Research on Chemical Mechanical Polishing Technology for Zirconium-Based Amorphous Alloys
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Experimental Method
2.3. Experimental Design
3. Results and Discussion
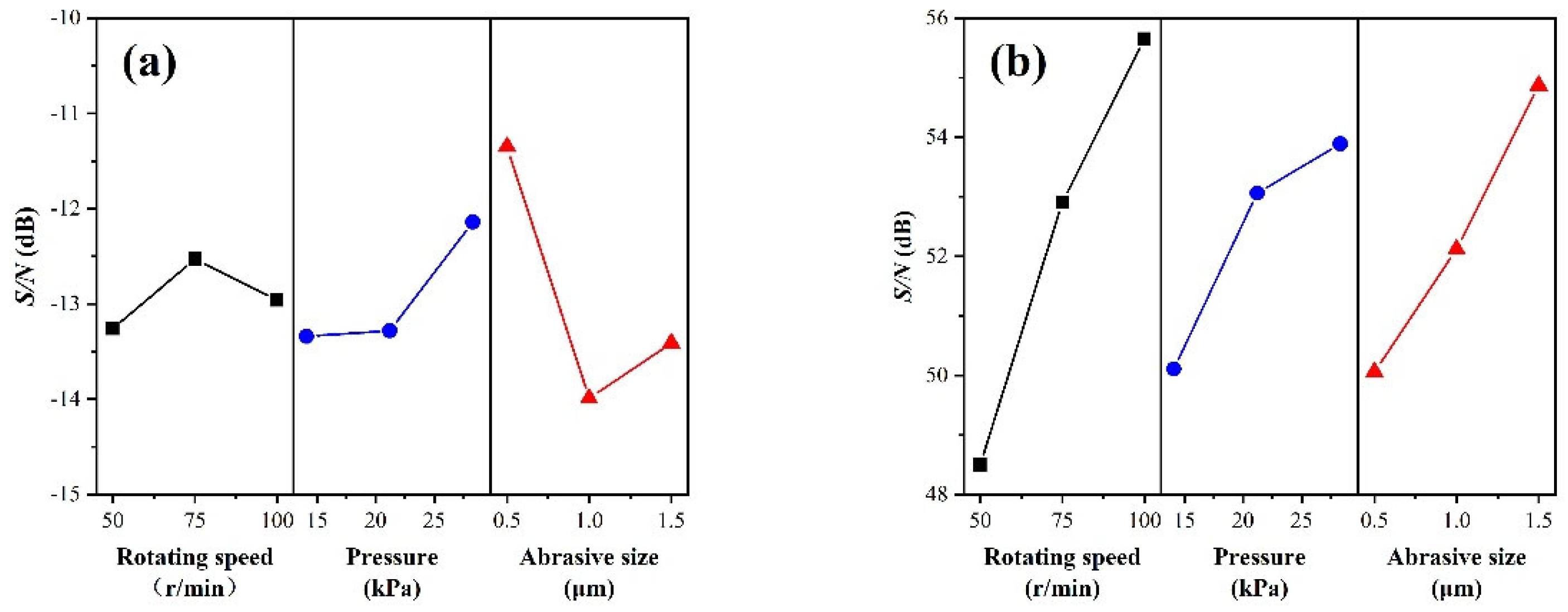
3.1. Optimal Factor Level Combination
3.2. Analysis of Variance
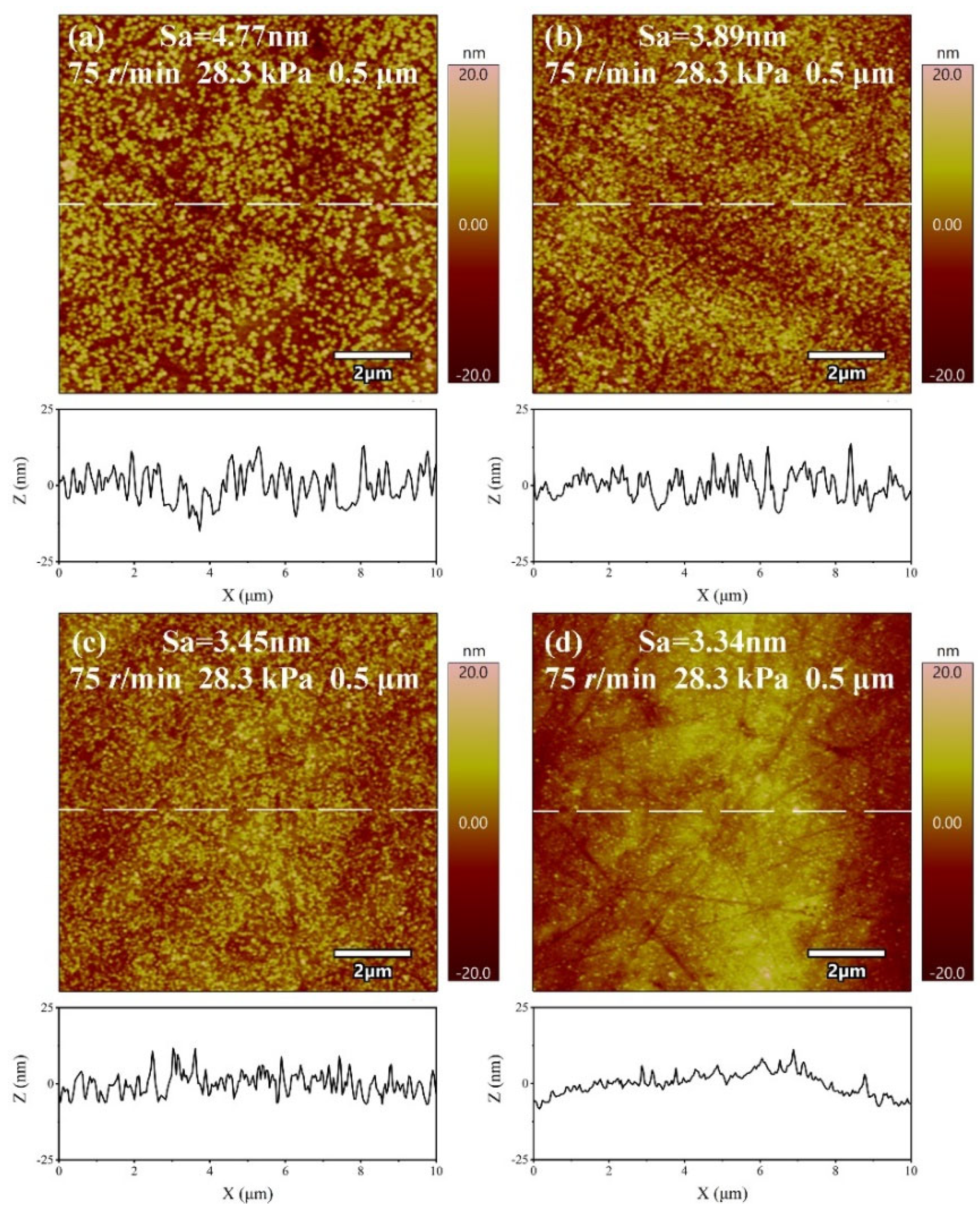
3.3. Effect of Size of Abrasive on Sa and MRR
3.4. Effect of H2O2 Concentration on Sa and MRR
3.5. XPS Analysis
4. Conclusions
- (1)
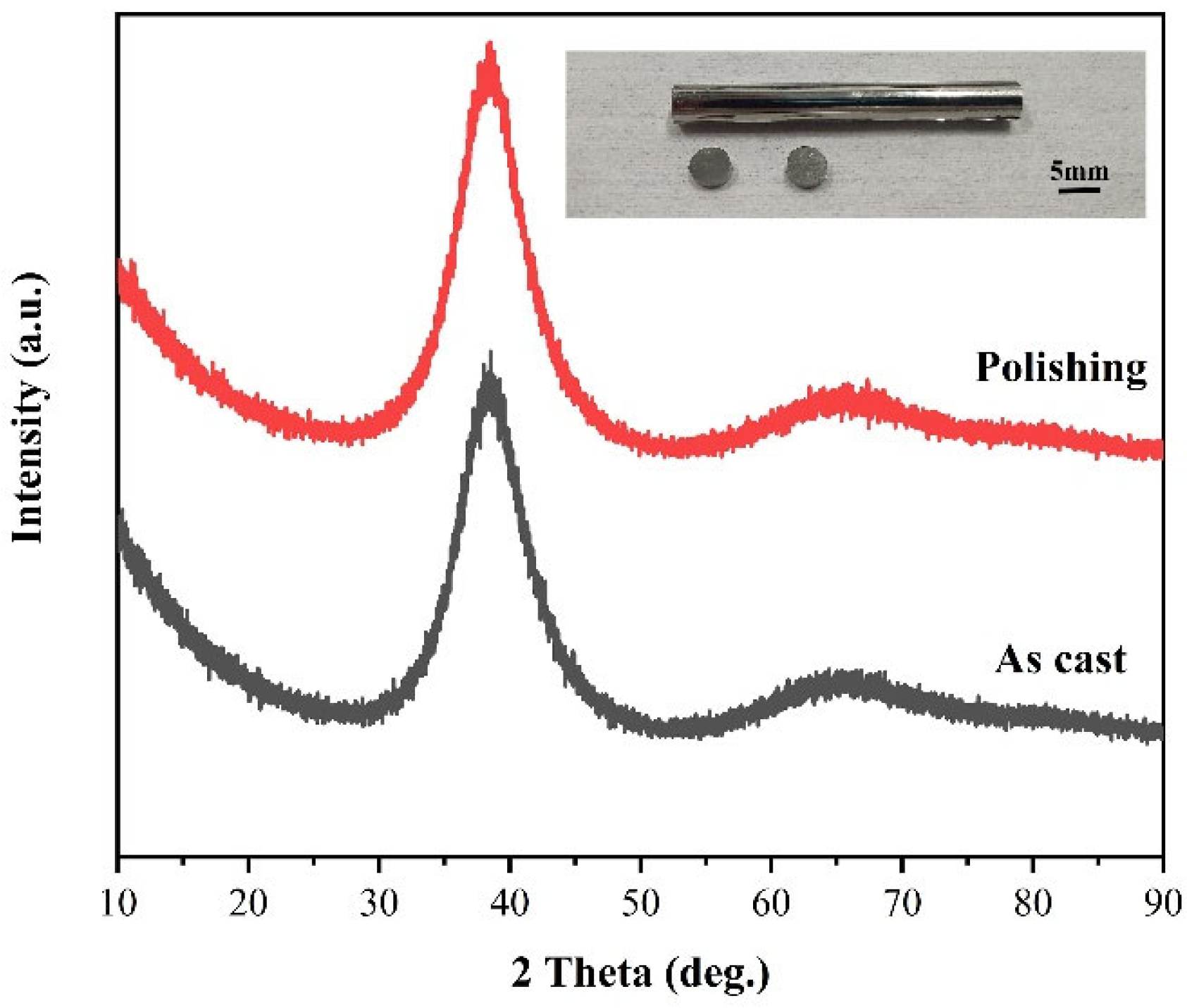
- The orthogonal experiments show that the polishing surface roughness Sa is minimized when the rotational speed of the polishing turntable is 75 r/min, the polishing pressure is 28.3 kPa, and the size of the abrasive is 0.5 μm. XRD patterns of samples before and after polishing are amorphous structures, indicating that chemical mechanical polishing can meet the requirements of efficient non-crystallization processing of amorphous alloys.
- (2)
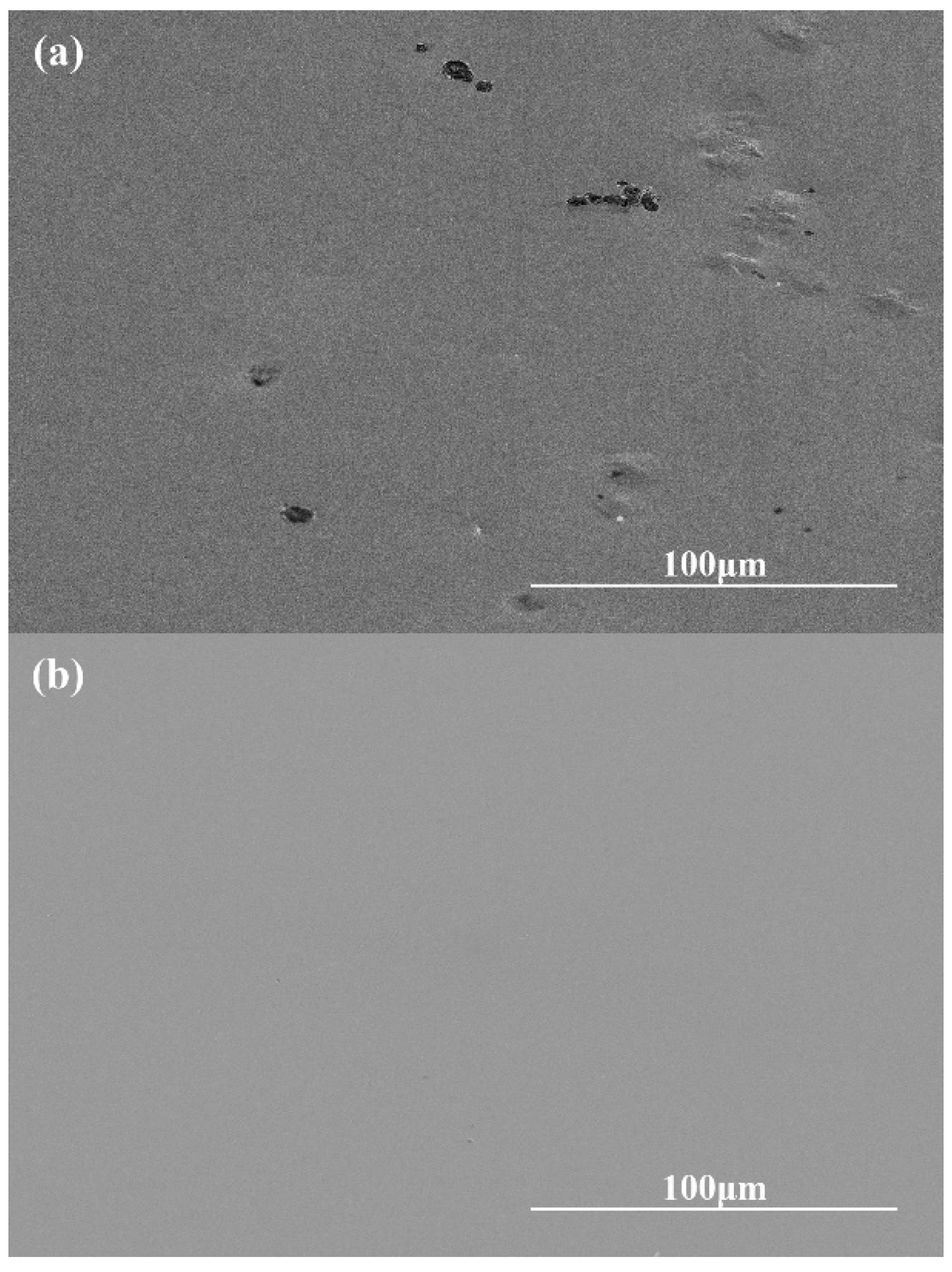
- The material removal rate and surface roughness decreased with the reduction of particle size. The material removal rate decreased from 812.57 ± 3.05 nm/min to 405.10 ± 7.09 nm/min, a reduction of 50.15%. The surface roughness decreased by 24.43% from 4.42 ± 0.61 nm to 3.34 ± 0.28 nm. With the increase of H2O2 concentration, the material removal rate decreased rapidly and then increased. When the concentration was 0.21 wt.%, the minimum value was 274.27 ± 6.10 nm/min, while the surface roughness decreased with the increase of concentration, reaching the minimum value of 3.34 ± 0.28 nm at 0.3 wt.%.
