Terahertz Combined with Metamaterial Microfluidic Chip for Troponin Antigen Detection
Abstract
:1. Introduction
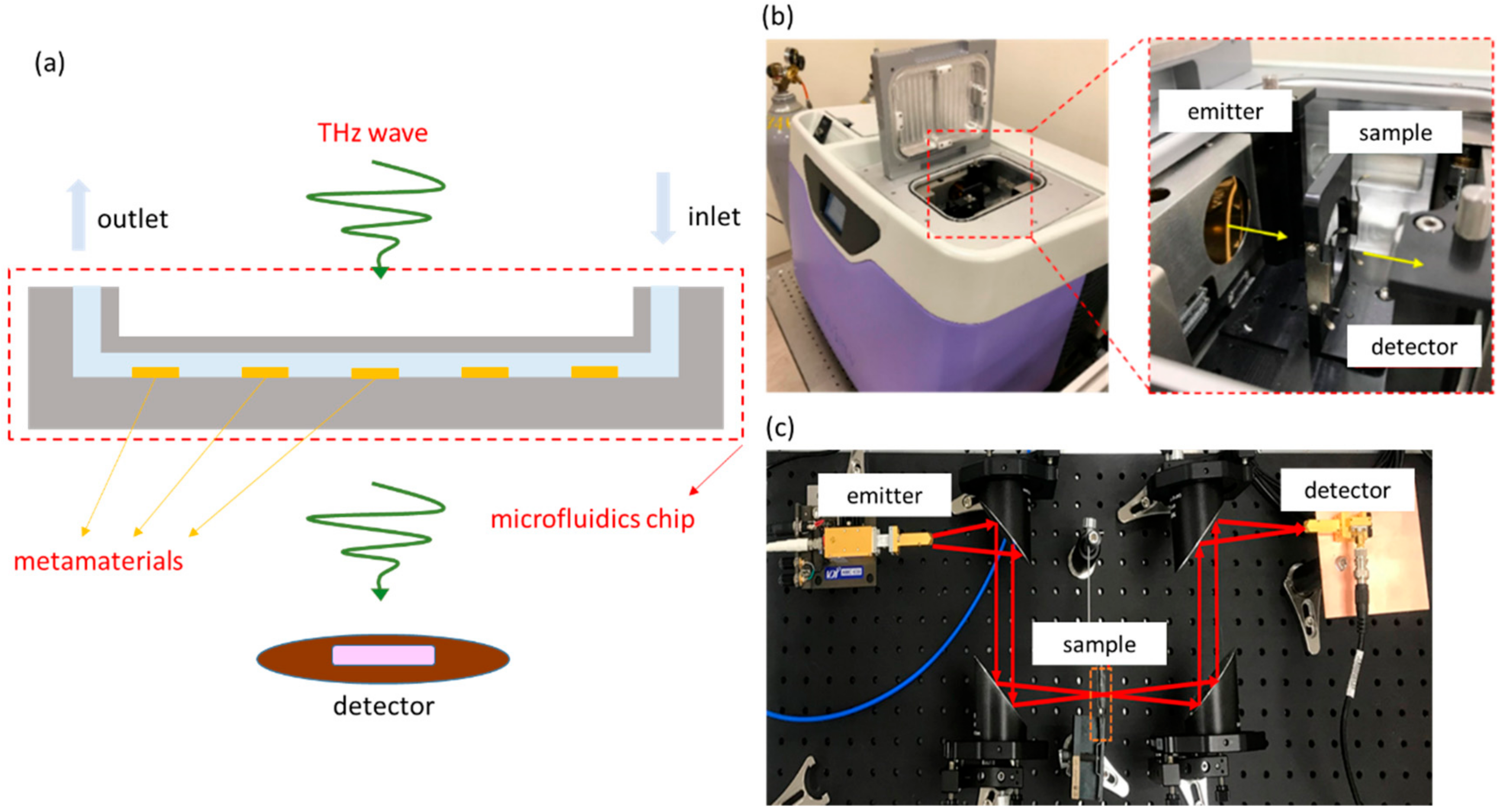
2. Microfluidic System, Metamaterials, and Experimental Setup
2.1. Microfluidic System and Metamaterial
2.2. Experimental Setup
3. Troponin Experiment
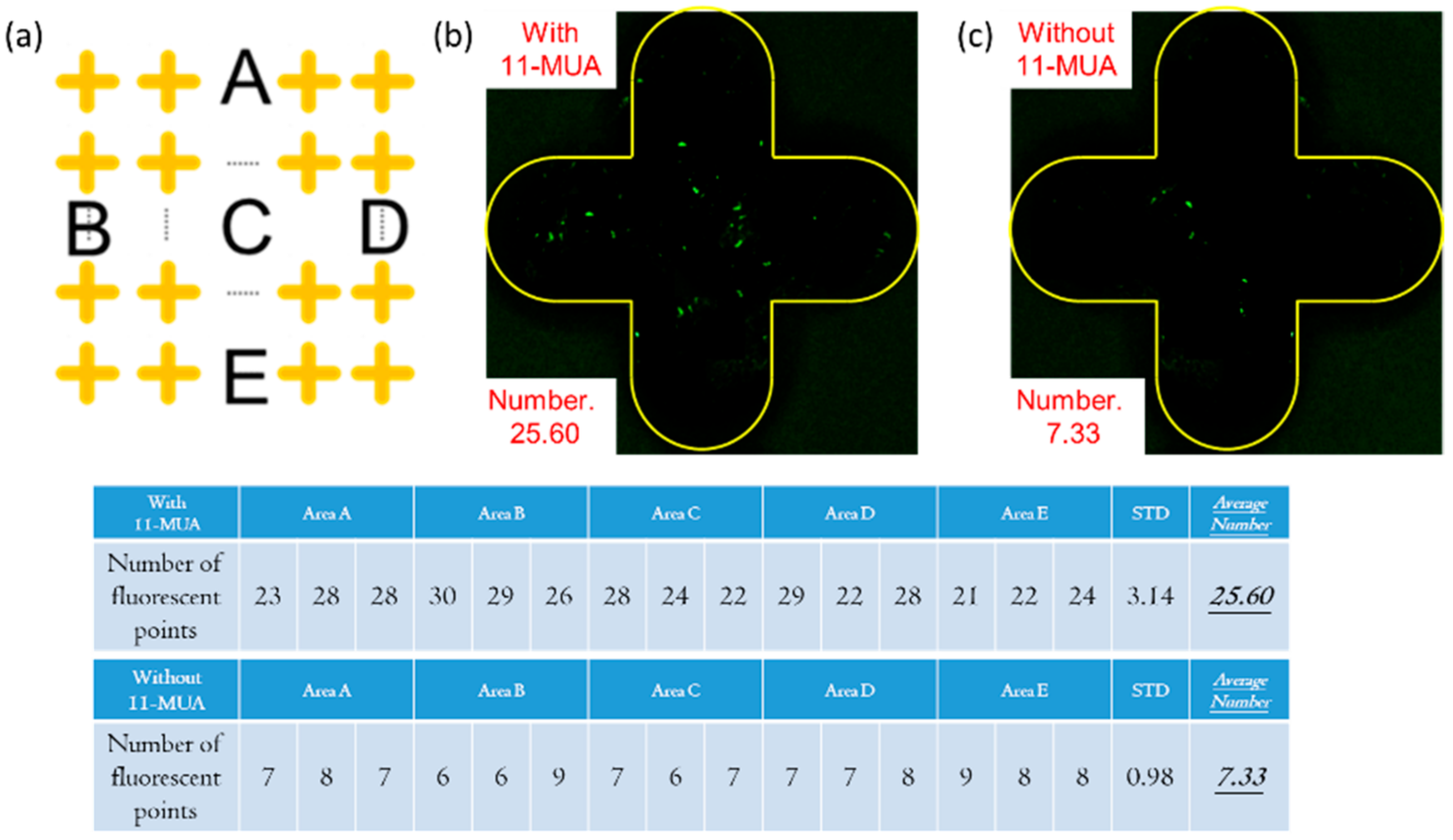
3.1. Silver Surface Modification Method
3.2. Surface Modification Process of Fluorescent Antibody Inspection
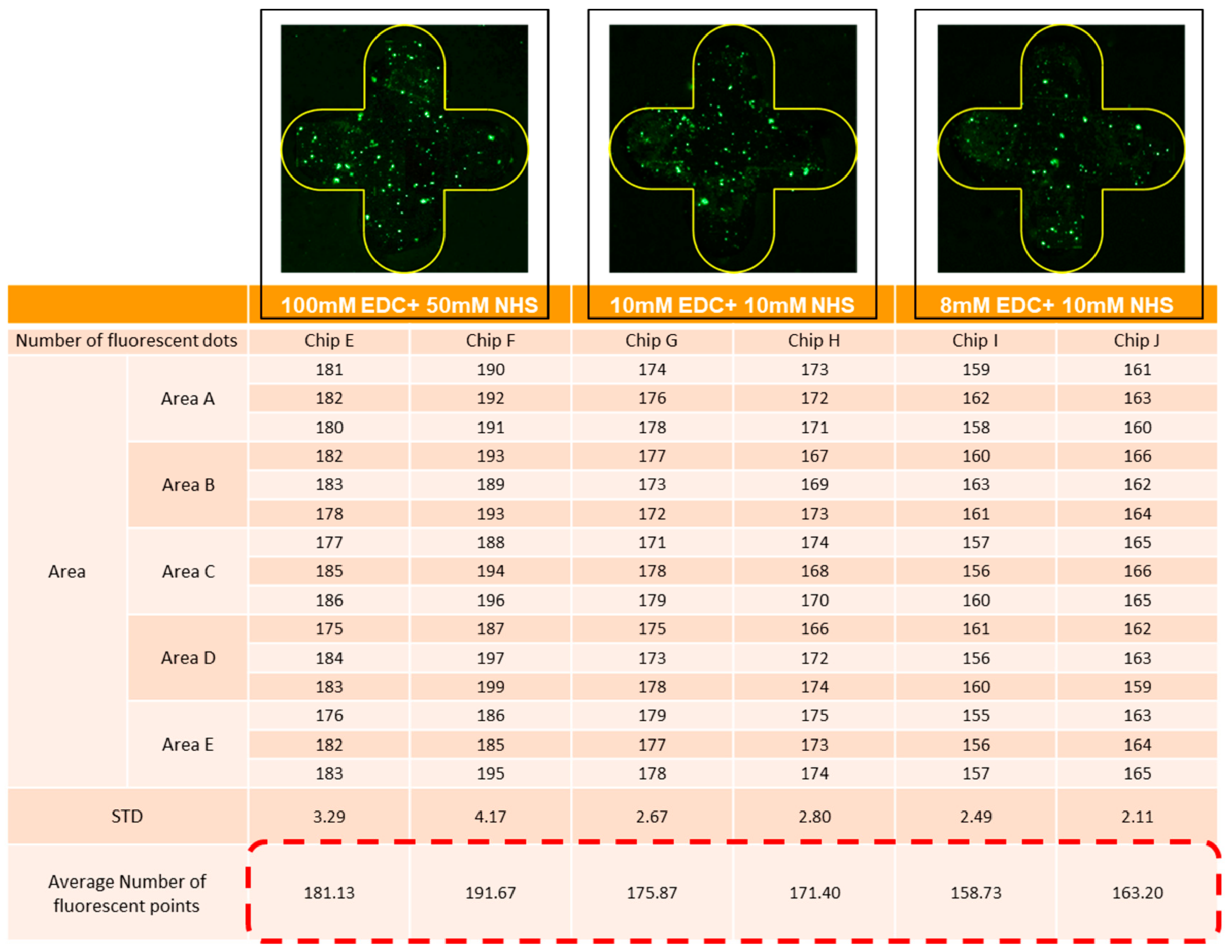
3.3. Experiment Parameter Optimization and Adjustment
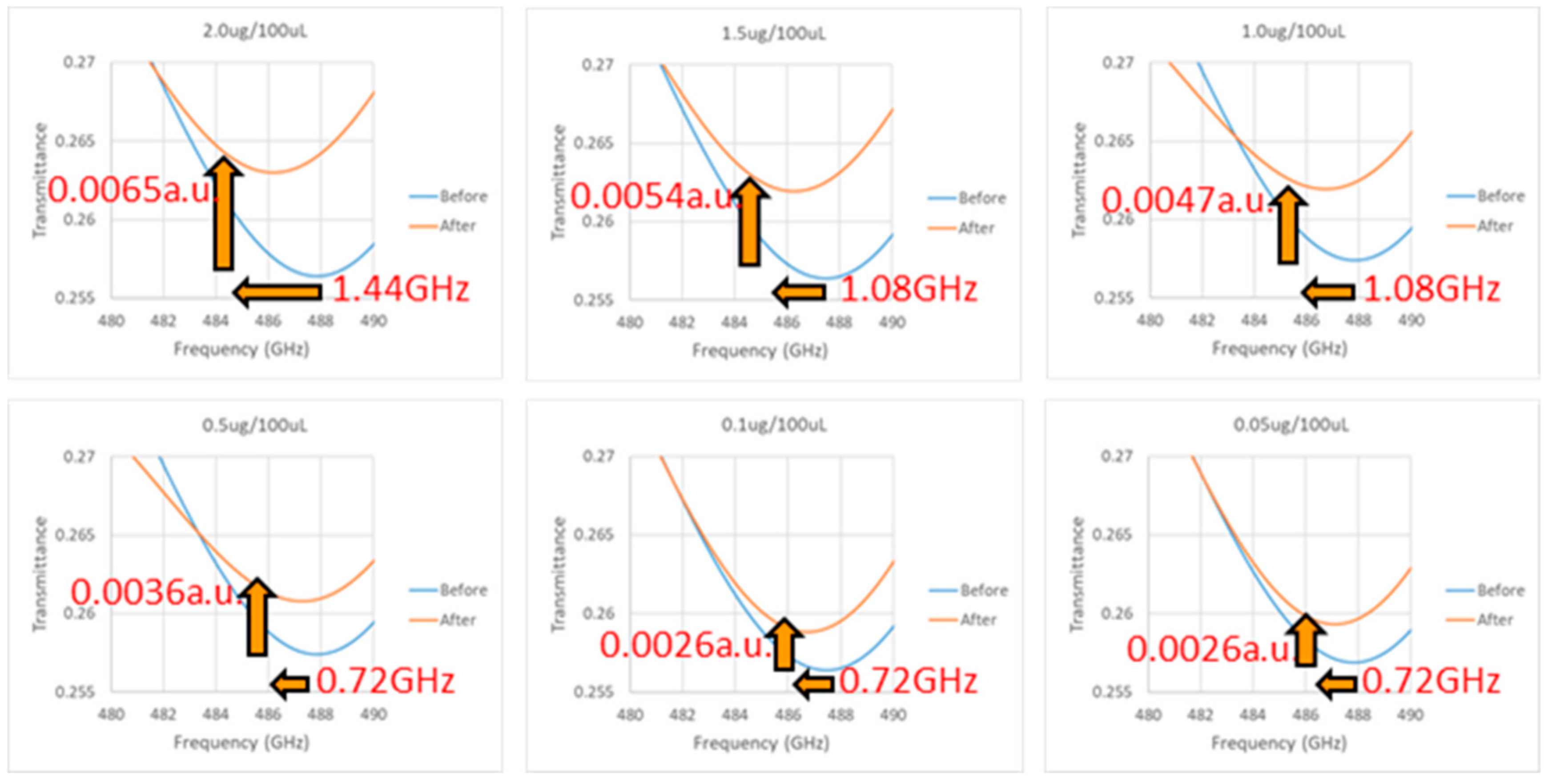
3.4. Experiment Results of Terahertz Detection of Troponin Antigen
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, A.; Zahid, A.; Fan, D.; Yang, X.; Imran, M.A.; Alomainy, A.; Abbasi, Q.H. State-of-the-art in terahertz sensing for food and water security–A comprehensive review. Trends Food Sci. Technol. 2019, 85, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Zeitler, J.A.; Kogermann, K.; Rantanen, J.; Rades, T.; Taday, P.F.; Pepper, M.; Aaltonen, J.; Strachan, C.J. Drug hydrate systems and dehydration processes studied by terahertz pulsed spectroscopy. Int. J. Pharm. 2007, 334, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical applications of terahertz spectroscopy and imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yeow, J.T.W. Nanotechnology-based terahertz biological sensing: A review of its current state and things to come. IEEE Nanotechnol. Mag. 2016, 10, 30–38. [Google Scholar] [CrossRef]
