Exploring Copper Oxide and Copper Sulfide for Non-Enzymatic Glucose Sensors: Current Progress and Future Directions
Abstract
:1. Introduction
2. Enzymatic to Non-Enzymatic Glucose Sensors
3. Copper Oxide Nanomaterials in Glucose Detection
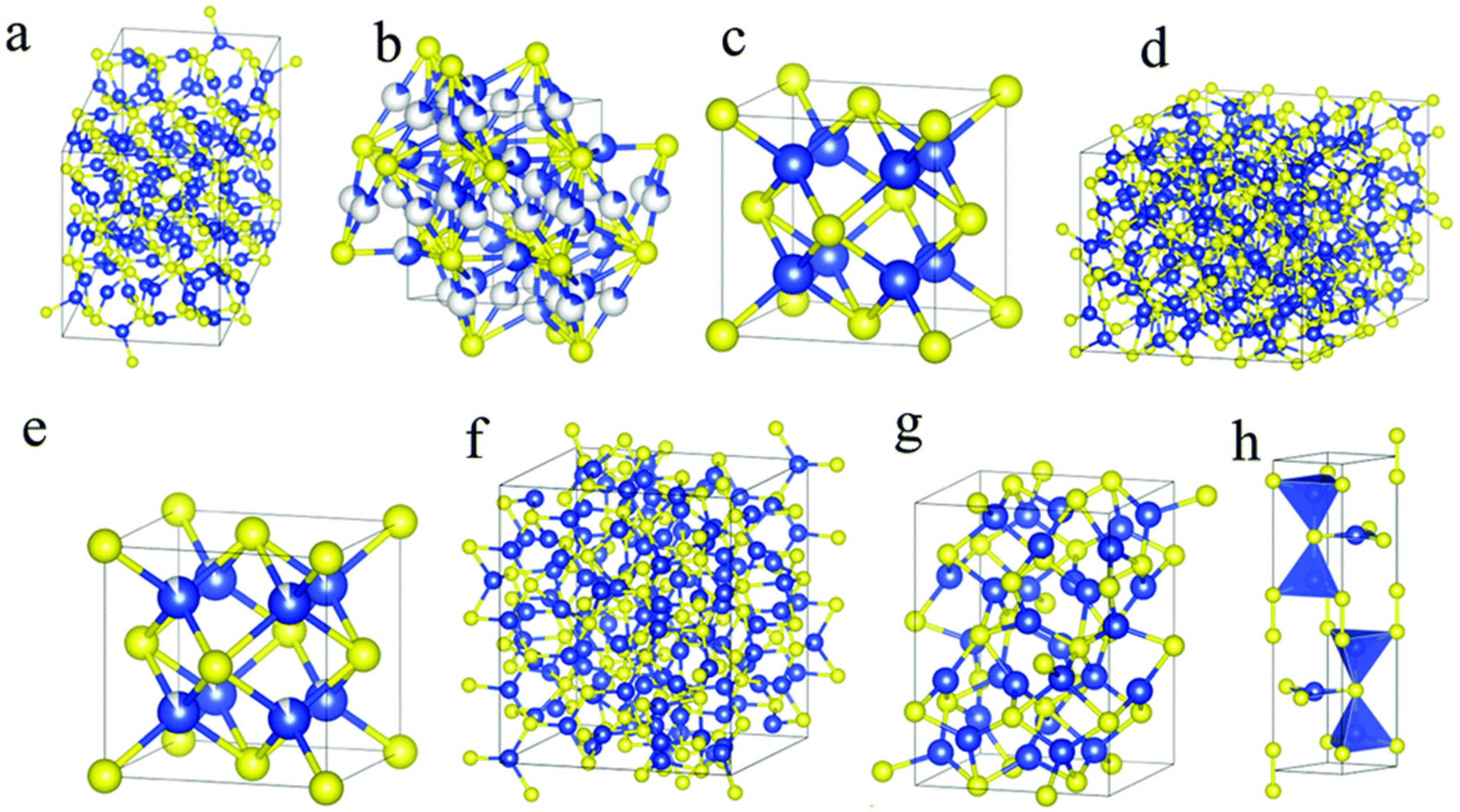
3.1. CuO Properties
- (a)
- (b)
- (c)
3.2. Preparation of CuO and CuO Composites
3.3. CuO and CuO Composites in Non-Enzymatic Glucose Sensors
3.3.1. CuO/C
3.3.2. CuO/Metal Oxides
3.3.3. CuO/Metals
3.3.4. CuO/Polymeric Nanocomposites
4. Copper Sulfide Nanomaterials in Glucose Detection
4.1. CuxSy Properties
4.2. Preparation of CuxSy and CuxSy Composites
4.3. CuxSy and CuxSy Composites in Glucose Sensors
4.3.1. CuS/C Nanohybrids
4.3.2. Metal and Non-Metal Doped CuS Nanomaterials
4.3.3. CuS-Based Mixed Metal Oxides
5. Comparison of Different Substrates for Use as Electrodes
6. Limitations of Copper Oxides and Sulfides in Glucose Detection
7. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 20 September 2022).
- Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 10 July 2022).