- (3)
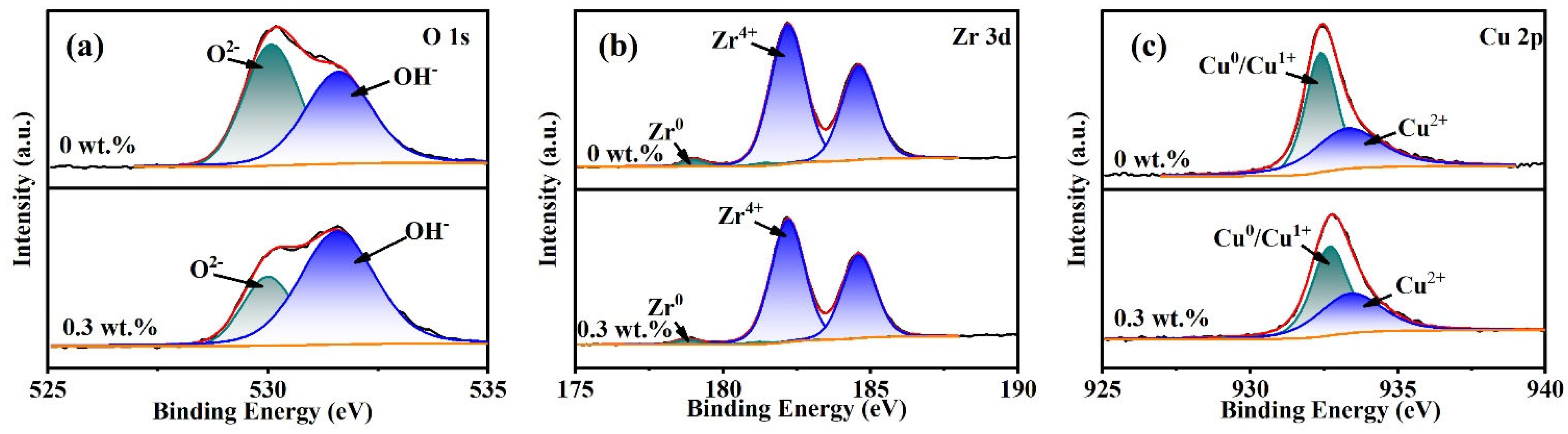
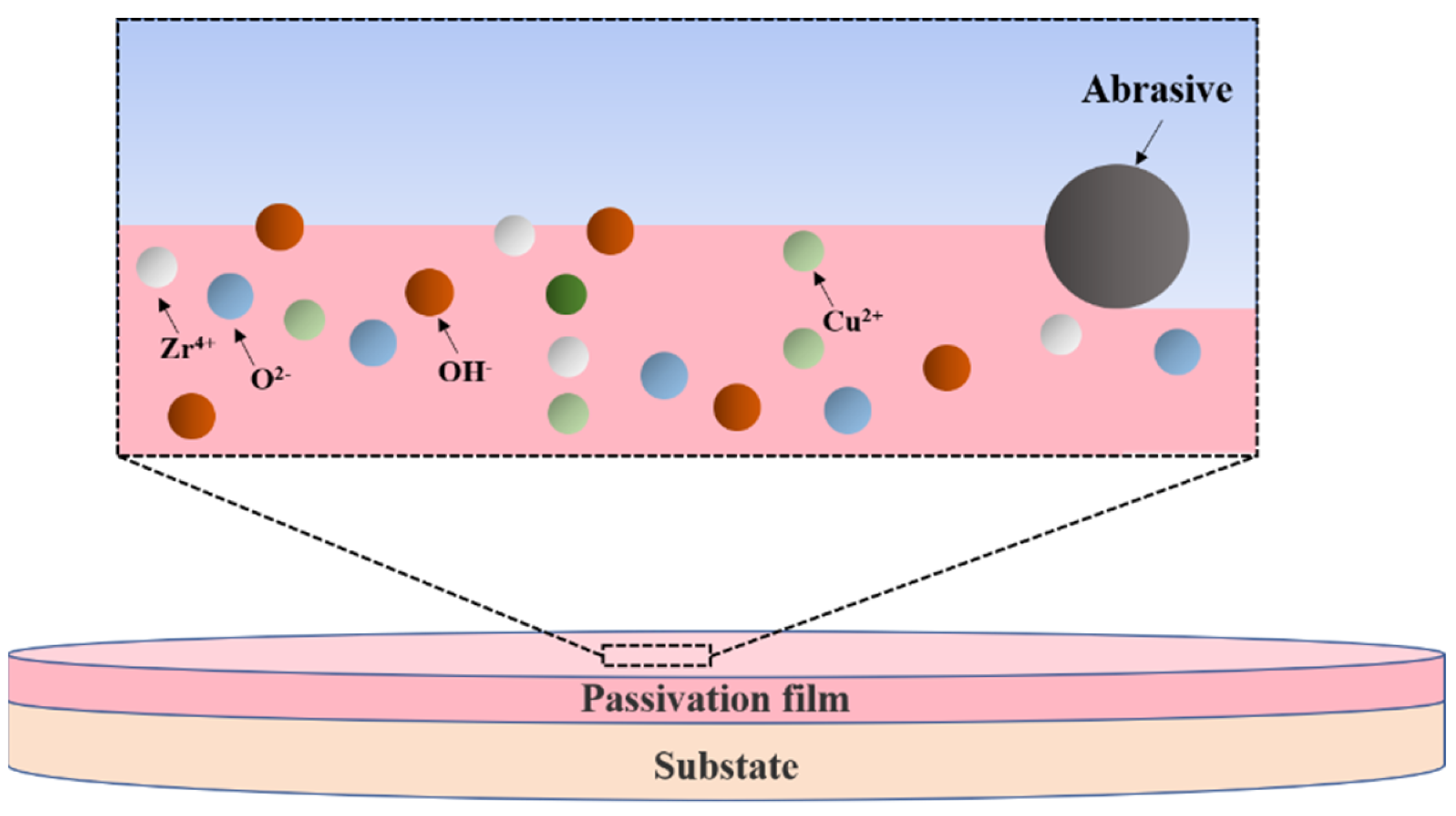
- XPS analysis shows that the oxide film on the sample surface is composed of oxides and hydroxides. With the addition of oxidants, the oxidation and wear resistance of the samples are enhanced, and the main components are transformed into hydroxides. At the same time, the contents of Zr4+ and Cu0/Cu1+ also increase. The results can provide some reference for chemical mechanical polishing zirconium-based amorphous alloys.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.-H.; Dong, C.; Shek, C. Bulk metallic glasses. Mater. Sci. Eng. R Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Grimberg, A.; Baur, H.; Bochsler, P.; Bühler, F.; Burnett, D.S.; Hays, C.C.; Heber, V.S.; Jurewicz, A.J.G.; Wieler, R. Solar wind neon from Genesis: Implications for the lunar noble gas record. Science 2006, 314, 1133–1135. [Google Scholar] [CrossRef]
- Schuh, C.A.; Hufnagel, T.C.; Ramamurty, U. Mechanical behavior of amorphous alloys. Acta Mater. 2007, 55, 4067–4109. [Google Scholar] [CrossRef]