- Tonouchi, M. Cutting-edge terahertz technology. Nat. Photonics 2007, 1, 97–105. [Google Scholar] [CrossRef]
- Menikh, A.; MacColl, R.; Mannella, C.A.; Zhang, X.-C. Terahertz biosensing technology: Frontiers and progress. Chemphyschem 2002, 3, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Pitchappa, P.; Manjappa, M.; Ho, C.P.; Singh, R.; Singh, N.; Lee, C. Active control of electromagnetically induced transparency analog in terahertz MEMS metamaterial. Adv. Opt. Mater. 2016, 4, 541–547. [Google Scholar] [CrossRef]
- Rotman, W. Plasma simulation by artificial dielectrics and parallel-plate media. IRE Trans. Antennas Propag. 1962, 10, 82–95. [Google Scholar] [CrossRef]
- Park, S.; Son, B.; Choi, S.; Kim, H.; Ahn, Y.H. Sensitive detection of yeast using terahertz slot antennas. Opt. Express 2014, 22, 30467–30472. [Google Scholar] [CrossRef] [PubMed]
- Kotsifaki, D.G.; Truong, V.G.; Chormaic, S.N. Fano-resonant, asymmetric, metamaterial-assisted tweezers for single nanoparticle trapping. Nano Lett. 2020, 20, 3388–3395. [Google Scholar] [CrossRef] [PubMed]
- Makimura, K.; Murayama, S.Y.; Yamaguchi, H. Detection of a wide range of medically important fungi by the polymerase chain reaction. J. Med. Microbiol. 