- Nichols, S.P.; Koh, A.; Storm, W.L.; Shin, J.H.; Schoenfisch, M.H. Biocompatible Materials for Continuous Glucose Monitoring Devices. Chem. Rev. 2013, 113, 2528–2549. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [PubMed]
- Mazurków, J.; Kusior, A.; Radecka, M. Nonenzymatic Glucose Sensors Based on Copper Sulfides: Effect of Binder-Particles Interactions in Drop-Casted Suspensions on Electrodes Electrochemical Performance. Sensors 2021, 21, 802. [Google Scholar] [CrossRef] [PubMed]
- CardioSecur Diabetes Mellitus—What You Need to Know. Available online: https://www.cardiosecur.com/magazine/specialist-articles-on-the-heart/diabetes-mellitus-nutrition-and-its-influence-on-the-heart (accessed on 20 July 2023).
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R.; Wilkins, E. Effect of Interference on Amperometric Glucose Biosensors with Cellulose Acetate Membranes. Electroanalysis 1994, 6, 677–682. [Google Scholar] [CrossRef]
- Kim, W.B.; Lee, S.H.; Cho, M.; Lee, Y. Facile and Cost-Effective CuS Dendrite Electrode for Non-Enzymatic Glucose Sensor. Sensors Actuators, B Chem. 2017, 249, 161–167. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Jeong, R.A.; Boo, H.; Park, J.; Kim, H.C.; Chung, T.D. Nonenzymatic Continuous Glucose Monitoring in Human Whole Blood Using Electrified Nanoporous Pt. Biosens. Bioelectron. 2012, 31, 284–291. [Google Scholar] [CrossRef]
- Renneberg, R.; Pfeiffer, D.; Lisdat, F.; Wilson, G.; Wollenberger, U.; Ligler, F.; Turner, A.P.F. Frieder Scheller and the Short History of Biosensors. Adv. Biochem. Eng. Biotechnol. 2007, 109, 1–18. [Google Scholar] [CrossRef]
- Alves, C.F.; Colombini, E.L.; Ribeiro, C.H.C. Sparse Sampling Action Values Initialized by a Compact Representation Technique. In Proceedings of the Seventh International Conference on Intelligent Systems Design and Applications (ISDA 2007), Rio de Janeiro, Brazil, 20–24 October 2007; pp. 729–734. [Google Scholar] [CrossRef]
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Tangkuaram, T.; Mercier, P.P.; Dassau, E.; Wang, J. Microneedle-Based Detection of Ketone Bodies along with Glucose and Lactate: Toward Real-Time Continuous Interstitial Fluid Monitoring of Diabetic Ketosis and Ketoacidosis. Anal. Chem. 2020, 92, 2291–2300. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef]
- Wang, G.; He, X.; Wang, L.; Gu, A.; Huang, Y.; Fang, B.; Geng, B.; Zhang, X. Non-Enzymatic Electrochemical Sensing of Glucose. Microchim. Acta 2013, 180, 161–186. [Google Scholar] [CrossRef]
- Gao, X.; Du, X.; Liu, D.; Gao, H.; Wang, P.; Yang, J. Core-Shell Gold-Nickel Nanostructures as Highly Selective and Stable Nonenzymatic Glucose Sensor for Fermentation Process. Sci. Rep. 2020, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, G.A.; Salim, H.; Hassan, I.U.; Awan, T.; Arshad, F.; Pedram, M.Z.; Ahmed, W.; Qurashi, A. Recent Advances in Non-Enzymatic Glucose Sensors Based on Metal and Metal Oxide Nanostructures for Diabetes Management—A Review. Front. Chem. 2021, 9, 748957. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Karuppiah, P.; Al-dhabi, N.A. Synthesis, Characterization, and Antimicrobial Activity of Copper Oxide Nanoparticles. J. Nanomater. 2014, 2014, 637858. [Google Scholar] [CrossRef]
- Busi, S.; Rajkumari, J. Chapter 15. Microbially Synthesized Nanoparticles as Next Generation Antimicrobials: Scope and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128165041. [Google Scholar]
- Keabadile, O.P.; Aremu, A.O.; Elugoke, S.E. Green and Traditional Synthesis of Copper Oxide Nanoparticles—Comparative Study. Nanomaterials 2020, 10, 2502. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Venkatachalam, K.; Wang, M.-H. Folic Acid Functionalized Starch Encapsulated Green Synthesized Copper Oxide Nanoparticles for Targeted Drug Delivery in Breast Cancer Therapy. Int. J. Biol. Macromol. 2020, 164, 2073–2084. [Google Scholar] [CrossRef]