- Johnson, W. Bulk amorphous metal—An emerging engineering material. JOM 2002, 54, 40–43. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Q.; Wang, J.; Li, P.; Yuan, J.; Lyu, B.; Wang, J.; Tokunaga, K.; Yao, G.; Luo, L.; et al. Effect of surface quality on hydrogen/helium irradiation behavior in tungsten. Nuclear Eng. Technol. 2022, 54, 1947–1953. [Google Scholar] [CrossRef]
- Han, D.; Wang, G.; Li, J.; Chan, K.; To, S.; Wu, F.; Gao, Y.; Zhai, Q. Cutting characteristics of Zr-based bulk metallic glass. J. Mater. Sci. Technol. 2015, 31, 153–158. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, H.; Zhang, G.; Chen, Y.; Ma, J.; Mo, R. Machinability and surface generation of Pd40Ni10Cu30P20 bulk metallic glass in single-point diamond turning. Micromachines 2019, 11, 4. [Google Scholar] [CrossRef]
- Hsieh, S.-F.; Chen, S.-L.; Lin, M.-H.; Ou, S.-F.; Lin, W.-T.; Huang, M.-S. Crystallization and carbonization of an electrical discharge machined Zr-based bulk metallic glass alloy. J. Mater. Res. 2013, 28, 3177–3184. [Google Scholar] [CrossRef]
- Huang, H.; Yan, J. On the surface characteristics of a Zr-based bulk metallic glass processed by microelectrical discharge machining. Appl. Surf. Sci. 2015, 355, 1306–1315. [Google Scholar] [CrossRef]
- Wessels, V.; Grigoryev, A.; Dold, C.; Wyen, C.-F.; Roth, R.; Weingärtner, E.; Pude, F.; Wegener, K.; Löffler, J.F. Abrasive waterjet machining of three-dimensional structures from bulk metallic glasses and comparison with other techniques. J. Mater. Res. 2012, 27, 1187–1192. [Google Scholar] [CrossRef]
