Investigation of Fusion between Nanosized Lipid Vesicles and a Lipid Monolayer Toward Formation of Giant Lipid Vesicles with Various Kinds of Biomolecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Formation of Large Unilamellar Vesicles (LUVs)
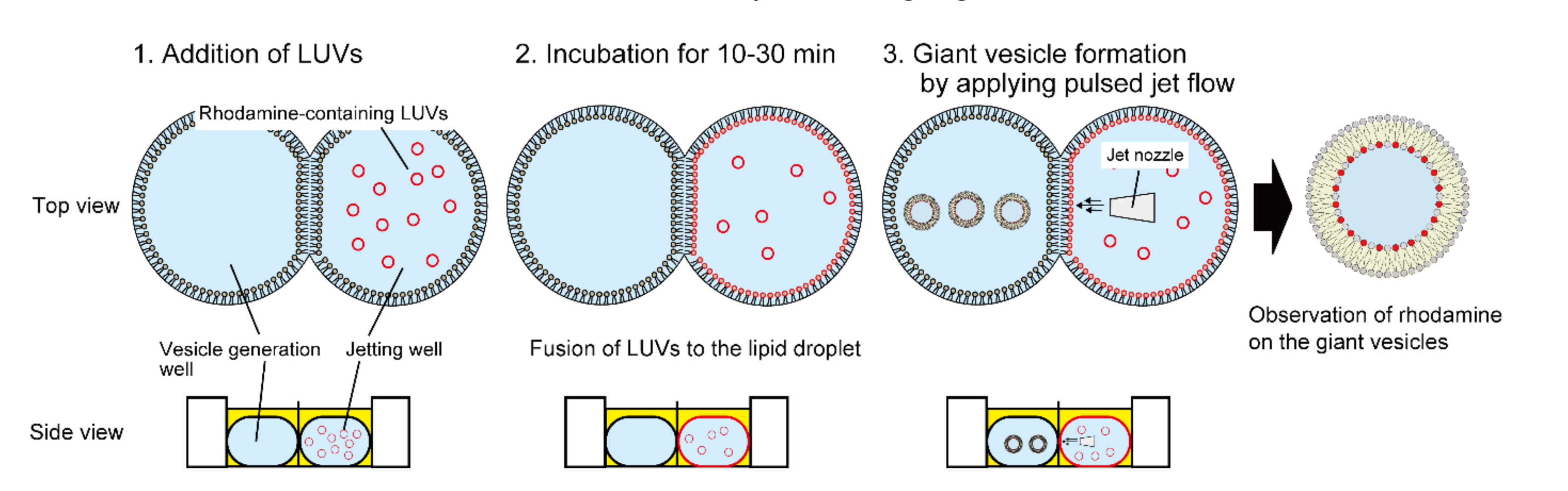
2.4. Fusion of LUVs to a Lipid Droplet
2.5. Fusion of LUVs to a Lipid Droplet at Different Incubation Times and Different Lipid Comportments
2.6. Fusion between LUVs and Giant Lipid Vesicles at Different Incubation Times and Lipid Comportments
2.7. CLSM Observation of the Giant Lipid Vesicles
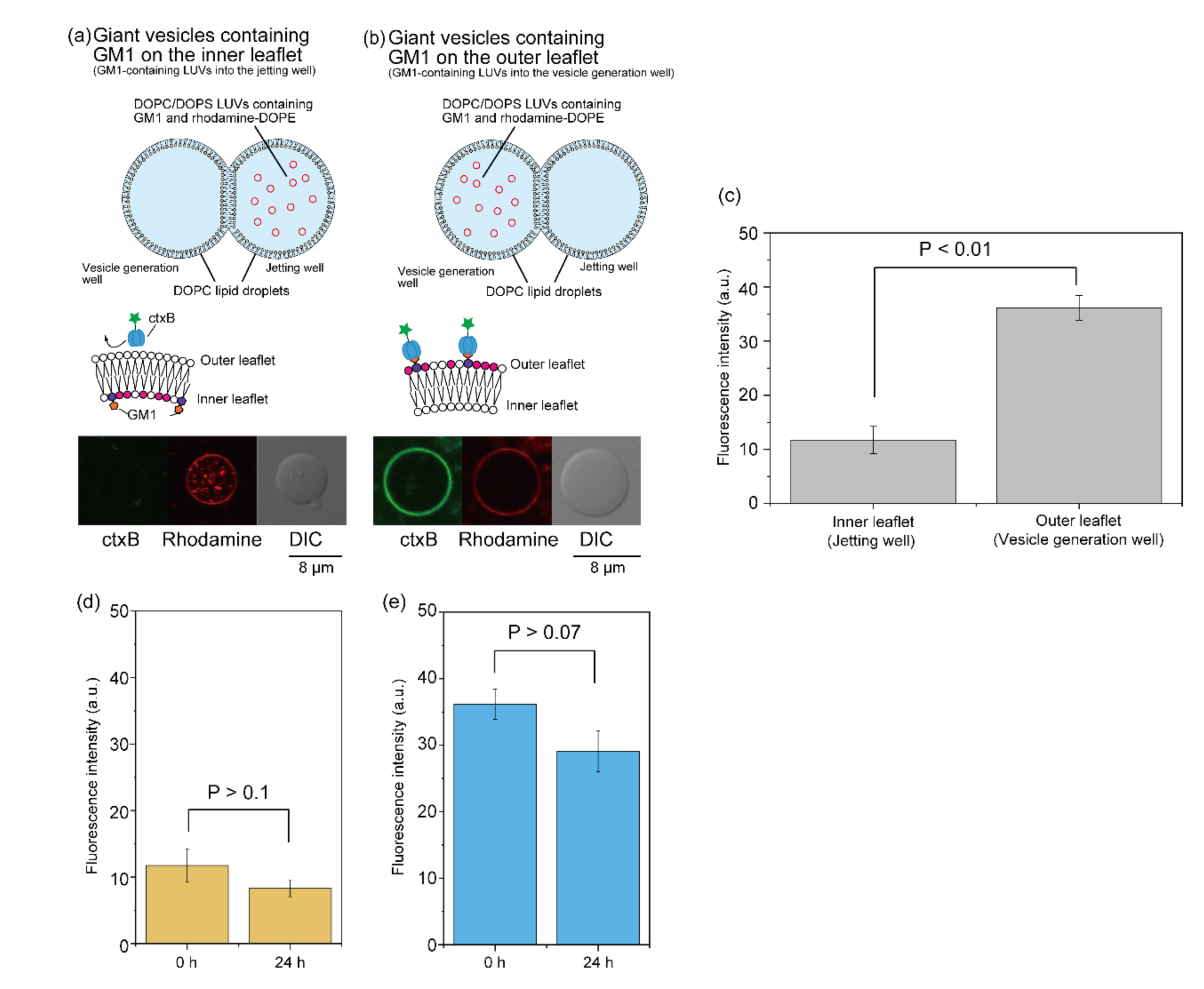
2.8. Formation of Asymmetric GM1 Vesicles
3. Results and Discussion
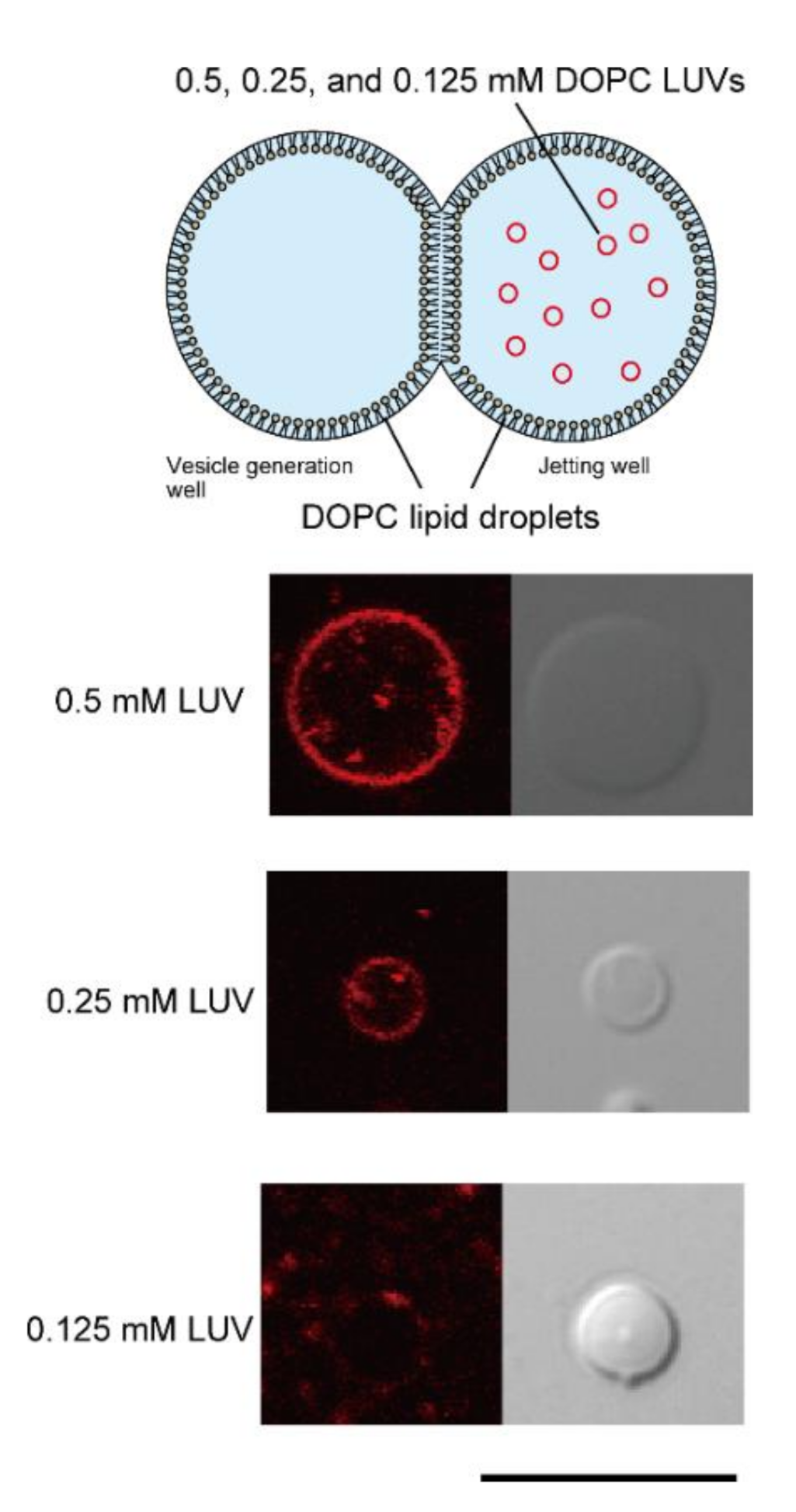
3.1. Determination of Optimal LUV Concentrations for DIB
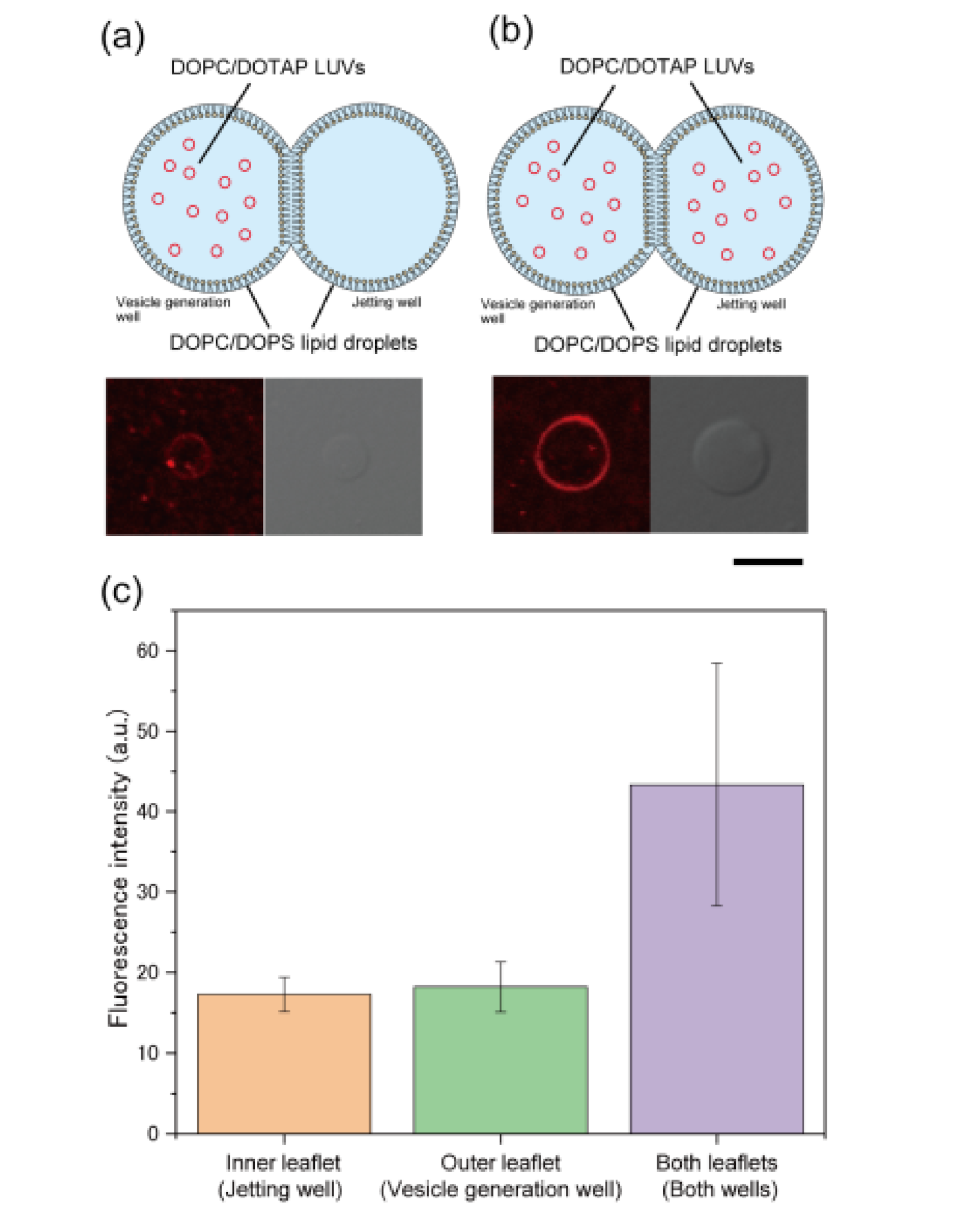
3.2. Fusion Properties of LUVs with Lipid Droplets
3.3. Formation of Asymmetric Lipid Vesicles with Rhodamine or GM1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van den Bogaart, G.; Meyenberg, K.; Risselada, H.J.; Amin, H.; Willig, K.I.; Hubrich, B.E.; Dier, M.; Hell, S.W.; Grubmüller, H.; Diederichsen, U.; et al. Membrane Protein Sequestering by Ionic Protein–lipid Interactions. Nature 2011, 479, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant Vesicles: Preparations and Applications. ChemBioChem 2010, 11, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S. Synthesizing Artificial Cells from Giant Unilamellar Vesicles: State-of-the Art in the