reaction is removed by dissolution.
4.3.2. Solid-Phase Oxidant Sodium Percarbonate (Na2CO3—1.5 H2O2)
4.3.3. Solid-Phase Oxidant Sodium Iodate (KIO3)
4.3.4. Solid-Phase Oxidant Sodium Chlorate (KClO3)
4.3.5. Solid-Phase Oxidant Sodium Permanganate (KMnO4)
5. Conclusions
- (1)
- After dry polishing SiC with all five solid-phase oxidants, oxygen was detected on the surface, but the percentage of oxygen atoms on the surface after polishing varied. The highest percentage of oxygen atoms was observed after dry polishing SiC with Na2CO3—1.5 H2O2 and the lowest percentage of oxygen atoms was observed on the surface after dry polishing with KClO3.
- (2)
- From the XRD results, it couldbe seen that the appearance of surface oxygen was due to the tribochemical reaction between the five solid-phase oxidants and the SiC in the polishing process. The reaction product was known to be silicon oxides, and the main substance was SiO2. In addition, under the action of friction, due to the high flash point temperature at the polishing interface, SiC reacted with H2O and generated a SiO2 oxide layer on the SiC surface.
- (3)
- The material removal rate was calculated by measuring the mass before and after polishing, and the highest material removal rate couldbe obtained after dry polishing of SiC with NaOH and the lowest material removal rate could be obtained after dry polishing with KClO3.
- (4)
- After polishing SiC with oxidant NaOH, soluble Na2SiO3 was generated. Therefore, more obvious scratches and pits appeared on the surface of SiC, and the roughness hada substantial increase.The surface roughness of the remaining four solid-phase oxidants didnot change significantly after polishing.
Author Contributions
Funding
Conflicts of Interest
References
- Lagudu, U.R.; Isono, S.; Krishnan, S.; Babu, S.V. Role of ionic strength in chemical mechanical polishing of silicon carbide using silica slurries. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 119–127. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, Z.; Guo, B. Material removal mechanism in ultrasonic vibration assisted polishing of micro cylindrical surface on SiC. Int. J. Mach. Tools Manuf. 2016, 103, 28–39. [Google Scholar] [CrossRef]
- Li, B. Investigation on Wide Bandgap Semiconductor Material. Equip. Electron. Prod. Manuf. 2010, 39, 5–10. [Google Scholar]
- Yamaoka, H.; Uruga, T.; Arakawa, E.; Matsuoka, M.; Ogasaka, Y.; Yamashita, K.; Ohtomo, N. Development and Surface Evaluation of Large SiC X-ray Mirrors for High-Brilliance Synchrotron Radiation. Jpn. J. Appl. Phys. 1994, 33, 6718–6726. [Google Scholar] [CrossRef]
- Jenny, J.R.; Skowronski, M.; Mitchel, W.C.; Hobgood, H.M.; Glass, R.C.; Augustine, G.; Hopkins, R.H. Vanadium related near-band-edge absorption bands in three SiC polytypes. J. Appl. Phys. 1995, 78, 3160–3163. [Google Scholar] [CrossRef]
- Zhuang, D.; Edgar, J.H. Wet etching of GaN, AlN, and SiC: Areview. Mater. Sci. Eng. R 2005, 48, 1–46. [Google Scholar] [CrossRef]
- Han, R. Research Status of Third generation semiconductor materials SiC planarization technology. Chem. Intermed. 2018, 11, 73–75. [Google Scholar]
- Jiang, P.; Wang, C.; Kang, R.; Guo, D. Design and realization of UML-based CMP bontrol system. Equip. Electron. Prod. Manuf. 2008, 8, 48–52. [Google Scholar]
- Zhang, W.; Yang, X.; Shang, C.; Hu, X.; Hu, Z. High Speed Lapping of SiC Ceramic Material with Solid (Fixed) Abrasives. J. China Ordnance 2005, 1, 225–228. [Google Scholar]
- Zhu, Y.; Wang, J.; Li, J. Research on the polishing of silicon substrate by fixed abrasive pad. China Mech. Eng. 2009, 20, 723–727. [Google Scholar]
- Zhu, Y.; He, J. A model of material removal rate with fixed abrasive pad. Dimond Abras. Eng. 2006, 153, 39–45. [Google Scholar]
