Depth Dose Enhancement in Orthovoltage Nanoparticle-Enhanced Radiotherapy: A Monte Carlo Phantom Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monte Carlo Simulation
2.2. Calculation of Dose Enhancement Ratio
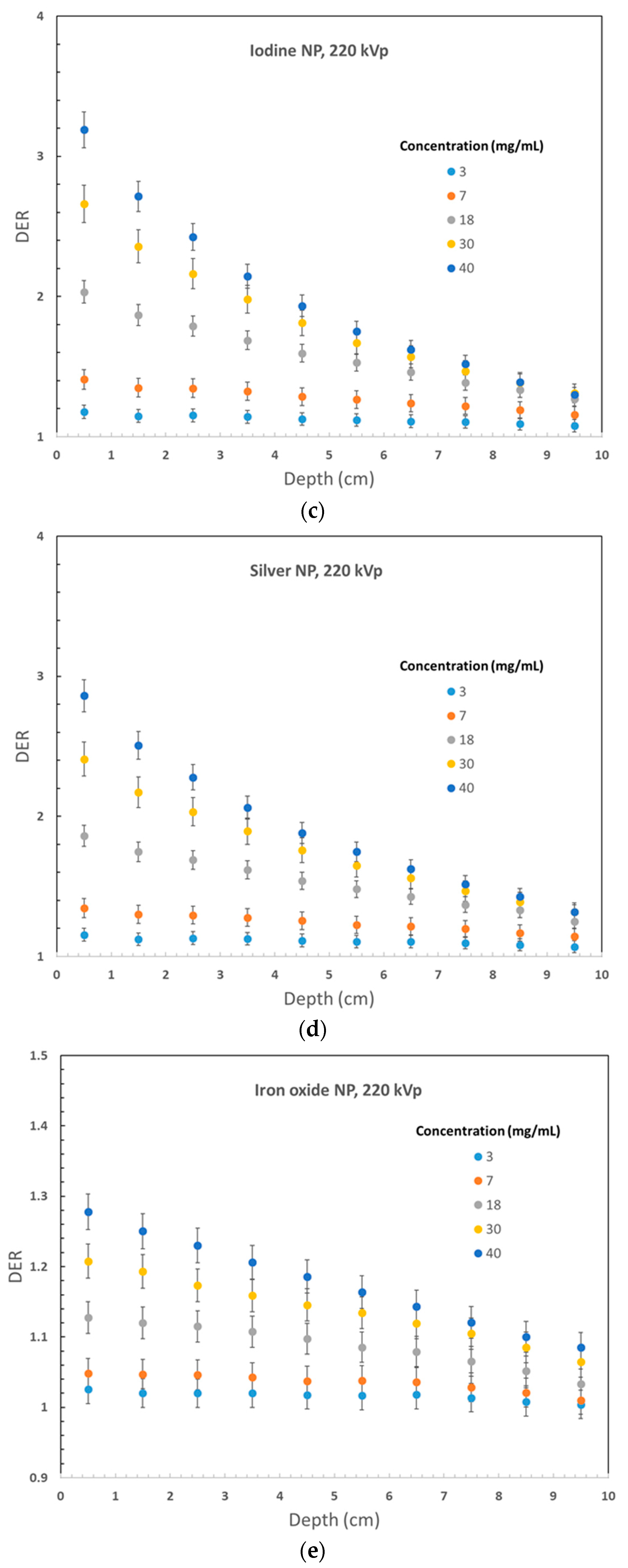
3. Results
4. Discussion
4.1. Dependence of Dose Enhancement on the Phantom Depth
4.2. Dependence of Dose Enhancement on the Nanoparticle Material
4.3. Dependences of Dose Enhancement on Nanoparticle Concentration
4.4. Dependences of Dose Enhancement on the Photon Beam Energy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rong, Y.; Zuo, L.; Shang, L.; Bazan, J.G. Radiotherapy treatment for nonmelanoma skin cancer. Expert Rev. Anticancer Ther. 2015, 15, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Veness, M.; Richards, S. Role of modern radiotherapy in treating skin cancer. Australas. J. Dermatol. 2003, 44, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Krema, H.; Herrmann, E.; Albert-Green, A.; Payne, D.; Laperriere, N.; Chung, C. Orthovoltage radiotherapy in the management of medial canthal basal cell carcinoma. Br. J. Ophthalmol. 2013, 97, 730–734. [Google Scholar] [CrossRef]
- Ogawa, R.; Yoshitatsu, S.; Yoshida, K.; Miyashita, T. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast. Reconstr. Surg. 2009, 124, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Khoshgard, K.; Hashemi, B.; Arbabi, A.; Rasaee, M.J.; Soleimani, M. Radiosensitization effect of folate-conjugated gold nanoparticles on HeLa cancer cells under orthovoltage superficial radiotherapy techniques. Phys. Med. Biol. 2014, 59, 2249. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Chow, J.C. Evaluation of Dosimetric Effect of Bone Scatter on Nanoparticle-Enhanced Orthovoltage Radiotherapy: A Monte Carlo Phantom Study. Nanomaterials 2022, 12, 2991. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.M.; Cho, S.H.; Reynoso, F.J.; Aliru, M.; Aziz, K.; Bodd, M.; Yang, X.; Ahmed, M.F.; Yasar, S.; Manohar, N.; et al. Radiosensitization of prostate cancers in vitro and in vivo to erbium-filtered orthovoltage x-rays using actively targeted gold nanoparticles. Sci. Rep. 2017, 7, 18044. [Google Scholar] [CrossRef] [Green Version]
- Rahman, W.N.; Bishara, N.; Ackerly, T.; He, C.F.; Jackson, P.; Wong, C.; Davidson, R.; Geso, M. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 136–142. [Google Scholar] [CrossRef]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Zein, R.; Sharrouf, W.; Selting, K. Physical properties of nanoparticles that result in improved cancer targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef]