1994, 40, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.; van der Vusse, G.J.; Simoons, M.L.; Kragten, J.A.; van Dieijen-Visser, M.P.; Hermens, W.T. Fatty acid-binding protein and the early detection of acute myocardial infarction. Clin. Chim. Acta 1998, 272, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Ahn, K.J.; Han, S.; Bahk, Y.M.; Park, N.; Kim, D.S. Colossal absorption of molecules inside single terahertz nanoantennas. Nano Lett. 2013, 13, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Li, C.-T.; Chen, H.-F.; Yen, T.-J. Demonstration of an ultrasensitive refractive-index plasmonic sensor by enabling its quadrupole resonance in phase interrogation. Opt. Lett. 2015, 40, 5152–5155. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Singh, R. Sensing with THz metamaterial absorbers. arXiv 2014, arXiv:1408.3711. [Google Scholar]
- Bertin, E.P. Qualitative and Semiquantitative Analysis. In Introduction to X-ray Spectrometric Analysis; Springer: Berlin/Heidelberg, Germany, 1978; pp. 255–278. [Google Scholar]
- Karabchevsky, A.; Tsapovsky, L.; Marks, R.S.; Abdulhalim, I. Study of immobilization procedure on silver nanolayers and detection of estrone with diverged beam surface plasmon resonance (SPR) imaging. Biosensors 2013, 3, 157–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, D.; Kaur, I.; Kumar, A. Ultrasensitive cardiac troponin I antibody based nanohybrid sensor for rapid detection of human heart attack. Int. J. Biol. Macromol. 