- Ye, Q.-L.; Wang, C.-Y.; Lai, Z.-J.; Ding, F.; Wang, J. An Investigation into the Erosion Mechanisms of Zr-based Bulk Metallic Glasses by Loose Abrasives. J. Mech. Eng. 2022, 58, 92–104. [Google Scholar]
- Yuan, J.-L.; Mao, M.-J.; Li, M.; Liu, S.; Hu, Z.-H.; Wu, F. Chemical and Mechanical Polishing Mechanism of Cemented Carbide Tool Material. Surf. Technol. 2019, 48, 260–267. [Google Scholar]
- Zhang, Z.; Liao, L.; Wang, X.; Xie, W.; Guo, D. Development of a novel chemical mechanical polishing slurry and its polishing mechanisms on a nickel alloy. Appl. Surf. Sci. 2020, 506, 144670. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, L.; Qin, N.; Qian, L. Effects of pH and H2O2 on the chemical mechanical polishing of titanium alloys. J. Mater. Process. Technol. 2021, 295, 117204. [Google Scholar] [CrossRef]
- Li, T.; Sun, H.; Wang, D.; Huang, J.; Li, D.; Lei, F.; Sun, D. High-performance chemical mechanical polishing slurry for aluminum alloy using hybrid abrasives of zirconium phosphate and alumina. Appl. Surf. Sci. 2021, 537, 147859. [Google Scholar] [CrossRef]
- Zhang, Q.-S.; Zhang, W.; Xie, G.-Q.; Inoue, A. Synthesis, structure and mechanical properties of Zr-Cu-based bulk metallic glass composites. Int. J. Miner. Metall. Mater. 2010, 17, 208–213. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Inoue, A. Unusual glass-forming ability of new Zr-Cu-based bulk glassy alloys containing an immiscible element pair. Mater. Trans. 2008, 49, 2743–2746. [Google Scholar] [CrossRef]
