Experimental Research on a Capsule Robot with Spring-Connected Legs
Abstract
:1. Introduction
2. Working Principle of CR
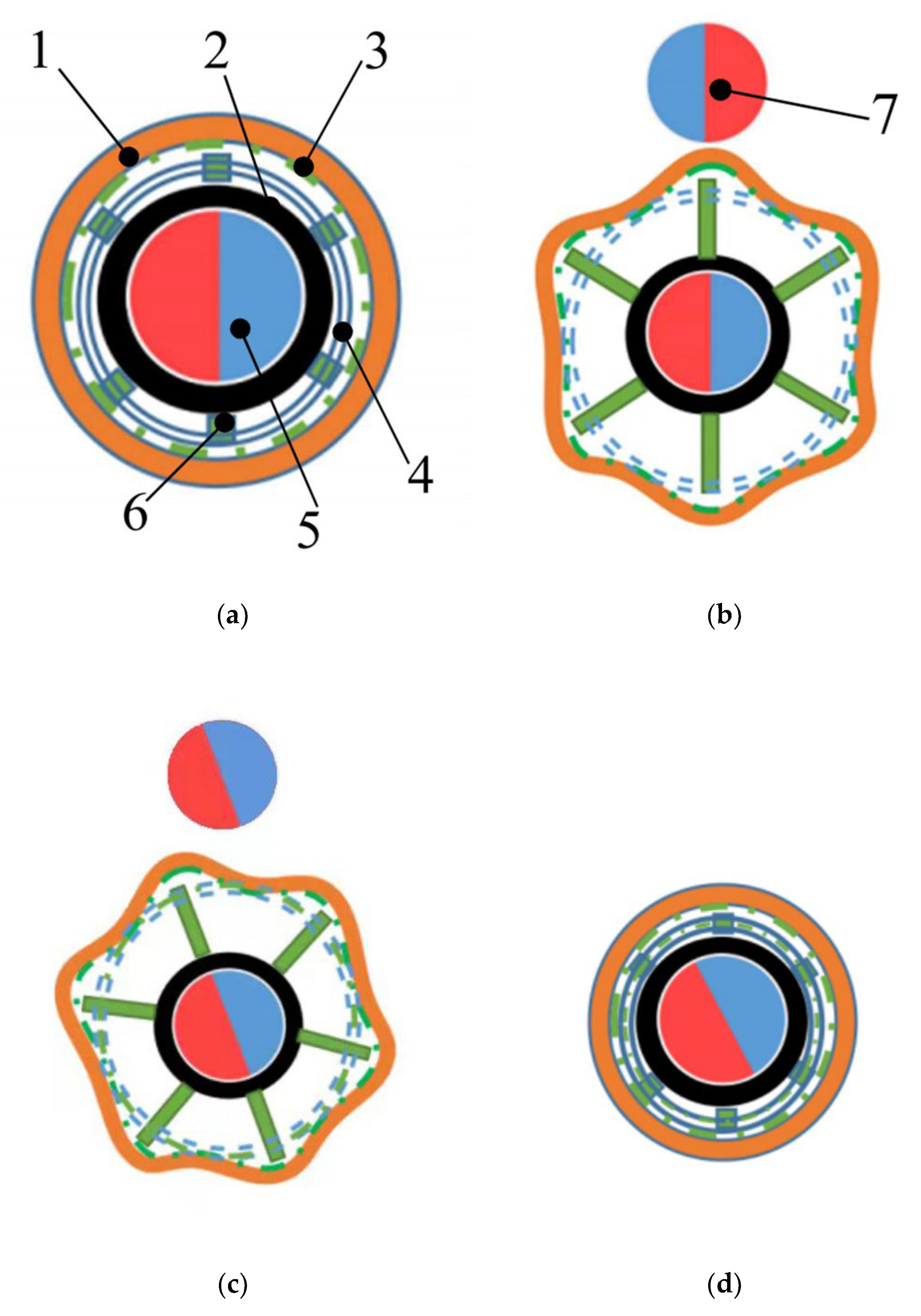
2.1. Structure of CR
2.2. Principle of Expanding Intestine and Biopsy
3. Measuring Experiment
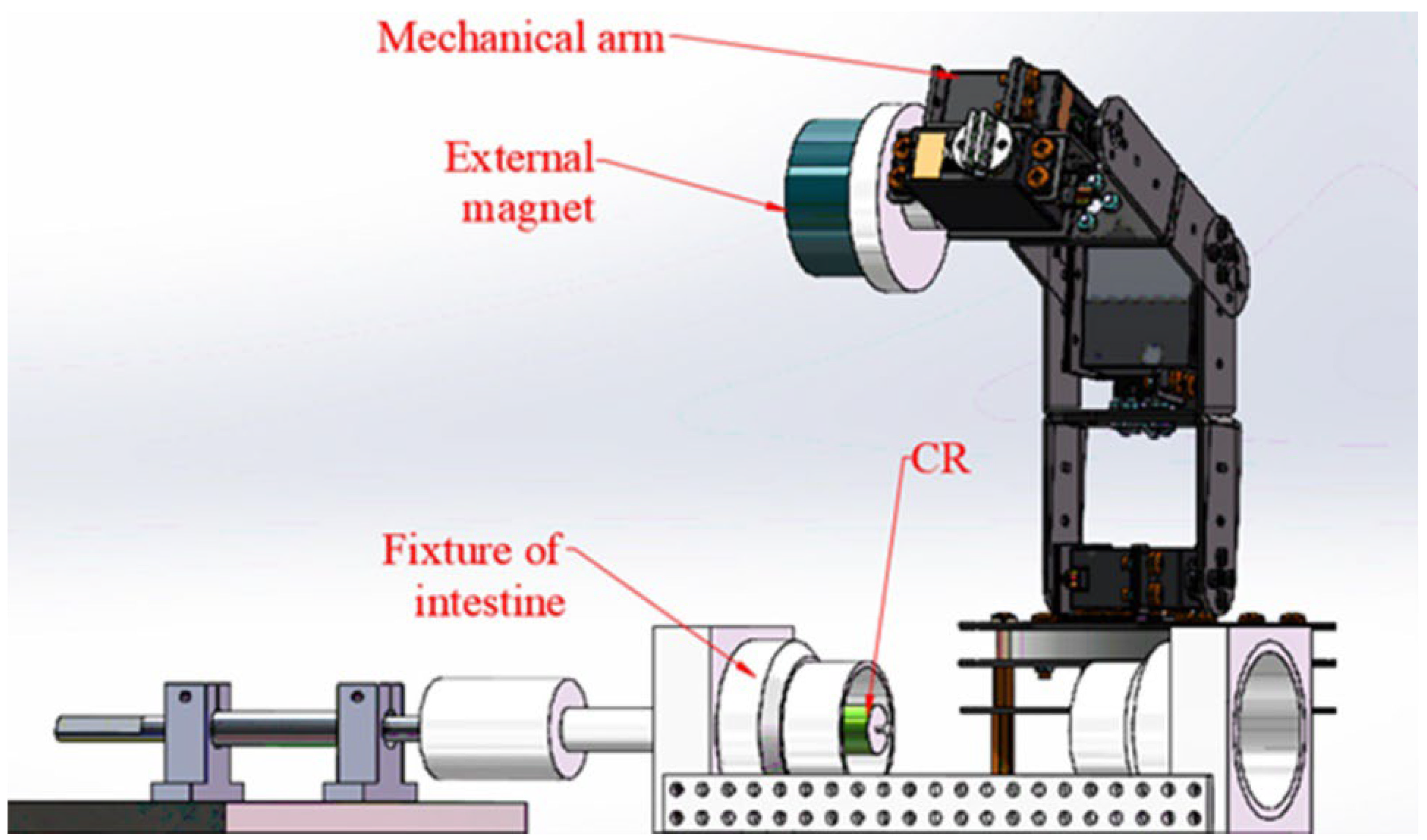
3.1. Magnetic Torque and Measuring System

3.2. Measuring Experiment and Result
4. Biopsy Experiment
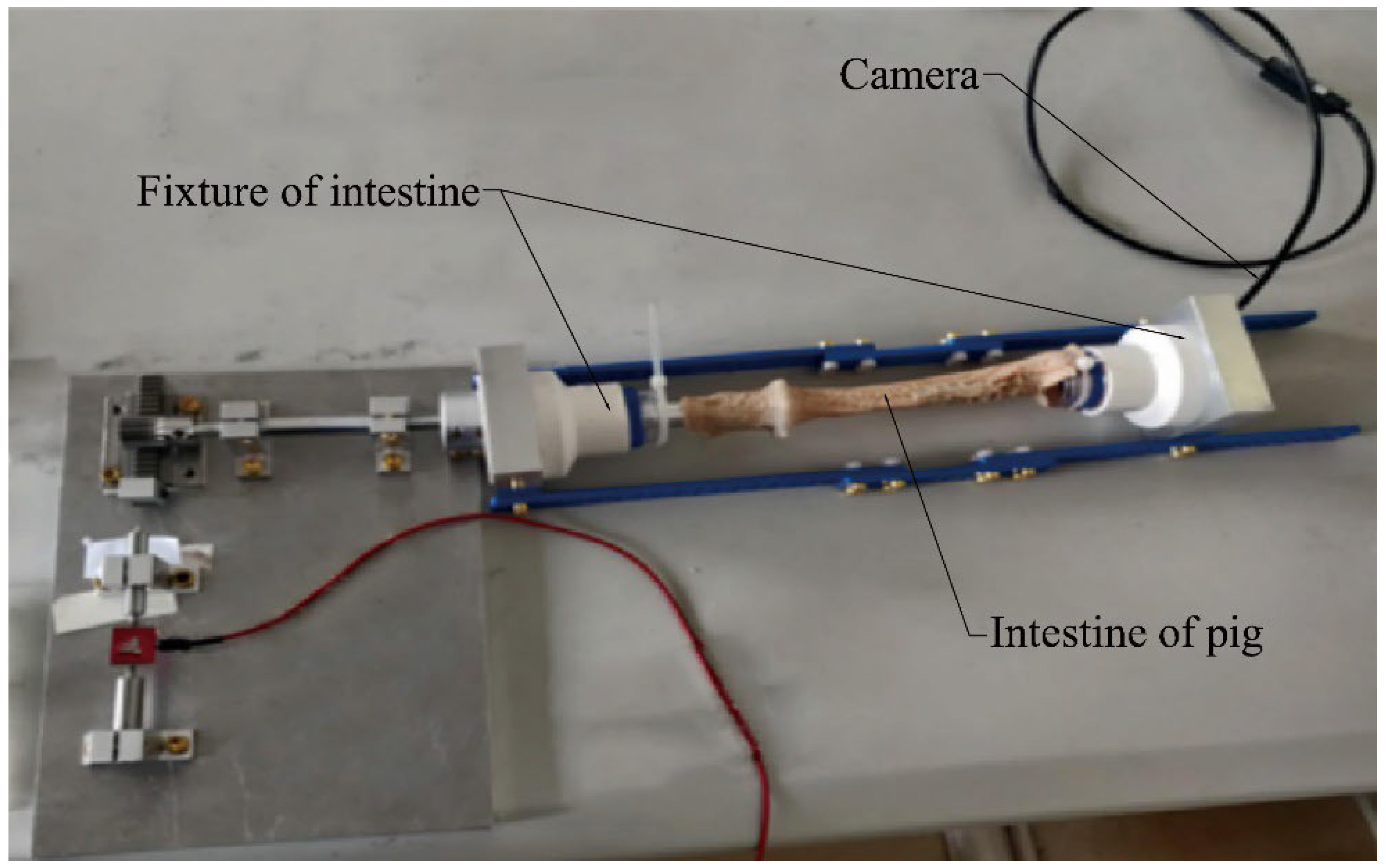