- Li, P.; Li, J.; Wang, J. Study on the lapping process of sapphire substrate through fixed abrasive. J. Synth. Cryst. 2013, 42, 2258–2264. [Google Scholar]
- Zhong, X. Development of Wmocr Polishing Plate Used in Dynamic Friction Polishing of Diamond; Dalian University of Technology: Dalian, China, 2015. [Google Scholar]
- Li, J. Recent advances and trends in tribology. Lubes Fuels 2007, 17, 10. [Google Scholar]
- Muratov, V.A.; Fischer, T.E. Tribochemical Polishing. Annu. Rev. Mater. Sci. 2002, 30, 27–51. [Google Scholar] [CrossRef]
- Zhu, Z.; Muratov, V.; Fischer, T.E. Tribochemical polishing of silicon carbide in oxidant solution. Wear 1999, 225–229, 848–856. [Google Scholar] [CrossRef]
- Kitaoka, S.; Tsuji, T.; Yamaguchi, Y. Tribochemical wear theory of non-oxide ceramics in high temperature and high-pressure water. Wear 1997, 205, 40–46. [Google Scholar] [CrossRef]
- Otani, Y.; Xu, J.; Adachi, K. First-Principles Molecular Dynamics Study of Silicon-Based Ceramics: Different Tribochemical Reaction Mechanisms during the Running-in Period of Silicon Nitride and Silicon Carbide. J. Phys. Chem. C 2020, 124, 20079–20089. [Google Scholar] [CrossRef]
- Zhou, F.; Kato, K.; Adachi, K. Friction and wear properties of CNx/SiC in water lubrication. Tribol. Lett. 2005, 18, 153–163. [Google Scholar] [CrossRef]
- Zhou, S.; Xiao, H. Study of high-temperature friction chemistry of silicon carbide and its complex-phase ceramics. China Ceram. Ind. 2002, 9, 16–20. [Google Scholar]
- Zhou, S.; Xiao, H. Tribo-chemistry and wear map of silicon carbide ceramics. J. Chin. Ceram. Soc. 2002, 30, 641–644. [Google Scholar]
- Liebau, F. Zeolites and clathrasils—Two distinct classes of framework silicates. Zeolites 1983, 3, 191–193. [Google Scholar] [CrossRef]
- Tieu, A.K.; Wan, S.; Kong, N. Excellent melt lubrication of alkali metal polyphosphate glass for high temperature applications. RSC Adv. 2014, 5, 1796–1800. [Google Scholar] [CrossRef] [Green Version]
- Tieu, A.K.; Kong, N.; Wan, S. The Influence of Alkali Metal Polyphosphate on the Tribological Properties of Heavily Loaded Steel on Steel Contacts at Elevated Temperatures. Adv. Mater. Interfaces 2015, 2, 1500032. [Google Scholar] [CrossRef]
- Matsuda, M.; Kato, K.; Hashimoto, A. Friction and Wear Properties of Silicon Carbide in Water from Different Sources. Tribol. Lett. 2011, 43, 33–41. [Google Scholar] [CrossRef]
- Presser, V. Oxidation and Wet Wear of Silicon Carbide; University Tübingen: Tübingen, Germany, 2009. [Google Scholar]
- Kuhlmann-Wilsdorf, D. Flash temperatures due to friction and Joule heat at asperity contacts. Wear 1985, 105, 187–198. [Google Scholar] [CrossRef]
- Quinn, T. Computational methods applied to oxidational wear. Wear 1996, 199, 169–180. [Google Scholar] [CrossRef]
- Tomizawa, H.; Fischer, T.E. Friction and Wear of Silicon Nitride and Silicon Carbide in Water: Hydrodynamic Lubrication at Low Sliding Speed Obtained by Tribochemical Wear. ASLE Trans. 1986, 30, 41–46. [Google Scholar] [CrossRef]
- Xu, J.; Kato, K. Formation of tribochemical layer of ceramics sliding in water and its role for low friction. Wear 2000, 245, 61–75. [Google Scholar] [CrossRef]
- Gates, R.S.; Hsu, S.M. Tribochemistry Between Water and Si3N4 and SiC: Induction Time Analysis. Tribol. Lett. 2004, 17, 399–407. [Google Scholar] [CrossRef]
- Qin, F.; Gu, D. Thermal Decomposition Reaction Kinetics of Sodium Percarbonate. J. East China Univ. Sci. Technol. (Nat. Sci.) 2001, 27, 214–216. [Google Scholar]
- Wang, H.; Zhao, H.; Qu, G. Research on Isothermal Decomposition Kinetics of Sodium Percarbonate. J. Henan Norm. Univ. (Nat. Sci.) 2001, 29, 58–61. [Google Scholar]
