Simulation and Synthesis of Cobalt (Co) Nanoparticles by Gamma Radiation Technique
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Procedure
2.3. Characterization
3. Results and Discussion
3.1. Experimental Process
Radiolytic Reduction Method
3.2. DLS Results
3.3. TEM Images of Colloidal Co Nanoparticles
3.4. XRD Analysis of Co Nanoparticles
3.5. Optical Properties
3.5.1. Experimental
3.5.2. Theoretical Model and Simulation
3.6. Interpretation between the Measured and Simulated Absorption Spectra
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greer, A.L. Nanostructured materials—From fundamentals to applications. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 1998; pp. 3–10. [Google Scholar]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Marignier, J.L.; Belloni, J.; Delcourt, M.O.; Chevalier, J.P. Microaggregates of non-noble metals and bimetallic alloys prepared by radiation-induced reduction. Nature 1985, 317, 344–345. [Google Scholar] [CrossRef]
- Belloni, J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry: Application to catalysis. Catal. Today 2006, 113, 141–156. [Google Scholar] [CrossRef]
- Jia, C.J.; Schüth, F. Colloidal metal nanoparticles as a component of designed catalyst. Phys. Chem. Chem. Phys. 2011, 13, 2457–2487. [Google Scholar] [CrossRef]
- Tao, F. Metal Nanoparticles for Catalysis: Advances and Applications; RSC Catalysis Series No. 17; Royal Society of Chemistry: London, UK, 2014; ISSN 1757-6725. [Google Scholar]
- Klabunde, K.J.; Richards, R. Nanoscale Materials in Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; ISBN 0-471-38395-3. [Google Scholar]
- Cowburn, R. Magnetic nanodots for device applications. J. Magn. Magn. Mater. 2002, 242, 505–511. [Google Scholar] [CrossRef]
- Brown, W.F., Jr. The Fundamental Theorem of Fine-Ferromagnetic-Particle Theory. J. Appl. Phys. 1968, 39, 993–994. [Google Scholar] [CrossRef]
- Zdorovets, M.; Kozlovskiy, A.; Shlimas, D.; Borgekov, D. Phase transformations in FeCo–Fe2CoO4/Co3O4-spinel nanostructures as a result of thermal annealing and their practical application. J. Mater. Sci. Mater. Electron. 2021, 32, 16694–16705. [Google Scholar] [CrossRef]
- Gharibshahi, E.; Young, B.D.; Bhalla, A.S.; Guo, R. Theory, simulation and experiment of optical properties of cobalt ferrite (CoFe2O4) nanoparticles. J. Mater. Sci. Technol. 2020, 57, 180–187. [Google Scholar] [CrossRef]
- Shakirzyanov, R.; Kozlovskiy, A.; Zdorovets, M.; Zheludkevich, A.; Shlimas, D.; Abmiotka, N.; Kazantsev, P.; Zubar, T.; Trukhanov, S.; Trukhanov, A. Impact of thermobaric conditions on phase content, magnetic and electrical properties of the CoFe2O4 ceramics. J. Alloys Compd. 2023, 954, 170083. [Google Scholar] [CrossRef]
- Petit, C.; Rusponi, S.; Brune, H. Magnetic properties of cobalt and cobalt–platinum nanocrystals investigated by magneto-optical Kerr effect. J. Appl. Phys. 2004, 95, 4251–4260. [Google Scholar] [CrossRef] [Green Version]
- Park, I.W.; Yoon, M.; Kim, Y.; Kim, Y.; Kim, J.; Kim, S.; Volkov, V. Synthesis of cobalt nanoparticles in polymeric membrane and their magnetic anisotropy. J. Magn. Magn. Mater. 2004, 272, 1413–1414. [Google Scholar] [CrossRef]