- Chakraborty, S.; Prakash, P.; Shah, J.; Mayya, C.; Singh, S.; Ranganathan, R.; Soppina, V.; Jones, E.V.; Misra, S.K. CuO Nanoparticles as Copper-Ion Reservoirs for Elesclomol-Mediated Intracellular Oxidative Stress: Implications for Anticancer Therapies. ACS Appl. Nano Mater. 2022, 5, 1607–1620. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Fouda, A.; Salem, S.S. Integration of Cotton Fabrics with Biosynthesized CuO Nanoparticles for Bactericidal Activity in the Terms of Their Cytotoxicity Assessment. Ind. Eng. Chem. Res. 2021, 60, 1553–1563. [Google Scholar] [CrossRef]
- Sibhatu, A.K.; Weldegebrieal, G.K.; Sagadevan, S.; Tran, N.N.; Hessel, V. Photocatalytic Activity of CuO Nanoparticles for Organic and Inorganic Pollutants Removal in Wastewater Remediation. Chemosphere 2022, 300, 134623. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, V.; Amiard, G.; Boninelli, S.; Scalese, S.; Miritello, M.; Sberna, P.M.; Impellizzeri, G.; Privitera, V. Photocatalytic Activity of CuO and Cu2O Nanowires. Mater. Sci. Semicond. Process. 2016, 42, 89–93. [Google Scholar] [CrossRef]
- Huang, C.; Tian, X.; Liu, J.; Dong, Z.; Wang, Y. The Assembly and Fabrication of Single CuO Nanowire Electronic Device Based on Controllable DWS-DEP Technology. IEEE Trans. Nanotechnol. 2015, 14, 101–107. [Google Scholar] [CrossRef]
- Rager, M.S.; Aytug, T.; Veith, G.M.; Joshi, P. Low-Thermal-Budget Photonic Processing of Highly Conductive Cu Interconnects Based on CuO Nanoinks: Potential for Flexible Printed Electronics. ACS Appl. Mater. Interfaces 2016, 8, 2441–2448. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Selective Detection of CO at Room Temperature with CuO Nanoplatelets Sensor for Indoor Air Quality Monitoring Manifested by Crystallinity. Appl. Surf. Sci. 2019, 466, 545–553. [Google Scholar] [CrossRef]
- Avinash, B.; Ravikumar, C.R.; Kumar, M.R.A.; Nagaswarupa, H.P.; Santosh, M.S.; Bhatt, A.S.; Kuznetsov, D. Nano CuO: Electrochemical Sensor for the Determination of Paracetamol and d-Glucose. J. Phys. Chem. Solids 2019, 134, 193–200. [Google Scholar] [CrossRef]
- Reddy, S.; Kumara Swamy, B.E.; Jayadevappa, H. CuO Nanoparticle Sensor for the Electrochemical Determination of Dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Sagadevan, S.; Pal, K.; Chowdhury, Z.Z. Fabrication of CuO Nanoparticles for Structural, Optical and Dielectric Analysis Using Chemical Precipitation Method. J. Mater. Sci. Mater. Electron. 2017, 28, 12591–12597. [Google Scholar] [CrossRef]
- Thampi, V.V.A.; Thanka Rajan, S.; Anupriya, K.; Subramanian, B. Functionalization of Fabrics with PANI/CuO Nanoparticles by Precipitation Route for Anti-Bacterial Applications. J. Nanoparticle Res. 2015, 17, 57. [Google Scholar] [CrossRef]
- Nahar, B.; Chaity, S.B.; Gafur, M.A.; Hossain, M.Z. Synthesis of Spherical Copper Oxide Nanoparticles by Chemical Precipitation Method and Investigation of Their Photocatalytic and Antibacterial Activities. J. Nanomater. 2023, 2023, 2892081. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Yuan, Z.; Sun, Y.; Chang, F.; Deng, H.; Xie, L.; Li, H. A Highly Sensitive Gas Sensor Based on CuO Nanoparticles Synthetized via a Sol–Gel Method. RSC Adv. 2016, 6, 79343–79349. [Google Scholar] [CrossRef]
- Masood, A.; Iqbal, T.; Afsheen, S.; Riaz, K.N.; Nabi, G.; Khan, M.I.; Al-Zaqri, N.; Warad, I.; Ahmed, H. Theoretical and Experimental Analysis of La-Doped CuO for Their Application an Efficient Photocatalyst. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Sivayogam, D.; Kartharinal Punithavathy, I.; Johnson Jayakumar, S.; Mahendran, N. Study on Structural, Electro-Optical and Optoelectronics Properties of CuO Nanoparticles Synthesis via Sol Gel Method. Mater. Today Proc. 2022, 48, 508–513. [Google Scholar] [CrossRef]
- Sankar, R.; Manikandan, P.; Malarvizhi, V.; Fathima, T.; Shivashangari, K.S.; Ravikumar, V. Green Synthesis of Colloidal Copper Oxide Nanoparticles Using Carica Papaya and Its Application in Photocatalytic Dye Degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Kumar, A.; Tarlochan, F. Highly Efficient Nonenzymatic Glucose Sensors Based on CuO Nanoparticles. Appl. Surf. Sci. 2019, 481, 712–722. [Google Scholar] [CrossRef]
- Silva, N.; Ramírez, S.; Díaz, I.; Garcia, A.; Hassan, N. Easy, Quick, and Reproducible Sonochemical Synthesis of CuO Nanoparticles. Materials 2019, 12, 804. [Google Scholar] [CrossRef]