- Galwey, A.K.; Hood, W.J. Thermal decomposition of sodium carbonate perhydrate in the solid state. Chem. Inf. 1979, 10, 1810–1815. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Zhao, Y. Study on the dealkalization of red mud by suspension and carbonation. Chin. J. Environ. Eng. 2009, 3, 2275–2280. [Google Scholar]
- Li, Z. A Study on the Origination of the Laboratory Method of Oxygen Preparation by Potassium Chlorate and Manganese Dioxide. Univ. Chem. 2020, 35, 268–273. [Google Scholar]
- Huang, H.; Li, Y. Research of the Thermal Decomposition Process of Potassium Permanganate. Chin. J. Chem. Educ. 2009, 30, 65–66. [Google Scholar]
- Tan, R.; Chen, G. A Study on the Mechanism of KMnO4 Thermolysis. J. Guizhou Norm. Univ. (Nat. Sci.) 1990, 1, 20–25. [Google Scholar]
- Mao, L. The Thermal Decomposition of KMnO4, KClO3 and Other Inorganic Solids; Guangxi Normal University: Guilin, China, 2008. [Google Scholar]
- Wang, G.; Zhong, X. Recovery and Utilization of By-products from Decomposition of Potassium Permanganate to Oxygen. Educ. Chem. 2000, 12, 41–42. [Google Scholar]
- Liu, H.; Shen, T.; Cao, W. Stability of the salt iodization agent potassium iodate: A differential thermal analysis. Chin. J. Endem. 2012, 31, 684–686. [Google Scholar]
- Chen, X.; Juan, L.I.; Deying, M.A. Fine Machining of Large-Diameter 6H-SiC Wafers. J. Mater. Sci. Technol. 2006, 22, 681–684. [Google Scholar]
- Ming, J.; Komanduri, R. On the finishing of Si3N4 balls for bearing applications. Wear 1998, 215, 267–278. [Google Scholar]
- Kitaoka, S.; Tsuji, T.; Katoh, T. Tribological Characteristics of SiC Ceramics in High-Temperature and High-Pressure Water. J. Am. Ceram. Soc. 1994, 77, 1851–1856. [Google Scholar] [CrossRef]
- Zhou, L. Chemomechanical polishing of silicon carbide. Jelectrochemsoc 1997, 144, 161–163. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Tan, S. Tribological properties of toughened SiC ceramics under water-lubricated sliding. J. Chin. Ceram. Soc. 1998, 26, 305–312. [Google Scholar]
- Hao, L. Study on Electrical Friction Characteristics of Silicon Carbide Ceramics during Polishing; Harbin Institute of Technology: Harbin, China, 2008. [Google Scholar]
- Jin, L.; Scheerer, H.; Andersohn, G. Experimental study on the tribo-chemical smoothening process between self-mated silicon carbide in a water-lubricated surface contact reciprocating test. Friction 2019, 7, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Scheerer, H.; Andersohn, G.; Oechsner, M. Tribological behavior of surface contact friction test with water lubrication at elevated temperatures. Tribol. Und Schmier. 2017, 64, 11–17. [Google Scholar]


| Factors | Speed of Polishing Tool n1 (r/min) | Speed of Polishing Head n2 (r/min) | Polishing Pressure P (psi) | Time t (h) |
|---|---|---|---|---|
| Parameters | 60 | 45 | 2 | 1.5 |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 6H-SiC | Na2CO3—1.5 H2O2 | NaOH | KIO3 | KClO3 | KMnO4 |
| Element | C (%) | Si (%) |
|---|---|---|
| Atomic percentage of SiC surface elements | 45.67 | 54.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, W.; Cao, X.; Xiao, W.; Wang, Z.; Su, J. Study on the Mechanism of Solid-Phase Oxidant Action in Tribochemical Mechanical Polishing of SiC Single Crystal Substrate. Micromachines 2021, 12, 1547. https://doi.org/10.3390/mi12121547
Qi W, Cao X, Xiao W, Wang Z, Su J. Study on the Mechanism of Solid-Phase Oxidant Action in Tribochemical Mechanical Polishing of SiC Single Crystal Substrate. Micromachines. 2021; 12(12):1547. https://doi.org/10.3390/mi12121547
Chicago/Turabian StyleQi, Wanting, Xiaojun Cao, Wen Xiao, Zhankui Wang, and Jianxiu Su. 2021. "Study on the Mechanism of Solid-Phase Oxidant Action in Tribochemical Mechanical Polishing of SiC Single Crystal Substrate" Micromachines 12, no. 12: 1547. https://doi.org/10.3390/mi12121547