- Masala, O.; Seshadri, R. Synthesis routes for large volumes of nanoparticles. Annu. Rev. Mater. Res. 2004, 34, 41–81. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.; Rehman, S.; Rahman, H.U.; Khan, Q. Synthesis and Application of Magnetic Nanoparticles; One Central Press (OCP): Manchester, UK, 2014; pp. 135–169. [Google Scholar]
- Shao, H.; Huang, Y.; Lee, H.; Suh, Y.J.; Kim, C.O. Cobalt nanoparticles synthesis from Co(CH3COO)2 by thermal decomposition. J. Magn. Magn. Mater. 2006, 304, e28–e30. [Google Scholar] [CrossRef]
- Raabe, J.; Pulwey, R.; Sattler, R.; Schweinbock, T.; Zweck, J.; Weiss, D. Magnetization pattern of ferromagnetic nanodisks. J. Appl. Phys. 2000, 88, 4437. [Google Scholar] [CrossRef]
- Puntes, V.F.; Krishnan, K.M.; Alivisatos, A.P. Colloidal Nanocrystal Shape and Size Control: The Case of Cobalt. Science 2001, 291, 2115–2117. [Google Scholar] [CrossRef]
- Lin, T.; Shao, H.; Guo, Z.; Luo, J.; Hao, J. Size- and shape-controlled synthesis of monodisperse Co nanoparticles from cobalt acetate by thermal decomposition. Rare Met. 2009, 28, 241–244. [Google Scholar] [CrossRef]
- Dong, X.; Choi, C.; Kim, B. Chemical synthesis of Co nanoparticles by chemical vapor condensation. Scr. Mater. 2002, 47, 857–861. [Google Scholar] [CrossRef]
- Wang, Z.; Choi, C.; Kim, B.; Kim, J.; Zhang, Z. Microstructure and magnetic property of Fe–Co nanoparticles prepared by chemical vapor condensation process. J. Alloys Compd. 2003, 351, 319–323. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B. Synthesis of monodisperse cobalt nanocrystals and their assembly into magnetic superlattices. J. Appl. Phys. 1999, 85, 4325–4330. [Google Scholar] [CrossRef]
- Hou, Y.; Kondoh, H.; Ohta, T. Self-Assembly of Co Nanoplatelets into Spheres: Synthesis and Characterization. Chem. Mater. 2005, 17, 3994–3996. [Google Scholar] [CrossRef]
- Lin, X.M.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Temperature Dependence of Morphology and Magnetic Properties of Cobalt Nanoparticles Prepared by an Inverse Micelle Technique. Langmuir 1998, 14, 7140–7146. [Google Scholar] [CrossRef]
- Yuanchun, Q.; Yanbao, Z.; Zhishen, W. Preparation of cobalt oxide nanoparticles and cobalt powders by solvothermal process and their characterization. Mater. Chem. Phys. 2008, 110, 457–462. [Google Scholar] [CrossRef]
- Wiedwald, U.; Spasova, M.; Salabas, E.L.; Ulmeanu, M.; Farle, M.; Frait, Z.; Rodriguez, A.F.; Arvanitis, D.; Sobal, N.S.; Hilgendorff, M.; et al. Ratio of orbital-to-spin magnetic moment in Co core-shell nanoparticles. Phys. Rev. B 2003, 68, 064424. [Google Scholar] [CrossRef]
- Puntes, V.F.; Zanchet, D.; Erdonmez, C.K.; Alivisatos, A.P. Synthesis of hcp-Co nanodisks. J. Am. Chem. Soc. 2002, 124, 12874–12880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Deka, J.R.; Cheng, C.J.; Lu, N.F.; Saikia, D.; Yang, Y.C.; Kao, H.M. Synthesis of highly dispersed ultra-small cobalt nanoparticles within the cage-type mesopores of 3D cubic mesoporous silica via double agent reduction method for catalytic hydrogen generation. Appl. Surf. Sci. 2019, 470, 764–772. [Google Scholar] [CrossRef]
- Kotelnikova, A.; Zubar, T.; Vershinina, T.; Panasyuk, M.; Kanafyev, O.; Fedkin, V.; Kubasov, I.; Turutin, A.; Trukhanov, S.; Tishkevich, D.; et al. The influence of saccharin adsorption on NiFe alloy film growth mechanisms during electrodeposition. RSC Adv. 2022, 12, 35722–35729. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zdorovets, M. Synthesis, structural, strength and corrosion properties of thin films of the type CuX (X = Bi, Mg, Ni). J. Mater. Sci. Mater. Electron. 2019, 30, 11819–11832. [Google Scholar] [CrossRef]