- Boran, F. Encapsulation of CuO Nanoparticles inside the Channels of the Multi-Walled Carbon Nanotubes Functionalized with Thermal Stress. Diam. Relat. Mater. 2021, 114, 108306. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Madhusudana Reddy, T.; Palakollu, V.N.; Karpoormath, R.; Subba Rao, Y.; Venkataprasad, G.; Gopal, T.V.; Gopal, P. Multi Walled Carbon Nanotubes Supported CuO-Au Hybrid Nanocomposite for the Effective Application towards the Electrochemical Determination of Acetaminophen and 4-Aminophenol. Synth. Met. 2019, 252, 29–39. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, G.; Yan, L.; Kong, L.; Zheng, H.; Mi, J.; Li, Z. Carbon Nanotube-Supported Cu-Based Catalysts for Oxidative Carbonylation of Methanol to Methyl Carbonate: Effect of Nanotube Pore Size. Catal. Sci. Technol. 2020, 10, 2615–2626. [Google Scholar] [CrossRef]
- Khater, D.Z.; Amin, R.S.; Mahmoud, M.; El-Khatib, K.M. Evaluation of Mixed Transition Metal (Co, Mn, and Cu) Oxide Electrocatalysts Anchored on Different Carbon Supports for Robust Oxygen Reduction Reaction in Neutral Media. RSC Adv. 2022, 12, 2207–2218. [Google Scholar] [CrossRef]
- Li, Z.; Yadav, R.M.; Sun, L.; Zhang, T.; Zhang, J.; Ajayan, P.M.; Wu, J. CuO/ZnO/C Electrocatalysts for CO2-to-C2+ Products Conversion with High Yield: On the Effect of Geometric Structure and Composition. Appl. Catal. A Gen. 2020, 606, 117829. [Google Scholar] [CrossRef]
- Li, Y.; Liang, G.; Wang, C.; Fang, Y.; Duan, H. Effect of Precipitated Precursor on the Catalytic Performance of Mesoporous Carbon Supported CuO-ZnO Catalysts. Crystals 2021, 11, 582. [Google Scholar] [CrossRef]
- Geetha, M.; Maurya, M.R.; Al-maadeed, S.; Muthalif, A.A.; Sadasivuni, K.K. High-Precision Nonenzymatic Electrochemical Glucose Sensing Based on CNTs/CuO Nanocomposite. J. Electron. Mater. 2022, 51, 4905–4917. [Google Scholar] [CrossRef]
- Cuara, E.; Sierra, U.; Mercado, A.; Barriga-Castro, E.D.; Cortés, A.; Gallardo-Vega, C.; Valle-Orta, M.; Fernández, S. Synthesis of Copper Oxides-Graphene Composites for Glucose Sensing. Carbon Trends 2021, 4, 100050. [Google Scholar] [CrossRef]
- Cai, B.; Zhou, Y.; Zhao, M.; Cai, H.; Ye, Z.; Wang, L.; Huang, J. Synthesis of ZnO–CuO Porous Core–Shell Spheres and Their Application for Non-Enzymatic Glucose Sensor. Appl. Phys. A 2015, 118, 989–996. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, H.-M.; Guo, L.; Zhang, L.; Zhao, H.-B.; Fang, X.; Li, S.; Wang, G. Facile Synthesis of CuO–Co3O4 Prickly-Sphere-like Composite for Non-Enzymatic Glucose Sensors. Rare Met. 2022, 41, 1911–1920. [Google Scholar] [CrossRef]
- Myung, N.; Kim, S.; Lee, C.; Kim, T.; Rajeshwar, K. Facile Synthesis of Pt-CuO Nanocomposite Films for Non-Enzymatic Glucose Sensor Application. J. Electrochem. Soc. 2016, 163, B180. [Google Scholar] [CrossRef]
- Viswanathan, P.; Wang, K.; Li, J.; Hong, J.-D. Multicore–Shell Ag–CuO Networked with CuO Nanorods for Enhanced Non-Enzymatic Glucose Detection. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 598, 124816. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dhar, S.; Debnath, K.; Majumder, T.; Mondal, S.P. Non-Enzymatic and Non-Invasive Glucose Detection Using Au Nanoparticle Decorated CuO Nanorods. Sens. Actuators B Chem. 2019, 283, 776–785. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, C.; Meng, X.; Chen, Z.; Li, Y.; Zhang, D.; Zhu, S. Design and Fabrication of Ag-CuO Nanoparticles on Reduced Graphene Oxide for Nonenzymatic Detection of Glucose. Sens. Actuators B Chem. 2018, 265, 435–442. [Google Scholar] [CrossRef]
- Felix, S.; Kollu, P.; Grace, A.N. Electrochemical Performance of Ag–CuO Nanocomposites towards Glucose Sensing. Mater. Res. Innov. 2019, 23, 27–32. [Google Scholar] [CrossRef]
- Dayakar, T.; Venkateswara Rao, K.; Park, J.; Krishna, P.; Swaroopa, P.; Ji, Y. Biosynthesis of Ag@CuO Core–Shell Nanostructures for Non-Enzymatic Glucose Sensing Using Screen-Printed Electrode. J. Mater. Sci. Mater. Electron. 2019, 30, 9725–9734. [Google Scholar] [CrossRef]