2017, 95, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Shoushtari, A.M.; Rabiee, M.; Uzun, L.; Mak, W.C.; Turner, A.P.F. An electrochemical immunosensor for cardiac Troponin I using electrospun carboxylated multi-walled carbon nanotube-whiskered nanofibres. Talanta 2018, 182, 178–186. [Google Scholar] [CrossRef] [PubMed]






| ∆Y(a.u.) | Antigen Concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2.0 mg/100 mL | 1.5 mg/100 mL | 1.0 mg/100 mL | ||||||||
| After Modification | 3 Chips (Each) | 0.0039 | 0.0038 | 0.0040 | 0.0041 | 0.0040 | 0.0040 | 0.0040 | 0.0038 | 0.0038 |
| Average | 0.0039 | 0.0040 | 0.0039 | |||||||
| After Antibody | 3 Chips (Each) | 0.0030 | 0.0029 | 0.0031 | 0.0030 | 0.0030 | 0.0030 | 0.0031 | 0.0028 | 0.0028 |
| Average | 0.0030 | 0.0030 | 0.0029 | |||||||
| After Antigen (Corresponding) | 3 Chips (Each) | 0.0023 | 0.0022 | 0.0023 | 0.0018 | 0.0017 | 0.0016 | 0.0012 | 0.0013 | 0.0011 |
| Average | 0.0023 | 0.0017 | 0.0012 | |||||||
| ∆Y(a.u.) | Antigen Concentration | |||||||||
| 0.5 mg/100 mL | 0.1 mg/100 mL | 0.05 mg/100 mL | ||||||||
| After Modification | 3 Chips (Each) | 0.0039 | 0.0037 | 0.0040 | 0.0039 | 0.0038 | 0.0039 | 0.0039 | 0.0041 | 0.0040 |
| Average | 0.0039 | 0.0039 | 0.0040 | |||||||
| After Antibody | 3 Chips (Each) | 0.0030 | 0.0029 | 0.0031 | 0.0028 | 0.0028 | 0.0031 | 0.0029 | 0.0030 | 0.0031 |
| Average | 0.0030 | 0.0029 | 0.0030 | |||||||