- Schiavi, P.; Altimari, P.; Pagnanelli, F.; Moscardini, E.; Toro, L. Synthesis of cobalt nanoparticles by electrodeposition onto aluminium foils. Chem. Eng. Trans. 2015, 43, 673–678. [Google Scholar]
- Ma, X.; Ma, Y.; Nolan, A.M.; Bai, J.; Xu, W.; Mo, Y.; Chen, H. Understanding the Polymorphism of Cobalt Nanoparticles Formed in Electrodeposition─An In Situ XRD Study. ACS Mater. Lett. 2023, 5, 979–984. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Morsi, M.A.; Abdelrazek, A. Structural, Optical, Morphological and Electrical Properties of CoCl2-filled Poly(vinyl chloride-co-vinyl acetate-co-2-hydroxypropyl acrylate) Terpolymer. Silicon 2018, 10, 1697–1704. [Google Scholar] [CrossRef]
- Clifford, D.M.; Castano, C.E.; Rojas, J.V. Highly magnetic Co nanoparticles fabricated by X-ray radiolysis. Radiat. Phys. Chem. 2018, 144, 111–115. [Google Scholar] [CrossRef]
- Athawale, A.; Majumdar, M.; Singh, H.; Navinkiran, K. Synthesis of Cobalt Oxide Nanoparticles/Fibres in Alcoholic Medium using y-ray Technique. Def. Sci. J. 2010, 60, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Kwon, B.J.; Park, J.H.; Hur, M.G.; Yang, S.D.; Jung, H. γ-ray Radiation Induced Synthesis and Characterization of α-Cobalt Hydroxide Nanoparticles. Bull. Korean Chem. Soc. 2010, 31, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Ershov, B.G.; Sukhov, N.L.; Janata, E. Formation, Absorption Spectrum, and Chemical Reactions of Nanosized Colloidal Cobalt in Aqueous Solution. J. Phys. Chem. B 2000, 104, 6138–6142. [Google Scholar] [CrossRef]
- Alrehaily, L.M.; Joseph, J.M.; Biesinger, M.C.; Guzonas, D.A.; Wren, J.C. Gamma-radiolysis-assisted cobalt oxide nanoparticle formation. Phys. Chem. Chem. Phys. 2013, 15, 1014–1024. [Google Scholar] [CrossRef]
- Gharibshahi, E.; Saion, E.; Ashraf, A.; Gharibshahi, L. Size-controlled and optical properties of platinum nanoparticles by gamma radiolytic synthesis. Appl. Radiat. Isot. 2017, 130, 211–217. [Google Scholar] [CrossRef]
- Farag, M.M.M. Effect of Gamma Radiation on the Microbial Synthesis of Metal Nanoparticles. Indian J. Microbiol. 2013, 52, 66–69. [Google Scholar]
- Li, T.; Park, H.G.; Choi, S.H. γ-Irradiation-induced preparation of Ag and Au nanoparticles and their characterizations. Mater. Chem. Phys. 2007, 105, 325–330. [Google Scholar] [CrossRef]
- Hosny, A.M.S.; Kashef, M.T.; Rasmy, S.A.; Aboul-Magd, D.S.; El-Bazza, Z. Antimicrobial activity of silver nanoparticles synthesized using honey and gamma radiation against silver-resistant bacteria from wounds and burns. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045009. [Google Scholar] [CrossRef] [Green Version]
- Gharibshahi, E.; Saion, E.; Johnston, R.L.; Ashraf, A. Theory and experiment of optical absorption of platinum nanoparticles synthesized by gamma radiation. Appl. Radiat. Isot. 2019, 147, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, G.Y. Radiation methods in nanotechnology. J. Eng. Phys. Thermophys. 2011, 84, 947. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Mendez, U.O. Radiation Synthesis of Materials and Compounds; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Rojas, J.; Higgins, M.M.; Gonzalez, M.T.; Castano, C. Single step radiolytic synthesis of iridium nanoparticles onto graphene oxide. Appl. Surf. Sci. 2015, 357, 2087–2093. [Google Scholar] [CrossRef]