- Xu, J.; Tang, M.; Liu, S.; Zhou, J.; Sheng, W.; Zhou, T.; Wu, J.; Song, K.; Wang, X.; Cheng, J.P. Ag-Doped CuO Microflowers on Multilayer Graphene for a Highly Sensitive Non-Enzymatic Glucose Sensor. J. Electron. Mater. 2022, 51, 995–1003. [Google Scholar] [CrossRef]
- Sreekumar, A.; Navaneeth, P.; Suneesh, P.V.; Nair, B.G.; Babu, T.G.S. A Graphite Pencil Electrode with Electrodeposited Pt-CuO for Nonenzymatic Amperometric Sensing of Glucose over a Wide Linear Response Range. Microchim. Acta 2020, 187, 113. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mukherjee, B.; Kumar, A.; Jarwal, D.K.; Ratan, S.; Kumar, C.; Jit, S. Superficial Fabrication of Gold Nanoparticles Modified CuO Nanowires Electrode for Non-Enzymatic Glucose Detection. RSC Adv. 2019, 9, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Siampour, H.; Abbasian, S.; Moshaii, A.; Amirsoleimani, A.R. Stable, Reproducible, and Binder-Free Gold/Copper Core–Shell Nanostructures for High-Sensitive Non-Enzymatic Glucose Detection. Sci. Rep. 2022, 12, 18945. [Google Scholar] [CrossRef]
- Yang, B.; Yu, Y.; Qiao, J.; Yuan, L.; Hu, X. Solution Plasma Method Direct Synthesis of Au/CuO Nanoparticles for Glucose Enzyme-Free Detection. J. Mater. Sci. Mater. Electron. 2020, 31, 12983–12990. [Google Scholar] [CrossRef]
- Esmaeeli, A.; Ghaffarinejad, A.; Zahedi, A.; Vahidi, O. Copper Oxide-Polyaniline Nanofiber Modified Fluorine Doped Tin Oxide (FTO) Electrode as Non-Enzymatic Glucose Sensor. Sens. Actuators B Chem. 2018, 266, 294–301. [Google Scholar] [CrossRef]
- Ghanbari, K.; Babaei, Z. Fabrication and Characterization of Non-Enzymatic Glucose Sensor Based on Ternary NiO/CuO/Polyaniline Nanocomposite. Anal. Biochem. 2016, 498, 37–46. [Google Scholar] [CrossRef]
- Fang, L.; Zhu, Q.; Cai, Y.; Liang, B.; Ye, X. 3D Porous Structured Polyaniline/Reduced Graphene Oxide/Copper Oxide Decorated Electrode for High Performance Nonenzymatic Glucose Detection. J. Electroanal. Chem. 2019, 841, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gu, A.; Wei, Y.; Fang, B. CuS Nanotubes for Ultrasensitive Nonenzymatic Glucose Sensors. Chem. Commun. 2008, 45, 5945–5947. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Cai, W. Synthesis and Biomedical Applications of Copper Sulfide Nanoparticles: From Sensors to Theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Zi, J.; Li, W. Enzyme-Free Sensing of Hydrogen Peroxide and Glucose at a CuS Nanoflowers Modified Glassy Carbon Electrode. Electrochim. Acta 2014, 115, 126–130. [Google Scholar] [CrossRef]
- Rawat, P.; Nigam, A.; Kala, S. Green Synthesis of Copper and Copper Sulfide Nanoparticles. In Proceedings of the AIP Conference Proceedings, Bikaner, India, 14–15 October 2019; Volume 2220. [Google Scholar]
- Yadav, S.; Bajpai, P.K. Synthesis of Copper Sulfide Nanoparticles: PH Dependent Phase Stabilization. Nano-Struct. Nano-Objects 2017, 10, 151–158. [Google Scholar] [CrossRef]
- Goble, R.J. Relationship Between Crystal Structure, Bonding and Cell Dimensions in the Copper Sulfides. Can. Mineral. 1985, 23, 61–76. [Google Scholar]
- Kolny-olesiak, J. Synthesis of Copper Sulphide-Based Hybrid Nanostructures and Their Application in Shape Control of Colloidal Semiconductor Nanocrystals. CrystEngComm 2014, 16, 9381–9390. [Google Scholar] [CrossRef]
- Abdullaeva, Z.; Omurzak, E.; Mashimo, T. Synthesis of Copper Sulfide Nanoparticles by Pulsed Plasma in Liquid Method. World Acad. Sci. Eng. Technol. 2013, 7, 1056–1059. [Google Scholar]
- Chen, L.; Hu, H.; Chen, Y.; Gao, J.; Li, G. Plasmonic Cu2-XS Nanoparticles: A Brief Introduction of Optical Properties and Applications. Mater. Adv. 2021, 2, 907–926. [Google Scholar] [CrossRef]
- Sun, S.; Song, X.; Kong, C.; Deng, D.; Yang, Z. Copper Sulfide Cages Wholly Exposed with Nanotwinned Building Blocks. CrystEngComm 2012, 14, 67–70. [Google Scholar] [CrossRef]
- Naumov, A.V.; Semenov, V.N.; Lukin, A.N.; Goncharov, E.G. Phase Composition of Copper Sulfide Films Produced from Copper Salt-Thiourea Complexes. Inorg. Mater. 2002, 38, 271–273. [Google Scholar] [CrossRef]
- Grozdanov, I. Optical Abnd Elecrical Properties of Copper Sulfide Films. J. Solid State Chem. 1995, 114, 469–475. [Google Scholar] [CrossRef]