4.1. Principle and Experiment System of Biopsy
- To test the effect of springs on the expansion of the folds of the intestine. In the meantime, to test whether the CR will clamp the intestine. For this purpose, we carry out the experiment of expanding the intestine.
- To test the biopsy effect of each action of the CR during a biopsy and the necessity of rotating the CR. For this purpose, we carry out the experiment of decomposed actions.
4.2. Expanding Intestine Experiment
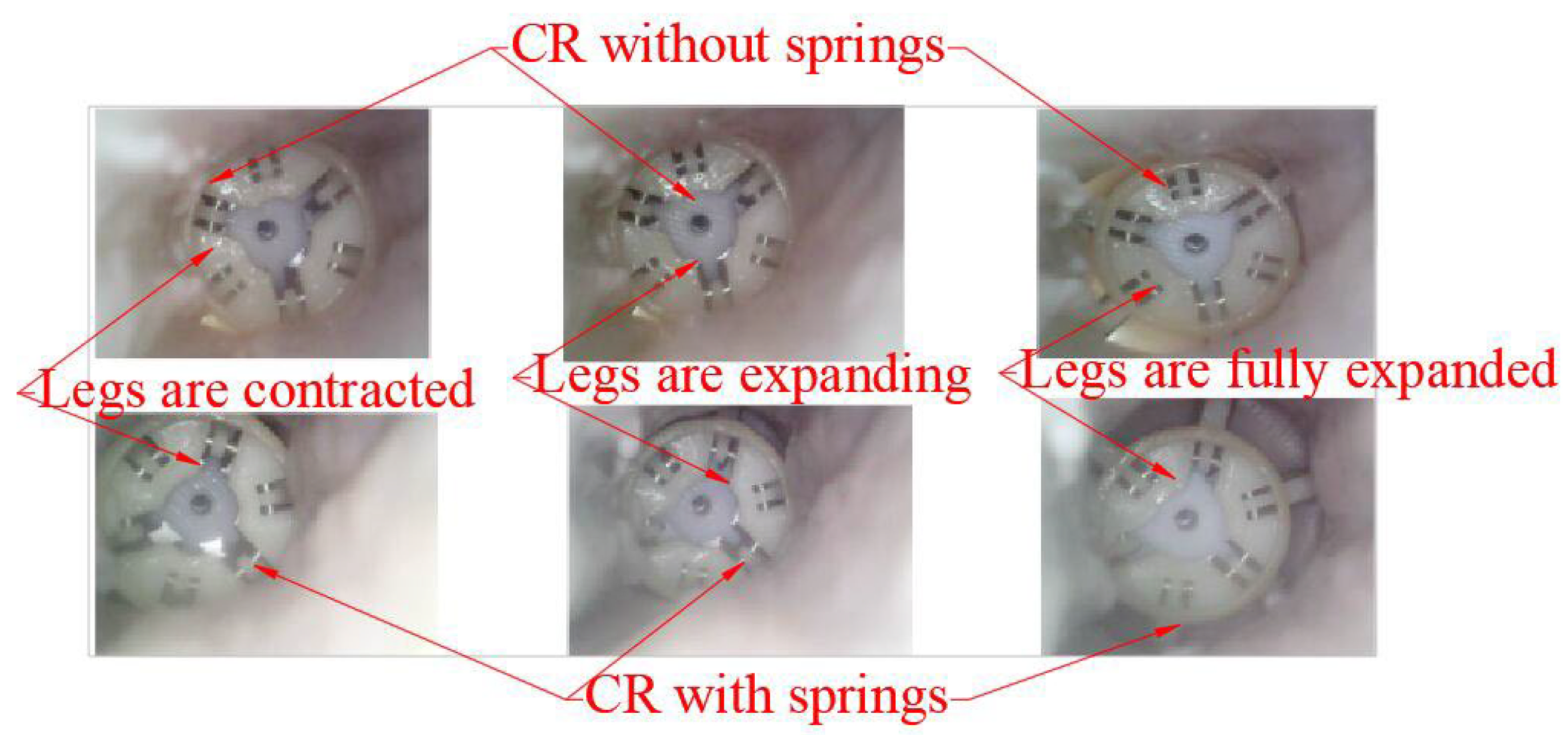
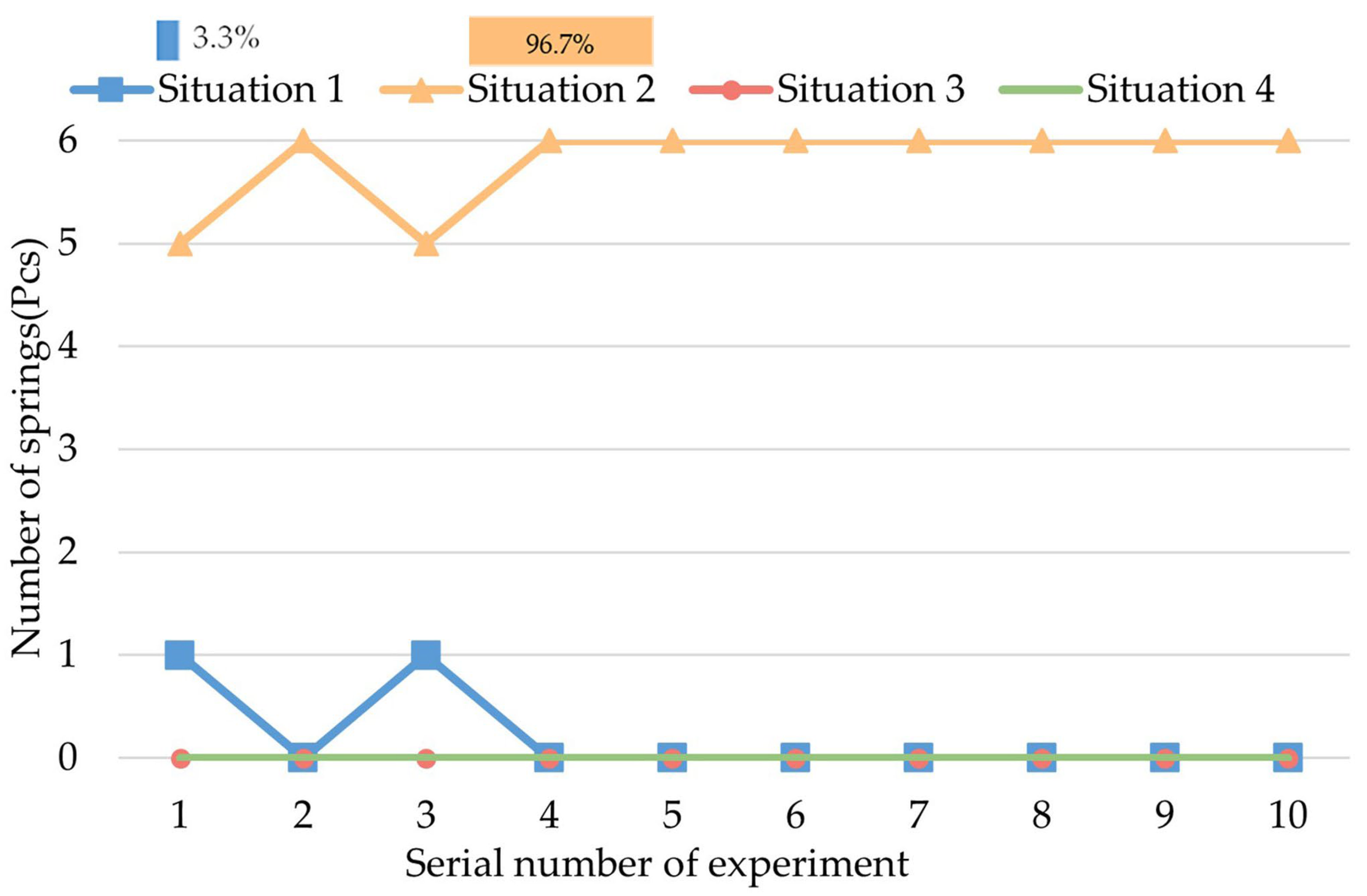
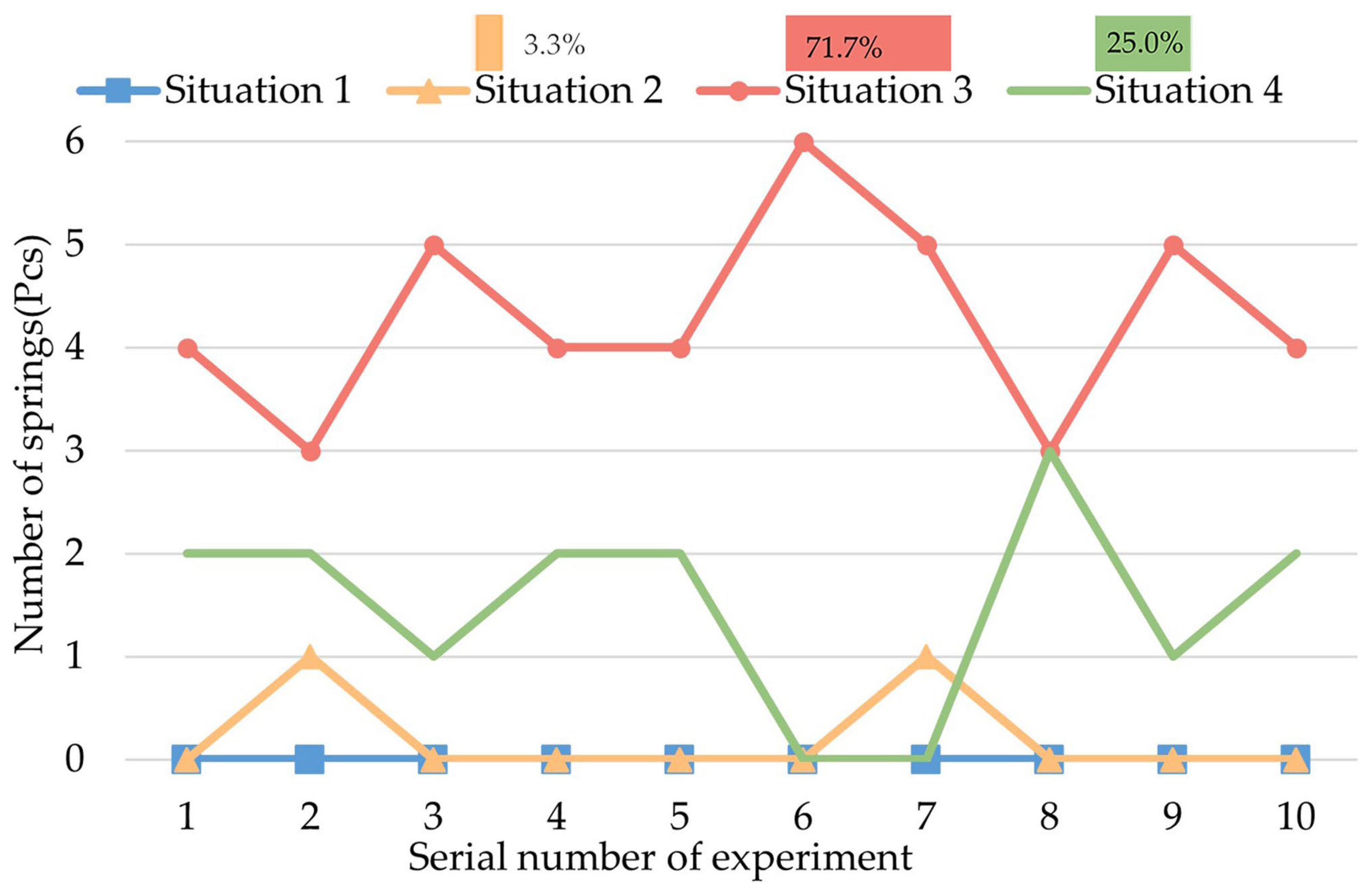
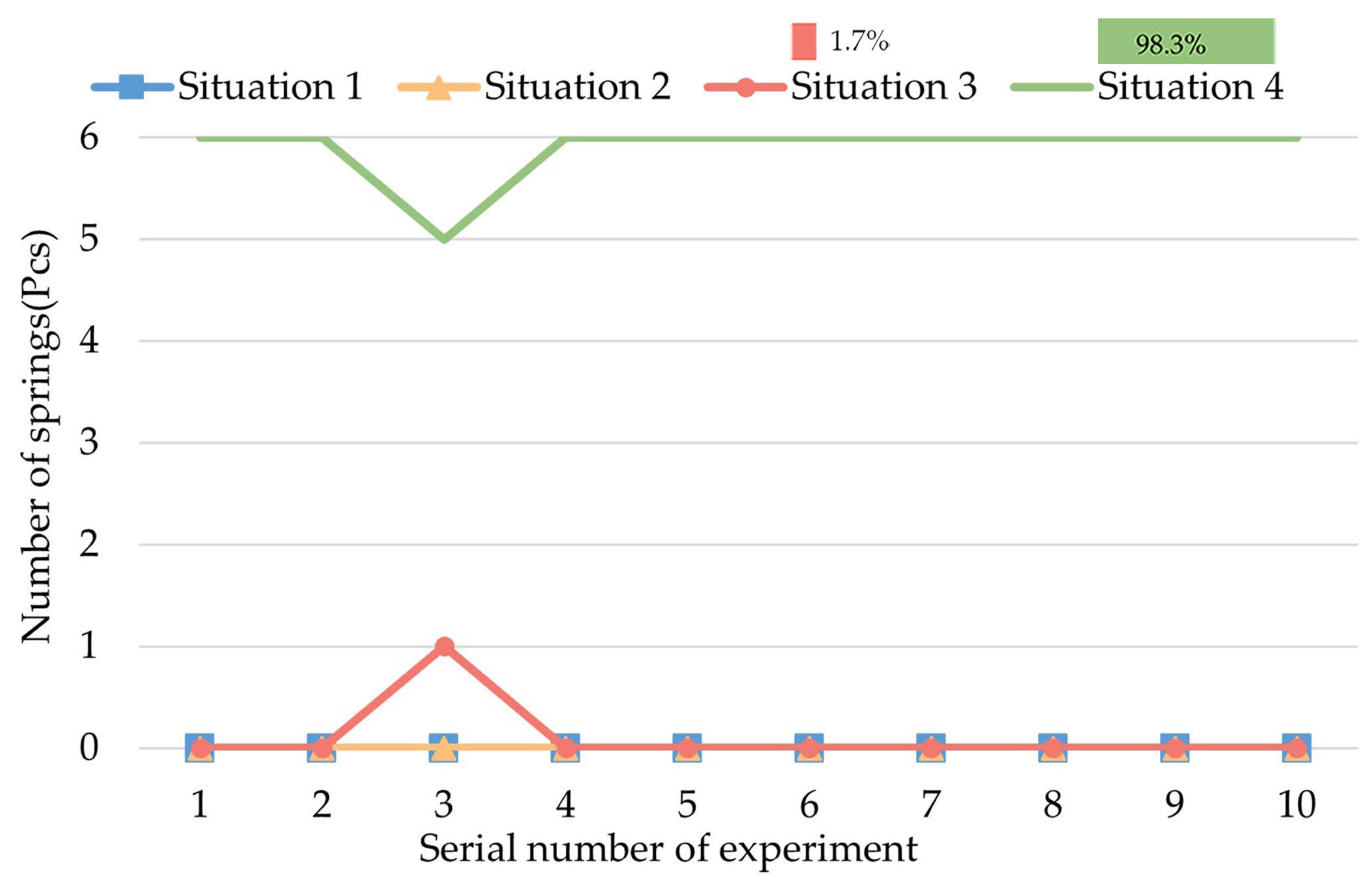
4.3. Experiment of Decomposed Actions
- A.
- The initial status is that the legs and springs are contracted.
- B.
- The process of expanding the legs and springs.
- C.
- The process of rotating the CR while the legs are expanded.
- D.
- The process of contracting the legs and springs.
5. Conclusions
- In this research, we designed an experiment system for a CR with spring-connected legs. This experiment system was used for measuring the torque and cutting force and testing the biopsy effect of the CR.
- We measured the torques and cutting force during a biopsy of this CR. When the axial distance between the external magnet and the internal magnet (D) was 70 mm, the torque exerted on the CR reached 1.620 N·mm at most, at this time, the cutting force of the CR was 0.203 N.
- We carried out several experiments to research the biopsy function of this CR. The biopsy effect of each decomposed action of the biopsy was tested. The most effective action was the rotation of the CR while the legs were fully expanded. Based on this experiment, we designed the most effective process for a biopsy.
6. Discussions of Future Work
- To reduce the pollution of the collected biopsy sample while the CR is moving inside the intestine, the capsule shell will be optimized so that the springs can be embedded in the shell.
- To save the internal space of the CR, the interior of the CR will be optimized. The micro-motor will be replaced by an internal magnet, which will cooperate with external magnets to expand the legs.