- Zheng, X.J.; Chow, J.C. Radiation dose enhancement in skin therapy with nanoparticle addition: A Monte Carlo study on kilovoltage photon and megavoltage electron beams. World J. Radiol. 2017, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, X.; Cheng, Y.; Chudal, L.; Pandey, N.K.; Zhang, J.; Ma, L.; Xi, Q.; Yang, G.; Chen, Y.; et al. Use of copper-cysteamine nanoparticles to simultaneously enable radiotherapy, oxidative therapy and immunotherapy for melanoma treatment. Signal Transduct. Target. Ther. 2020, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Guidelli, E.J.; Baffa, O. Influence of photon beam energy on the dose enhancement factor caused by gold and silver nanoparticles: An experimental approach. Med. Phys. 2014, 41, 032101. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.W. Fifty years of Monte Carlo simulations for medical physics. Phys. Med. Biol. 2006, 51, R287. [Google Scholar] [CrossRef]
- Gray, T.; Bassiri, N.; David, S.; Patel, D.Y.; Stathakis, S.; Kirby, N.; Mayer, K.M. A detailed experimental and Monte Carlo analysis of gold nanoparticle dose enhancement using 6 MV and 18 MV external beam energies in a macroscopic scale. Appl. Radiat. Isot. 2021, 171, 109638. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Alerstam, E.; Svensson, T.; Andersson-Engels, S. Parallel computing with graphics processing units for high-speed Monte Carlo simulation of photon migration. J. Biomed. Opt. 2008, 13, 060504. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Gu, X.; Graves, Y.J.; Folkerts, M.; Jiang, S.B. GPU-based fast Monte Carlo simulation for radiotherapy dose calculation. Phys. Med. Biol. 2011, 56, 7017. [Google Scholar] [CrossRef]
- Chow, J.C.L. Depth dose enhancement on flattening-filter-free photon beam: A Monte Carlo study in nanoparticle-enhanced radiotherapy. Appl. Sci. 2020, 10, 7052. [Google Scholar] [CrossRef]
- Spina, A.; Chow, J.C.L. Dosimetric Impact on the Flattening Filter and Addition of Gold Nanoparticles in Radiotherapy: A Monte Carlo Study on Depth Dose Using the 6 and 10 MV FFF Photon Beams. Materials 2022, 15, 7194. [Google Scholar] [CrossRef]
- Mahdavi, M.; KhademAbolfazli, M.; Mahdavi, S.R.; Ataei, G. Effect of gold nanoparticle on percentage depth dose enhancement on megavoltage energy in MAGICA polymer gel dosimeter. J. Biomed. Phys. Eng. 2013, 3, 37. [Google Scholar] [PubMed]
- Ranjbar, H.; Shamsaei, M.; Ghasemi, M.R. Investigation of the dose enhancement factor of high intensity low mono-energetic X-ray radiation with labeled tissues by gold nanoparticles. Nukleonika 2010, 55, 307–312. [Google Scholar]