- Cuevas, A.; Romero, R.; Leinen, D.; Dalchiele, E.A.; Ramos-Barrado, J.R.; Martin, F. Effect of the Stoichiometry of CuxS Thin Films on the Optical and Electrical Properties and the Solar Thermal Performance. Sol. Energy Mater. Sol. Cells 2015, 134, 199–208. [Google Scholar] [CrossRef]
- Ortiz De Solorzano, I.; Prieto, M.; Mendoza, G.; Alejo, T.; Irusta, S.; Sebastian, V.; Arruebo, M. Microfluidic Synthesis and Biological Evaluation of Photothermal Biodegradable Copper Sulfide Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 21545–21554. [Google Scholar] [CrossRef] [PubMed]
- Mane, R.S.; Lokhande, C.D. Chemical Deposition Method for Metal Chalcogenide Thin Films. Mater. Chem. Phys. 2000, 65, 1–31. [Google Scholar] [CrossRef]
- Cheng, J.; Pan, Y.; Zhu, J.; Li, Z.; Pan, J.; Ma, Z. Hybrid Network CuS Monolith Cathode Materials Synthesized via Facile in Situ Melt-Diffusion for Li-Ion Batteries. J. Power Sources 2014, 257, 192–197. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, S.W.; Kim, E.J.; Park, J. In-Situ Growth of Copper Sulfide Nanocrystals on Multiwalled Carbon Nanotubes and Their Application as Novel Solar Cell and Amperometric Glucose Sensor Materials. Nano Lett. 2007, 7, 778–784. [Google Scholar] [CrossRef]
- Yadav, S.; Shrivas, K.; Bajpai, P.K. Role of Precursors in Controlling the Size, Shape and Morphology in the Synthesis of Copper Sulfide Nanoparticles and Their Application for Fluorescence Detection. J. Alloys Compd. 2019, 772, 579–592. [Google Scholar] [CrossRef]
- Wang, M.; Xie, F.; Li, W.; Chen, M.; Zhao, Y. Preparation of Various Kinds of Copper Sulfides in a Facile Way and the Enhanced Catalytic Activity by Visible Light. J. Mater. Chem. A 2013, 1, 8616–8621. [Google Scholar] [CrossRef]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A Review of Methods for Synthesis of Nanostructured Metals with Emphasis on Iron Compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- De Mello Donegá, C.; Liljeroth, P.; Vanmaekelbergh, D. Physicochemical Evaluation of the Hot-Injection Method, a Synthesis Route for Monodisperse Nanocrystals. Small 2005, 1, 1152–1162. [Google Scholar] [CrossRef]
- Xie, Y.; Carbone, L.; Nobile, C.; Grillo, V.; D’Agostino, S.; Della Sala, F.; Giannini, C.; Altamura, D.; Oelsner, C.; Kryschi, C.; et al. Metallic-like Stoichiometric Copper Sulfide Nanocrystals: Phase- and Shape-Selective Synthesis, near-Infrared Surface Plasmon Resonance Properties, and Their Modeling. ACS Nano 2013, 7, 7352–7369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Mikrovalov, V.; Ambro, G.; Orel, Z.C. Microwave-Assisted Non-Aqueous Synthesis of ZnO Nanoparticles Microwave-Assisted Non-Aqueous Synthesis of ZnO Nanoparticles. Mater. Tehnol. 2011, 45, 173–177. [Google Scholar]
- Kar, P.; Farsinezhad, S.; Zhang, X.; Shankar, K. Anodic Cu2S and CuS Nanorod and Nanowall Arrays: Preparation, Properties and Application in CO2 Photoreduction. Nanoscale 2014, 6, 14305–14318. [Google Scholar] [CrossRef] [PubMed]
- DeMeo, D.; MacNaughton, S.; Sonkusale, S.; Vandervelde, T. Electrodeposited Copper Oxide and Zinc Oxide Core-Shell Nanowire Photovoltaic Cells. In Nanowires-Implementations and Applications; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef]
- Capek, I. Preparation of Metal Nanoparticles in Water-in-Oil (w/o) Microemulsions. Adv. Colloid Interface Sci. 2004, 110, 49–74. [Google Scholar] [CrossRef]
- Erol, M.; Boccaccini, A.R. Nanoscaled Bioactive Glass Particles and Nanofibres. Bioact. Glas. Mater. Prop. Appl. 2011, 129–161. [Google Scholar] [CrossRef]
- Modan, E.M.; Plăiașu, A.G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. Ann. Univ. Dunarea Jos Galati Fasc. IX Metall. Mater. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Suslick, K.S. Applications of Ultrasound to Materials Chemistry. MRS Bull. 1995, 20, 29–34. [Google Scholar] [CrossRef]