Development of Microfluidic Technology. BioEssays 2012, 34, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Takeuchi, S. Giant Liposome Formation toward the Synthesis of Well-Defined Artificial Cells. J. Mater. Chem. B 2017, 5, 5911–5923. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, K.; Suzuki, H.; Takeuchi, S. Lipid Bilayer Formation by Contacting Monolayers in a Microfluidic Device for Membrane Protein Analysis. Anal. Chem. 2006, 78, 8169–8174. [Google Scholar] [CrossRef]
- Holden, M.A.; Needham, D.; Bayley, H. Functional Bionetworks from Nanoliter Water Droplets. J. Am. Chem. Soc. 2007, 129, 8650–8655. [Google Scholar] [CrossRef]
- Heron, A.J.; Thompson, J.R.; Cronin, B.; Bayley, H.; Wallace, M.I. Simultaneous Measurement of Ionic Current and Fluorescence from Single Protein Pores. J. Am. Chem. Soc. 2009, 131, 1652–1653. [Google Scholar] [CrossRef]
- Kawano, R.; Tsuji, Y.; Sato, K.; Osaki, T.; Kamiya, K.; Hirano, M.; Ide, T.; Miki, N.; Takeuchi, S. Automated Parallel Recordings of Topologically Identified Single Ion Channels. Sci. Rep. 2013, 3, 1995. [Google Scholar] [CrossRef]
- Huang, S.; Romero-Ruiz, M.; Castell, O.K.; Bayley, H.; Wallace, M.I. High-Throughput Optical Sensing of Nucleic Acids in a Nanopore Array. Nat. Nanotechnol. 2015, 10, 986–991. [Google Scholar] [CrossRef] [Green Version]