- In addition to the biopsy function, the drug-delivery function will be researched, and we will store medicine inside the springs and test whether the medicine can be released to a lesion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Toennies, J.L.; Tortora, G.; Simi, M.; Valdastri, P.; Webster, R.J. Swallowable medical devices for diagnosis and surgery: The state of the art. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2016, 224, 1397–1414. [Google Scholar] [CrossRef] [Green Version]
- Camarillo, D.B.; Krummel, T.M.; Salisbury, J.K. Robotic technology in surgery: Past, present, and future. Am. J. Surg. 2004, 188, 2S–15S. [Google Scholar] [CrossRef] [PubMed]
- Meron, G.D. The development of the swallowable video capsule (M2A). Gastrointest. Endosc. 2000, 52, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Shamsudhin, N.; Zverev, V.I.; Keller, H.; Pane, S.; Egolf, P.W.; Nelson, B.J.; Tishin, A.M. Magnetically guided capsule endoscopy. Med. Phys. 2017, 44, e91–e111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Banerjee, S.; Barth, B.A.; Bhat, Y.M.; Chauhan, S.; Gottlieb, K.T.; Konda, V.; Maple, J.T.; Murad, F.; Pfau, P.R.; et al. Wireless capsule endoscopy. Gastrointest. Endosc. 2013, 78, 805–815. [Google Scholar] [CrossRef]
- Valdastri, P.; Simi, M.; Webster, R.J., III. Advanced Technologies for Gastrointestinal Endoscopy. Annu. Rev. Biomed. Eng. 2012, 14, 397–429. [Google Scholar] [CrossRef] [Green Version]
- Alsunaydih, F.N.; Yuce, M.R. Next-generation ingestible devices: Sensing, locomotion and navigation. Physiol. Meas. 2021, 42, 04TR01. [Google Scholar] [CrossRef]
- Moglia, A.; Menciassi, A.; Schurr, M.O.; Dario, P. Wireless capsule endoscopy: From diagnostic devices to multipurpose robotic systems. Biomed. Microdevices 2007, 9, 235–243. [Google Scholar] [CrossRef]
- Ciuti, G.; Menciassi, A.; Dario, P. Capsule endoscopy: From current achievements to open challenges. IEEE Rev. Biomed. Eng. 2011, 4, 59–72. [Google Scholar] [CrossRef]
- Manh Cuong, H.; Viet Ha, L.; Kim Tien, N.; Van Du, N.; Kim, J.; Choi, E.; Bang, S.; Kang, B.; Park, J.-O.; Kim, C.-S. A Robotic Biopsy Endoscope with Magnetic 5-DOF Locomotion and a Retractable Biopsy Punch. Micromachines 2020, 11, 98. [Google Scholar] [CrossRef]
- Son, D.; Gilbert, H.; Sitti, M. Magnetically Actuated Soft Capsule Endoscope for Fine-Needle Biopsy. Soft Robot. 2020, 7, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Jeon, D.; ASME. Design of a biopsy module for the capsule type endoscope. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Orlando, FL, USA, 5–11 November 2005; pp. 19–22. [Google Scholar]
- Kong, K.C.; Cha, J.; Jeon, D.; Cho, D.I.D.; IEEE. A rotational micro biopsy device for the capsule endoscope. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Edmonton, AB, Canada, 2–6 August 2005; pp. 3057–3061. [Google Scholar]
- Kong, K.; Yim, S.; Choi, S.; Jeon, D. A Robotic Biopsy Device for Capsule Endoscopy. J. Med. Devices-Trans. ASME 2012, 6, 031004. [Google Scholar] [CrossRef]
- Simi, M.; Gerboni, G.; Menciassi, A.; Valdastri, P. Magnetic Torsion Spring Mechanism for a Wireless Biopsy Capsule. J. Med. Devices-Trans. ASME 2013, 7, 041009. [Google Scholar] [CrossRef]
- Viet Ha, L.; Jin, Z.; Leon-Rodriguez, H.; Lee, C.; Choi, H.; Van Du, N.; Go, G.; Ko, S.-Y.; Park, J.-O.; Park, S. Electromagnetic field intensity triggered micro-biopsy device for active locomotive capsule endoscope. Mechatronics 2016, 36, 112–118. [Google Scholar] [CrossRef]
- Le, V.H.; Hernando, L.-R.; Lee, C.; Choi, H.; Jin, Z.; Kim Tien, N.; Go, G.; Ko, S.-Y.; Park, J.-O.; Park, S. Shape memory alloy-based biopsy device for active locomotive intestinal capsule endoscope. Proc. Inst. Mech. Eng. Part H-J. Eng. Med. 2015, 229, 255–263. [Google Scholar] [CrossRef]
- Yim, S.; Gultepe, E.; Gracias, D.H.; Sitti, M. Biopsy using a Magnetic Capsule Endoscope Carrying, Releasing, and Retrieving Untethered Microgrippers. Ieee Trans. Biomed. Eng. 2014, 61, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-W.; Yan, G.-Z.; Liu, H.; Jiang, P.-P.; Wang, Z.-W. Design of Micro Biopsy Device for Wireless Autonomous Endoscope. Int. J. Precis. Eng. Manuf. 2014, 15, 2317–2325. [Google Scholar] [CrossRef]
- Park, S.; Koo, K.-i.; Bang, S.M.; Park, J.Y.; Song, S.Y.; Cho, D.D. A novel microactuator for microbiopsy in capsular endoscopes. J. Micromech. Microeng. 2008, 18, 025032. [Google Scholar] [CrossRef]