- Qian, L.; Mao, J.; Tian, X.; Yuan, H.; Xiao, D. In Situ Synthesis of CuS Nanotubes on Cu Electrode for Sensitive Nonenzymatic Glucose Sensor. Sens. Actuators B Chem. 2013, 176, 952–959. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Wang, Z.; Kang, Y.M.; Bando, Y.; Yamauchi, Y. Elaborately Assembled Core-Shell Structured Metal Sulfides as a Bifunctional Catalyst for Highly Efficient Electrochemical Overall Water Splitting. Nano Energy 2018, 47, 494–502. [Google Scholar] [CrossRef]
- Lin, J.; Tao, F.; Wang, L.; Chen, L.; Ying, Y.; Zhang, L.; Liu, H.; Xia, M. Solvothermal Synthesis of Sphere-like CuS Microcrystals and Improvement as Nonenzymatic Glucose Sensor. J. Mater. Sci. 2013, 48, 5509–5516. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Kim, H.Y.; Kim, B.S. A Novel CuS Microflower Superstructure Based Sensitive and Selective Nonenzymatic Glucose Detection. Sens. Actuators B Chem. 2016, 233, 93–99. [Google Scholar] [CrossRef]
- Karikalan, N.; Karthik, R.; Chen, S.M.; Karuppiah, C.; Elangovan, A. Sonochemical Synthesis of Sulfur Doped Reduced Graphene Oxide Supported CuS Nanoparticles for the Non-Enzymatic Glucose Sensor Applications. Sci. Rep. 2017, 7, 2494. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. A Non-Enzymatic Glucose Sensor Based on the CuS Nanoflakes-Reduced Graphene Oxide Nanocomposite. Anal. Methods 2018, 10, 381–388. [Google Scholar] [CrossRef]
- Cao, M.; Wang, H.; Kannan, P.; Ji, S.; Wang, X.; Zhao, Q.; Linkov, V.; Wang, R. Highly Efficient Non-Enzymatic Glucose Sensor Based on CuxS Hollow Nanospheres. Appl. Surf. Sci. 2019, 492, 407–416. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, L.; Fan, Z. Biomass-Derived Carbon Materials with Structural Diversities and Their Applications in Energy Storage. Sci. China Mater. 2018, 61, 133–158. [Google Scholar] [CrossRef]
- Ghorai, S.; Sarkar, A.; Raoufi, M.; Panda, A.B.; Schönherr, H.; Pal, S. Enhanced Removal of Methylene Blue and Methyl Violet Dyes from Aqueous Solution Using a Nanocomposite of Hydrolyzed Polyacrylamide Grafted Xanthan Gum and Incorporated Nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 4766–4777. [Google Scholar] [CrossRef]
- Keerthi, M.; Mutharani, B.; Chen, S.M.; Ranganathan, P. Carbon Fibers Coated with Urchin-like Copper Sulfide for Nonenzymatic Voltammetric Sensing of Glucose. Microchim. Acta 2019, 186, 807. [Google Scholar] [CrossRef]
- Mai, L.N.T.; Tran, T.H.; Bui, Q.B.; Nhac-Vu, H.T. A Novel Nanohybrid of Gold Nanoparticles Anchored Copper Sulfide Nanosheets as Sensitive Sensor for Nonenzymatic Glucose Detection. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123936. [Google Scholar] [CrossRef]
- Xu, G.R.; Ge, C.; Liu, D.; Jin, L.; Li, Y.C.; Zhang, T.H.; Rahman, M.M.; Li, X.B.; Kim, W. In-Situ Electrochemical Deposition of Dendritic Cu-Cu2S Nanocomposites onto Glassy Carbon Electrode for Sensitive and Non-Enzymatic Detection of Glucose. J. Electroanal. Chem. 2019, 847, 113177. [Google Scholar] [CrossRef]
- Sharma, K.P.; Shin, M.; Awasthi, G.P.; Poudel, M.B.; Kim, H.J.; Yu, C. Chitosan Polymer Matrix-Derived Nanocomposite (CuS/NSC) for Non-Enzymatic Electrochemical Glucose Sensor. Int. J. Biol. Macromol. 2022, 206, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zou, X.; Liu, Q.; Li, S.; Kang, C.; Xiang, W. A Highly Sensitive Non-Enzymatic Glucose Sensor Based on CuS Nanosheets Modified Cu2O/CuO Nanowire Arrays. Electrochim. Acta 2020, 334, 135630. [Google Scholar] [CrossRef]
- Yu, C.; Cui, J.; Wang, Y.; Zheng, H.; Zhang, J.; Shu, X.; Liu, J.; Zhang, Y.; Wu, Y. Porous HKUST-1 Derived CuO/Cu2O Shell Wrapped Cu(OH)2 Derived CuO/Cu2O Core Nanowire Arrays for Electrochemical Nonenzymatic Glucose Sensors with Ultrahigh Sensitivity. Appl. Surf. Sci. 2018, 439, 11–17. [Google Scholar] [CrossRef]
- Myeni, N.; Perla, V.K.; Ghosh, S.K.; Mallick, K. Organic Matrix Stabilized Copper Sulfide Nanoparticles: Synthesis, Characterization and Application in Glucose Recognition. Mater. Today Commun. 2020, 25, 101291. [Google Scholar] [CrossRef]
- Huang, W.; Liu, F.; Huang, Y.; Yang, W.; Zhong, H.; Peng, J. Facile One-Pot Synthesis of Hollow-Structured CuS/Cu2S Hybrid for Enhanced Electrochemical Determination of Glucose. Electrochemistry 2021, 89, 340–347. [Google Scholar] [CrossRef]