- Villar, G.; Graham, A.D.; Bayley, H. A Tissue-Like Printed Material. Science 2013, 340, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funakoshi, K.; Suzuki, H.; Takeuchi, S. Formation of Giant Lipid Vesiclelike Compartments from a Planar Lipid Membrane by a Pulsed Jet Flow. J. Am. Chem. Soc. 2007, 129, 12608–12609. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, J.C.; Richmond, D.L.; Li, T.H.; Liu, A.P.; Parekh, S.H.; Fletcher, D.A. Unilamellar Vesicle Formation and Encapsulation by Microfluidic Jetting. Proc. Natl. Acad. Sci. USA 2008, 105, 4697–4702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Li, Q.-H.; Bayley, H. Orientation of the Monomeric Porin OmpG in Planar Lipid Bilayers. ChemBioChem 2008, 9, 3029–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupin, A.; Simmel, F.C. Signalling and Differentiation in Emulsion-Based Multi-Compartmenalized In Vitro Gene Ciruits. Nat. Chem. 2018, 11, 32–39. [Google Scholar] [CrossRef]
- Bachler, S.; Haidas, D.; Ort, M.; Duncombe, T.A.; Dittrich, P.S. Microfluidic Platform Enables Tailored Translocation and Reaction Cascades in Nanoliter Droplet Networks. Commun. Biol. 2020, 3, 769. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Kawano, R.; Osaki, T.; Akiyoshi, K.; Takeuchi, S. Cell-Sized Asymmetric Lipid Vesicles Facilitate the Investigation of Asymmetric Membranes. Nat. Chem. 2016, 8, 881–889. [Google Scholar] [CrossRef]
- Schrempf, H.; Schmidt, O.; Kümmerlen, R.; Hinnah, S.; Müller, D.; Betzler, M.; Steinkamp, T.; Wagner, R. A Prokaryotic Potassium Ion Channel with Two Predicted Transmembrane Segments from Streptomyces Lividans. EMBO J. 1995, 14, 5170–5178. [Google Scholar] [CrossRef] [Green Version]