- Taguchi, G. Quality engineering (Taguchi methods) for the development of electronic circuit technology. IEEE Trans. Reliab. 1995, 44, 225–229. [Google Scholar] [CrossRef]
- Roy, R.K. Design of Experiments Using the Taguchi Approach: 16 Steps to Product and Process Improvement; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Tsai, F.; Yan, B.; Kuan, C.; Huang, F. A Taguchi and experimental investigation into the optimal processing conditions for the abrasive jet polishing of SKD61 mold steel. Int. J. Mach. Tools Manuf. 2008, 48, 932–945. [Google Scholar] [CrossRef]
- Mori, T. Taguchi Methods: Benefits, Impacts, Mathematics, Statistics, and Applications; ASME Press: New York, NY, USA, 2011. [Google Scholar]
- Nalbant, M.; Gökkaya, H.; Sur, G. Application of Taguchi method in the optimization of cutting parameters for surface roughness in turning. Mater. Des. 2007, 28, 1379–1385. [Google Scholar] [CrossRef]
- Preston, F. The theory and design of plate glass polishing machines. J. Glass Technol. 1927, 11, 214–256. [Google Scholar]
- Yang, W.P.; Tarng, Y. Design optimization of cutting parameters for turning operations based on the Taguchi method. J. Mater. Process. Technol. 1998, 84, 122–129. [Google Scholar] [CrossRef]
- Hang, W.; Wei, L.; Debela, T.T.; Chen, H.; Zhou, L.; Yuan, J.; Ma, Y. Crystallographic orientation effect on the polishing behavior of LiTaO3 single crystal and its correlation with strain rate sensitivity. Ceram. Int. 2022, 48, 7766–7777. [Google Scholar] [CrossRef]