- Le, V.H.; Van Du, N.; Lee, C.; Go, G.; Park, J.-O.; Park, S. Miniaturized biopsy module using gripper tool for active locomotive capsule endoscopee. Mechatronics 2017, 44, 52–59. [Google Scholar] [CrossRef]
- Gao, J.; Yan, G.; Wang, Z.; He, S.; Xu, F.; Jiang, P.; Liu, D. Design and Testing of a Motor-Based Capsule Robot Powered by Wireless Power Transmission. Ieee-Asme Trans. Mechatron. 2016, 21, 683–693. [Google Scholar] [CrossRef]
- Munoz, F.; Alici, G.; Li, W. A Magnetically Actuated Drug Delivery System for Robotic Endoscopic Capsules. J. Med. Devices-Trans. ASME 2016, 10, 011004. [Google Scholar] [CrossRef] [Green Version]
- Sreekumar, M.; Nagarajan, T.; Singaperumal, M.; Zoppi, M.; Molfino, R. Critical review of current trends in shape memory alloy actuators for intelligent robots. Ind. Robot-Int. J. Robot. Res. Appl. 2007, 34, 285–294. [Google Scholar] [CrossRef]
- Rehan, M.; Al-Bahadly, I.; Thomas, D.G.; Avci, E. Capsule robot for gut microbiota sampling using shape memory alloy spring. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Soto, F.; Purcell, E.; Ozen, M.O.; Sinawang, P.D.; Wang, J.; Akin, D.; Demirci, U. Robotic Pill for Biomarker and Fluid Sampling in the Gastrointestinal Tract. Adv. Intell. Syst. 2022, 4, 2200030. [Google Scholar] [CrossRef]
- Ye, D.; Xue, J.; Yuan, S.; Zhang, F.; Song, S.; Wang, J.; Meng, M.Q.H. Design and Control of a Magnetically-Actuated Capsule Robot With Biopsy Function. Ieee Trans. Biomed. Eng. 2022, 69, 2905–2915. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.S.; Hoang, M.C.; Jeong, S.; Kim, J.S.; Lee, C.; Kang, B.; Lee, J.; Son, Y.D.; Bang, S.; et al. Redundant Electromagnetic Control of an Endoscopic Magnetic Capsule Driven by Multiple Electromagnets Configuration. IEEE Trans. Ind. Electron. 2022, 69, 11370–11382. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Z.H.; Fu, Q.; Guo, S.X. Design and implementation of a novel wireless modular capsule robotic system in pipe. Med. Biol. Eng. Comput. 2020, 58, 2305–2324. [Google Scholar] [CrossRef]
- Chen, W.; Sui, J.; Wang, C. Magnetically Actuated Capsule Robots: A Review. IEEE Access 2022, 10, 88398–88420. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Bi, C.; Cappelleri, D.J.; Ciuti, G.; Conn, A.T.; Faivre, D.; Habibi, N.; Hosovsky, A.; Iacovacci, V.; Khalil, I.S.M.; et al. Magnetic Actuation Methods in Bio/Soft Robotics. Adv. Funct. Mater. 2021, 31, 2005137. [Google Scholar] [CrossRef]
- Yang, X.; Shang, W.F.; Lu, H.J.; Liu, Y.T.; Yang, L.; Tan, R.; Wu, X.Y.; Shen, Y.J. An agglutinate magnetic spray transforms inanimate objects into millirobots for biomedical applications. Sci. Robot. 2020, 5, eabc8191. [Google Scholar] [CrossRef]
- Sun, Z.-J.; Gu, W.; Xin, Y.; Lin, J. Preliminary study of a novel capsule robot with spring-connected legs. Adv. Mech. Eng. 2022, 14, 16878132221085126. [Google Scholar] [CrossRef]
- Zhen-Jun, S. Research on Locomotion of Capsule Endoscope Actuated by Force/Torque between Permanent Magnets. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2015. [Google Scholar]





| D/mm | T/N·mm | FC/N |
|---|---|---|
| 70 | 1.62 | 2.16 |
| 75 | 1.29 | 1.72 |
| 80 | 1.095 | 1.46 |
| 85 | 0.758 | 1.01 |
| Group Number * | Preparation Step | Intermediate Step | Last Step |
|---|---|---|---|
| I | Set CR in the initial status (legs are contracted), cover the CR with the intestine | Move the intestine back and forth to simulate the movement of the CR inside the intestine. | Remove the intestine carefully and expand the legs to observe and record the sampling situation of the springs. |
| II | Fully expand the legs and stand for 30 s (increase the standing time by 30 s each time). | ||
| III | Expand and then contract the legs. | ||
| IV | Expand the legs, rotate CR for a round, and contract the legs. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Sun, Z.-J.; Gu, W.; Yu, L. Experimental Research on a Capsule Robot with Spring-Connected Legs. Micromachines 2022, 13, 2042. https://doi.org/10.3390/mi13122042
Xin Y, Sun Z-J, Gu W, Yu L. Experimental Research on a Capsule Robot with Spring-Connected Legs. Micromachines. 2022; 13(12):2042. https://doi.org/10.3390/mi13122042
Chicago/Turabian StyleXin, Yesheng, Zhen-Jun Sun, Wenjin Gu, and Lei Yu. 2022. "Experimental Research on a Capsule Robot with Spring-Connected Legs" Micromachines 13, no. 12: 2042. https://doi.org/10.3390/mi13122042