- Kawrakow, I.; Rogers, D.W. The EGSnrc Code System; NRC Report PIRS-701; NRC: Ottawa, ON, Canada, 2000; p. 17. [Google Scholar]
- Rogers, D.W.; Walters, B.R.; Kawrakow, I. BEAMnrc Users Manual; NRCC Report PIRS-0509; NRC: Ottawa, ON, Canada, 2009; Volume 509, p. 12. [Google Scholar]
- Chow, J.C.L.; Jiang, R. Bone and mucosal dosimetry in skin radiation therapy: A Monte Carlo study using kilovoltage photon and megavoltage electron beams. Phys. Med. Biol. 2012, 57, 3885–3899. [Google Scholar] [CrossRef]
- Cho, S.H. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: A preliminary Monte Carlo study. Phys. Med. Biol. 2005, 50, N163. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.L.; Krishnan, S.; Cho, S.H. Estimation of microscopic dose enhancement factor around gold nanoparticles by Monte Carlo calculations. Med. Phys. 2010, 37, 3809–3816. [Google Scholar] [CrossRef]
- Roeske, J.C.; Nunez, L.; Hoggarth, M.; Labay, E.; Weichselbaum, R.R. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol. Cancer Res. Treat. 2007, 6, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhattarai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1277–1283. [Google Scholar] [CrossRef] [Green Version]
- Berking, C.; Hauschild, A.; Kölbl, O.; Mast, G.; Gutzmer, R. Basal cell carcinoma—Treatments for the commonest skin cancer. Dtsch. Ärzteblatt Int. 2014, 111, 389. [Google Scholar]
- Wolstenholme, V.; Glees, J.P. The role of kilovoltage X-rays in the treatment of skin cancers. Eur. Oncol. Dis. 2006, 1, 32–35. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical translation of gold nanoparticles. Drug Deliv. Transl. Res. 2023, 13, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Khizar, S.; Elkalla, E.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Magnetic nanoparticles: Multifunctional tool for cancer therapy. Expert Opin. Drug Deliv. 2023, 20, 189–204. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, J.C.L.; Jubran, S. Depth Dose Enhancement in Orthovoltage Nanoparticle-Enhanced Radiotherapy: A Monte Carlo Phantom Study. Micromachines 2023, 14, 1230. https://doi.org/10.3390/mi14061230
Chow JCL, Jubran S. Depth Dose Enhancement in Orthovoltage Nanoparticle-Enhanced Radiotherapy: A Monte Carlo Phantom Study. Micromachines. 2023; 14(6):1230. https://doi.org/10.3390/mi14061230
Chicago/Turabian StyleChow, James C. L., and Sama Jubran. 2023. "Depth Dose Enhancement in Orthovoltage Nanoparticle-Enhanced Radiotherapy: A Monte Carlo Phantom Study" Micromachines 14, no. 6: 1230. https://doi.org/10.3390/mi14061230