- Fu, G.; Chandra, A.; Guha, S.; Subhash, G. A plasticity-based model of material removal in chemical-mechanical polishing (CMP). IEEE Trans. Semicond. Manuf. 2001, 14, 406–417. [Google Scholar]
- Luo, J.; Dornfeld, D.A. Material removal mechanism in chemical mechanical polishing: Theory and modeling. IEEE Trans. Semicond. Manuf. 2001, 14, 112–133. [Google Scholar]
- Li, Y. Microelectronic Applications of Chemical Mechanical Planarization; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Mani, G.; Feldman, M.D.; Oh, S.; Agrawal, C.M. Surface modification of cobalt–chromium–tungsten–nickel alloy using octadecyltrichlorosilanes. Appl. Surf. Sci. 2009, 255, 5961–5970. [Google Scholar] [CrossRef]
- Kawashima, A.; Ohmura, K.; Yokoyama, Y.; Inoue, A. The corrosion behaviour of Zr-based bulk metallic glasses in 0.5 M NaCl solution. Corros. Sci. 2011, 53, 2778–2784. [Google Scholar] [CrossRef]
- Olsson, C.-O.; Landolt, D. Atmospheric oxidation of a Nb–Zr alloy studied with XPS. Corros. Sci. 2004, 46, 213–224. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]




| Parameters | Values |
|---|---|
| Density (g/cm3) | 7.18 |
| Hardness (MPa) | 7.20 |
| Yield strength (MPa) | 1880 [16] |
| Fracture strength (MPa) | 1903 [16] |
| Tg (K, Glass transition temperature) | 683 [17] |
| Tx (K, Crystallization temperature) | 791 [17] |