| After Antigen (Corresponding) | 3 Chips (Each) | 0.0007 | 0.0006 | 0.0006 | 0.0002 | 0.0002 | 0.0003 | 0.0000 | 0.0001 | 0.0000 |
| Average | 0.0006 | 0.0002 | 0.0000 | |||||||
| Initial Position | After Binding | Change | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X Axis (GHz) | Y Axis (a.u.) | X Axis (GHz) | Y Axis (a.u.) | ∆X (GHz) | ∆Y (a.u.) | ∆X (GHz) | ∆Y (a.u.) | ||
| Resonant Position | Transmittance | Resonant Position | Transmittance | ||||||
| Concentration 2.0 μg/100 μL | Chip 1 | 487.84 | 0.2561 | 486.40 | 0.2628 | 1.44 | 0.0067 | 1.44 | 0.0065 |
| Chip 2 | 487.84 | 0.2573 | 486.40 | 0.2635 | 1.44 | 0.0062 | |||
| Chip 3 | 487.84 | 0.2564 | 486.40 | 0.2629 | 1.44 | 0.0065 | |||
| Concentration 1.5 μg/100 μL | Chip 1 | 487.48 | 0.2565 | 486.40 | 0.2618 | 1.08 | 0.0053 | 1.08 | 0.0054 |
| Chip 2 | 487.84 | 0.2560 | 486.76 | 0.2618 | 1.08 | 0.0058 | |||
| Chip 3 | 487.48 | 0.2567 | 486.40 | 0.2618 | 1.08 | 0.0051 | |||
| Concentration 1.0 μg/100 μL | Chip 1 | 487.84 | 0.2575 | 486.76 | 0.2619 | 1.08 | 0.0044 | 1.08 | 0.0047 |
| Chip 2 | 487.48 | 0.2578 | 486.40 | 0.2625 | 1.08 | 0.0047 | |||
| Chip 3 | 487.84 | 0.2571 | 486.76 | 0.2621 | 1.08 | 0.0050 | |||
| Concentration 0.5 μg/100 μL | Chip 1 | 487.84 | 0.2573 | 487.12 | 0.2609 | 0.72 | 0.0036 | 0.72 | 0.0036 |
| Chip 2 | 487.12 | 0.2559 | 486.40 | 0.2593 | 0.72 | 0.0034 | |||
| Chip 3 | 487.48 | 0.2563 | 486.76 | 0.2602 | 0.72 | 0.0039 | |||
| Concentration 0.1 μg/100 μL | Chip 1 | 487.48 | 0.2563 | 486.76 | 0.2587 | 0.72 | 0.0024 | 0.72 | 0.0026 |