- Khairullina, E.M.; Panov, M.S.; Andriianov, V.S.; Ratautas, K.; Tumkin, I.I.; Račiukaitis, G. High Rate Fabrication of Copper and Copper–Gold Electrodes by Laser-Induced Selective Electroless Plating for Enzyme-Free Glucose Sensing. RSC Adv. 2021, 11, 19521–19530. [Google Scholar] [CrossRef]
- Yadav, H.M.; Lee, J.-J. One-Pot Synthesis of Copper Nanoparticles on Glass: Applications for Non-Enzymatic Glucose Detection and Catalytic Reduction of 4-Nitrophenol. J. Solid State Electrochem. 2019, 23, 503–512. [Google Scholar] [CrossRef]
- Tumkin, I.I.; Khairullina, E.M.; Panov, M.S.; Yoshidomi, K.; Mizoshiri, M. Copper and Nickel Microsensors Produced by Selective Laser Reductive Sintering for Non-Enzymatic Glucose Detection. Materials 2021, 14, 2493. [Google Scholar] [CrossRef]
- Lin, S.; Feng, W.; Miao, X.; Zhang, X.; Chen, S.; Chen, Y.; Wang, W.; Zhang, Y. A Flexible and Highly Sensitive Nonenzymatic Glucose Sensor Based on DVD-Laser Scribed Graphene Substrate. Biosens. Bioelectron. 2018, 110, 89–96. [Google Scholar] [CrossRef]
- Settu, K.; Chiu, P.-T.; Huang, Y.-M. Laser-Induced Graphene-Based Enzymatic Biosensor for Glucose Detection. Polymers 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]


| Electrode Material | Sensitivity (µA mM−1 cm−2) | Linear Range (mM) | Detection Limit (µM) | Reference |
|---|---|---|---|---|
| Ag–CuO/rGO | 214.37 | 10–28 | 0.76 | [55] |
| Ag–CuO | 2528.6 | 0.01–1 | 1.5 | [56] |
| Ag@CuO | 3763.44 | 1–9.2 | 0.006 | [57] |
| Ag/CuO/MLG | 1527 | 0.01–6.0 | 3.8 | [58] |
| Pt-CuO/GPE | 2035 | 3.125–18.75 | 0.1 | [59] |
| Au/CuO NWs | 4398.8 | 0.0005–5.9 | 0.5 | [60] |
| Au@Cu2O | 1601 | 0.005–2.1 | 0.6 | [61] |
| Au/CuO | 63.66 | 3–18 | 0.22 | [62] |
| Au/CuO | 172.45 | 0.002–1 | 0.22 | [62] |
| Electrode Composition | Sensitivity | Linear Detection Range (mM) | Limit of Detection (µM) | Reference |
|---|---|---|---|---|
| CuS nanotubes | 7.842 µA mM−1 | 0.00005–0.005 | - | [66] |
| CuS dendrite | 8337 µA mM−1 cm−2 | 0.001–4.9 | 0.05 | [9] |
| CuS nanotubes/Cu | 3135 µA mM−1 cm−2 | 0.0002–2.5 | 0.045 | [97] |
| Sphere-like CuS microcrystals | 117.3 µA mM−1 cm−2 | 0.0001–12 | 0.015 | [99] |
| CuS MF | 1007 µA mM−1 cm−2 | 0.02–5.4 | 2.0 | [100] |
| Cu7S4 | 3728.7 µA mM−1 cm−2 | 0.001–2.0 | 0.023 | [103] |
| S-rGO/CuS | 429.4 µA µM−1 cm−2 | 0.0001–20.17 | 0.032 | [101] |
| rGO/CuSFs | 53.5 µA µM−1 cm−2 | 0.001–2 | 0.19 | [102] |
| CuS/Cu2O/CuO/Cu | 4262 µA µM−1 cm−2 | 0.002–4.096 | - | [110] |
| CuS/Cu2S | 321.3 µA µM−1 cm−2 | 0.003–1.1 | 1.1 | [113] |
| CuS/XGCNFs | 23.69 µA µM−1 cm−2 | 0.158–1.221 | 0.019 | [106] |
| Cu2S | 38.21 µA µM−1 cm−2 | - | 2.42 | [112] |
| Au–CuxS/3DCF | 0.059 mA µM−1 cm−2 | 0.00198–0.97656 | 7.62 | [107] |
| Dendritic Cu-Cu2S | 5.02 mA mM−1 cm−2 | 0.0001–0.5 | 0.33 | [108] |
| CuS/NSC | 13.62 mA mM−1 cm−2 | 0.16–11.0 | 2.72 | [109] |
| Substrate | Electrode Material | Sensitivity (μA mM−1 cm−2) | Linear Range (µM) | Detection Limit (µM) | Reference |
|---|---|---|---|---|---|
| Glass | Cu on glass | 719 | 10–1000 | 1.97 | [114] |
| Glass | Cu on glass | 145.521 | 10–200 | 2.87 | [115] |
| Glass-ceramics | Cu on glass-ceramics | 911 | 3–1000 | 0.75 | [114] |
| Glass-ceramics | Cu on glass-ceramics | 1110 | 3–3000 | 0.91 | [116] |
| Polyimide (PI) foil | Graphene-Cu on PI | 1518 | 1–4540 | 0.35 | [117] |
| Polyimide film | Glucose oxidase/chitosan-modified graphene | 43.15 | 0–8000 | 431 | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miya, N.; Machogo-Phao, L.F.E.; Ntsendwana, B. Exploring Copper Oxide and Copper Sulfide for Non-Enzymatic Glucose Sensors: Current Progress and Future Directions. Micromachines 2023, 14, 1849. https://doi.org/10.3390/mi14101849
Miya N, Machogo-Phao LFE, Ntsendwana B. Exploring Copper Oxide and Copper Sulfide for Non-Enzymatic Glucose Sensors: Current Progress and Future Directions. Micromachines. 2023; 14(10):1849. https://doi.org/10.3390/mi14101849
Chicago/Turabian StyleMiya, Nonkululeko, Lerato F. Eugeni Machogo-Phao, and Bulelwa Ntsendwana. 2023. "Exploring Copper Oxide and Copper Sulfide for Non-Enzymatic Glucose Sensors: Current Progress and Future Directions" Micromachines 14, no. 10: 1849. https://doi.org/10.3390/mi14101849