- Oshima, A.; Hirano-Iwata, A.; Mozumi, H.; Ishinari, Y.; Kimura, Y.; Niwano, M. Reconstitution of Human Ether-a-Go-Go-Related Gene Channels in Microfabricated Silicon Chips. Anal. Chem. 2013, 85, 4363–4369. [Google Scholar] [CrossRef]
- Gotanda, M.; Kamiya, K.; Osaki, T.; Fujii, S.; Misawa, N.; Miki, N.; Takeuchi, S. Sequential genaration of asymmteric lipid vesicles using a pulsed-jetting method in rotational wells. Sens. Actuators B Chem. 2018, 261, 392–397. [Google Scholar] [CrossRef]
- Gotanda, M.; Kamiya, K.; Osaki, T.; Miki, N.; Takeuchi, S. Automatic Generation System of Cell-Sized Liposomes. Sens. Actuators B Chem. 2019, 292, 57–63. [Google Scholar] [CrossRef]
- Przybylo, M.; Sýkora, J.; Humpolíčová, J.; Benda, A.; Zan, A.; Hof, M. Lipid Diffusion in Giant Unilamellar Vesicles Is More than 2 Times Faster than in Supported Phospholipid Bilayers under Identical Conditions. Langmuir 2006, 22, 9096–9099. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, K.H.; Choi, M.C.; Choi, S.Q. Fluorescence Recovery after Merging a Droplet to Measure the Two-Dimensional Diffusion of a Phospholipid Monolayer. J. Vis. Exp. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trier, S.; Henriksen, J.R.; Andresen, T.L. Membrane Fusion of PH-Sensitive Liposomes—A Quantitative Study Using Giant Unilamellar Vesicles. Soft Matter 2011, 7, 9027–9034. [Google Scholar] [CrossRef]