| Level | A. Rotating Speed (r/min) | B. Pressure (kPa) | C. Abrasive Size (μm) |
|---|---|---|---|
| 1 | 50 | 14.1 | 0.5 |
| 2 | 75 | 21.2 | 1.0 |
| 3 | 100 | 28.3 | 1.5 |
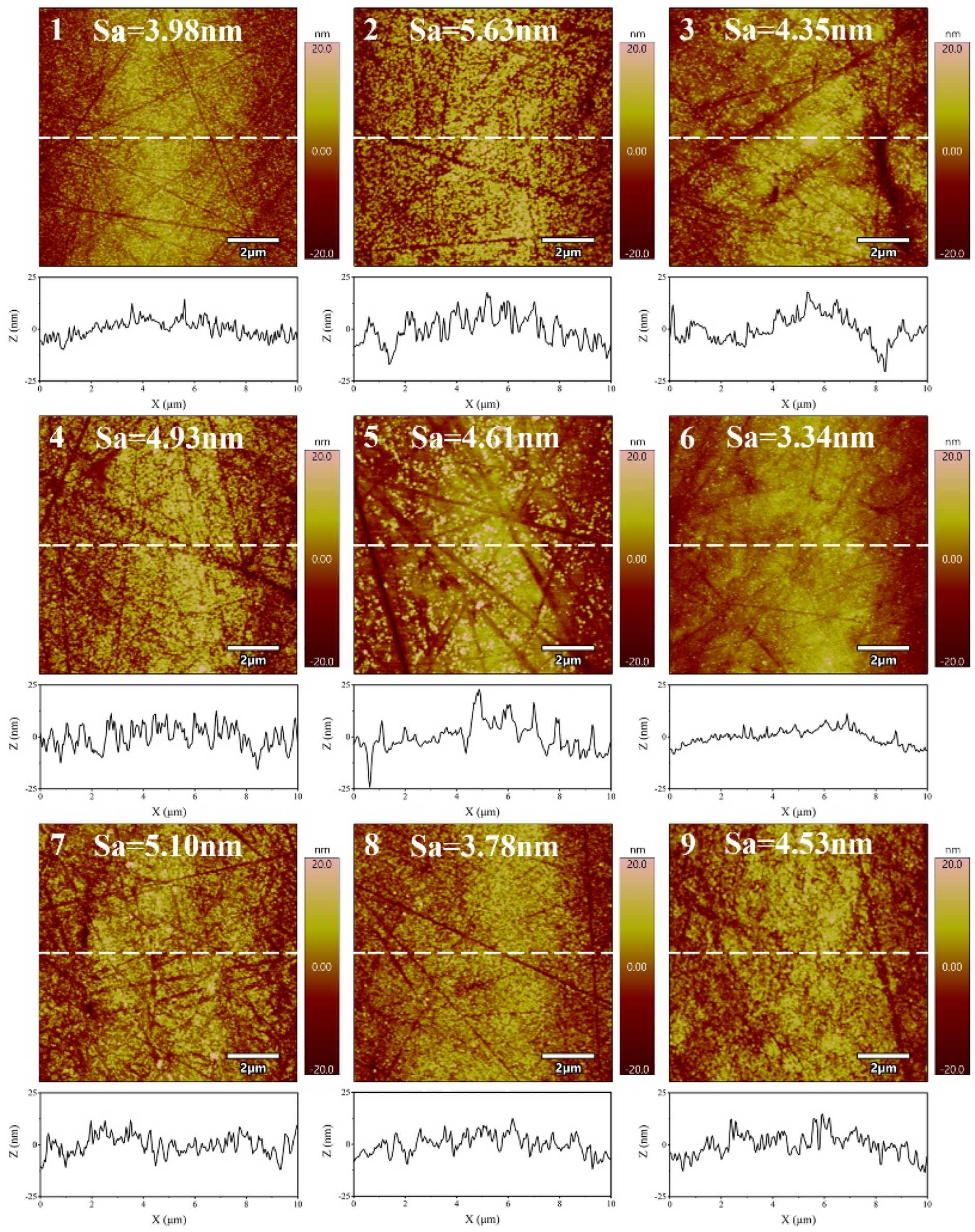
| Experiment. No. | Control Factor | Surface Roughness Sa (nm) | MRR (nm/min) | S/N Ratio(dB) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | 1 | 2 | 3 | Mean (nm) | Sa | MRR | ||
| 1 | 1 | 1 | 1 | 3.93 | 3.78 | 4.22 | 3.98 | 157.63 | −12.00 | 43.95 |
| 2 | 1 | 2 | 2 | 5.64 | 5.57 | 5.67 | 5.63 | 280.58 | −15.00 | 48.96 |
| 3 | 1 | 3 | 3 | 4.20 | 4.57 | 4.29 | 4.35 | 425.20 | −12.78 | 52.57 |
| 4 | 2 | 1 | 2 | 4.87 | 5.20 | 4.68 | 4.93 | 333.38 | −13.84 | 50.46 |
| 5 | 2 | 2 | 3 | 4.55 | 4.73 | 4.55 | 4.61 | 640.76 | −13.27 | 56.13 |
| 6 | 2 | 3 | 1 | 3.45 | 3.15 | 3.42 | 3.34 | 405.10 | −10.49 | 52.15 |
| 7 | 3 | 1 | 3 | 5.33 | 4.50 | 5.47 | 5.10 | 624.21 | −14.18 | 55.91 |
| 8 | 3 | 2 | 1 | 3.88 | 3.89 | 3.58 | 3.78 | 505.99 | −11.56 | 54.08 |
| 9 | 3 | 3 | 2 | 4.80 | 4.22 | 4.58 | 4.53 | 703.81 | −13.14 | 56.95 |
| Factor | D.F. | S.S. | M.S. | F Value | F0.05(2,2) |
|---|---|---|---|---|---|
| A | 2 | 0.80 | 0.40 | 1.87 | 19 |
| B | 2 | 2.76 | 1.38 | 6.43 | 19 |
| C | 2 | 11.61 | 5.81 | 27.04 | 19 |
| Error | 2 | 0.43 | 0.21 | - | - |
| Total | 8 | 15.61 | - | - | - |
| Factor | D.F. | S.S. | M.S. | F Value | F0.05(2,2) |
|---|---|---|---|---|---|
| A | 2 | 157,216 | 78,608 | 14.82 | 19 |