| Chip 2 | 487.12 | 0.2558 | 486.40 | 0.2585 | 0.72 | 0.0027 | |||
| Chip 3 | 487.48 | 0.2572 | 486.76 | 0.2600 | 0.72 | 0.0028 | |||
| Concentration 0.05 μg/100 μL | Chip 1 | 487.84 | 0.2573 | 487.12 | 0.2597 | 0.72 | 0.0024 | 0.72 | 0.0026 |
| Chip 2 | 487.48 | 0.2566 | 486.76 | 0.2593 | 0.72 | 0.0027 | |||
| Chip 3 | 487.84 | 0.2558 | 487.12 | 0.2584 | 0.72 | 0.0026 | |||
| Concentration 0.0 μg/100 μL | Chip 1 | 487.48 | 0.2561 | 486.76 | 0.2588 | 0.72 | 0.0027 | 0.72 | 0.0026 |
| Chip 2 | 487.84 | 0.2571 | 487.12 | 0.2598 | 0.72 | 0.0027 | |||
| Chip 3 | 487.12 | 0.2569 | 486.40 | 0.2592 | 0.72 | 0.0023 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-S.; Huang, S.-T.; Hsu, S.-F.; Tang, K.-Y.; Yen, T.-J.; Yao, D.-J. Terahertz Combined with Metamaterial Microfluidic Chip for Troponin Antigen Detection. Micromachines 2022, 13, 2257. https://doi.org/10.3390/mi13122257
Lin Y-S, Huang S-T, Hsu S-F, Tang K-Y, Yen T-J, Yao D-J. Terahertz Combined with Metamaterial Microfluidic Chip for Troponin Antigen Detection. Micromachines. 2022; 13(12):2257. https://doi.org/10.3390/mi13122257
Chicago/Turabian StyleLin, Yen-Shuo, Shih-Ting Huang, Shen-Fu Hsu, Kai-Yuan Tang, Ta-Jen Yen, and Da-Jeng Yao. 2022. "Terahertz Combined with Metamaterial Microfluidic Chip for Troponin Antigen Detection" Micromachines 13, no. 12: 2257. https://doi.org/10.3390/mi13122257