- Sonnino, S.; Mauri, L.; Chigorno, V.; Prinetti, A. Gangliosides as Components of Lipid Membrane Domains. Glycobiology 2007, 17, 1R–13R. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richmond, D.L.; Schmid, E.M.; Martens, S.; Stachowiak, J.C.; Liska, N.; Fletcher, D.A. Forming Giant Vesicles with Controlled Membrane Composition, Asymmetry, and Contents. Proc. Natl. Acad. Sci. USA 2011, 108, 9431–9436. [Google Scholar] [CrossRef] [Green Version]
- Hwang, W.L.; Chen, M.; Cronin, B.; Holden, M.A.; Bayley, H. Asymmetric Droplet Interface Bilayers. J. Am. Chem. Soc. 2008, 130, 5878–5879. [Google Scholar] [CrossRef]
- Kamiya, K. Development of Artificial Cell Models Using Microfluidic Technology and Synthetic Biology. Micromachines 2020, 11, 559. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiya, K.; Arisaka, C.; Suzuki, M. Investigation of Fusion between Nanosized Lipid Vesicles and a Lipid Monolayer Toward Formation of Giant Lipid Vesicles with Various Kinds of Biomolecules. Micromachines 2021, 12, 133. https://doi.org/10.3390/mi12020133
Kamiya K, Arisaka C, Suzuki M. Investigation of Fusion between Nanosized Lipid Vesicles and a Lipid Monolayer Toward Formation of Giant Lipid Vesicles with Various Kinds of Biomolecules. Micromachines. 2021; 12(2):133. https://doi.org/10.3390/mi12020133
Chicago/Turabian StyleKamiya, Koki, Chika Arisaka, and Masato Suzuki. 2021. "Investigation of Fusion between Nanosized Lipid Vesicles and a Lipid Monolayer Toward Formation of Giant Lipid Vesicles with Various Kinds of Biomolecules" Micromachines 12, no. 2: 133. https://doi.org/10.3390/mi12020133