| B | 2 | 31,587 | 15,794 | 2.98 | 19 |
| C | 2 | 65,211 | 32,606 | 6.15 | 19 |
| Error | 2 | 10,611 | 5306 | - | - |
| Total | 8 | 264,626 | - | - | - |
| Experiment. No. | Surface Roughness Sa (nm) | S/N Ratio (dB) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | Mean (nm) | ||
| 1 | 3.71 | 3.26 | 3.58 | 3.42 | −10.94 |
| 2 | 3.96 | 3.13 | 3.22 | 3.44 | −10.78 |
| 3 | 3.49 | 3.17 | 3.22 | 3.29 | −10.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, W.; Song, C.; Yin, Z.; Liu, Y.; Wang, Q.; Wang, Y.; Ma, Y.; Zeng, Q. Research on Chemical Mechanical Polishing Technology for Zirconium-Based Amorphous Alloys. Micromachines 2023, 14, 584. https://doi.org/10.3390/mi14030584
Hang W, Song C, Yin Z, Liu Y, Wang Q, Wang Y, Ma Y, Zeng Q. Research on Chemical Mechanical Polishing Technology for Zirconium-Based Amorphous Alloys. Micromachines. 2023; 14(3):584. https://doi.org/10.3390/mi14030584
Chicago/Turabian StyleHang, Wei, Chao Song, Ziliang Yin, Ye Liu, Qifan Wang, Yinggang Wang, Yi Ma, and Qiaoshi Zeng. 2023. "Research on Chemical Mechanical Polishing Technology for Zirconium-Based Amorphous Alloys" Micromachines 14, no. 3: 584. https://doi.org/10.3390/mi14030584
APA StyleHang, W., Song, C., Yin, Z., Liu, Y., Wang, Q., Wang, Y., Ma, Y., & Zeng, Q. (2023). Research on Chemical Mechanical Polishing Technology for Zirconium-Based Amorphous Alloys. Micromachines, 14(3), 584. https://doi.org/10.3390/mi14030584