- Zhang, Z.; Nenoff, T.M.; Leung, K.; Ferreira, S.R.; Huang, J.Y.; Berry, D.T.; Provencio, P.P.; Stumpf, R. Room-temperature synthesis of Ag− Ni and Pd− Ni alloy nanoparticles. J. Phys. Chem. C 2010, 114, 14309–14318. [Google Scholar] [CrossRef]
- Grand, J.; Ferreira, S.R.; De Waele, V.; Mintova, S.; Nenoff, T.M. Nanoparticle Alloy Formation by Radiolysis. J. Phys. Chem. C 2018, 122, 12573–12588. [Google Scholar] [CrossRef]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.L.; Delcourt, M.O. Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New J. Chem. 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- Clifford, D.; Castano, C.; Rojas, J. Supported transition metal nanomaterials: Nanocomposites synthesized by ionizing radiation. Radiat. Phys. Chem. 2017, 132, 52–64. [Google Scholar] [CrossRef]
- Čubová, K.; Čuba, V. Synthesis of inorganic nanoparticles by ionizing radiation–A review. Radiat. Phys. Chem. 2019, 158, 153–164. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Chen, F.; Alemu, N.; Johnston, R.L. Collective plasmon modes in a compositionally asymmetric nanoparticle dimer. AIP Adv. 2011, 1, 032134. [Google Scholar] [CrossRef] [Green Version]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Liao, C.H.; Huang, C.W.; Wu, J.C.S. Hydrogen Production from Semiconductor-based Photocatalysis via Water Splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef] [Green Version]
- Alenaizi, R.; Radiman, S.; Rahman, I.A.; Mohamed, F. Zwitterionic betaine-cholesterol system: Effects of sonication duration and aging on vesicles stability. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 662–669. [Google Scholar] [CrossRef]
- ISO13321; Methods for Determination of Particle Size Distribution Part 8: Photon Correlation Spectroscopy. International Organisation for Standardisation (ISO): Geneva, Switzerland, 1996.
- Lawson, C.L.; Hanson, R.J. Solving Least Squares Problems; SIAM: Philadelphia, PA, USA, 1995. [Google Scholar]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, Y.; Banerjee, D.; Datta, A.; Das, S.; Guin, R.; Saha, A. Gamma irradiation route to synthesis of highly re-dispersible natural polymer capped silver nanoparticles. Radiat. Phys. Chem. 2010, 79, 1240–1246. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Vasiliev, A.N.; Balagurov, A.M.; Szymczak, H. Magnetic state of the structural separated anion-deficient La0.70Sr0.30MnO2.85 manganite. J. Exp. Theor. Phys. 2011, 113, 819–825. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Egizbek, K.; Zdorovets, M.V.; Ibragimova, M.; Shumskaya, A.; Rogachev, A.A.; Ignatovich, Z.V.; Kadyrzhanov, K. Evaluation of the efficiency of detection and capture of manganese in aqueous solutions of FeCeOx nanocomposites doped with Nb2O5. Sensors 2020, 20, 4851. [Google Scholar] [CrossRef]
- Abedini, A.; Larki, F.; Saion, E.; Zakaria, A.; Hussein, M.Z. Influence of dose and ion concentration on formation of binary Al–Ni alloy nanoclusters. Radiat. Phys. Chem. 2012, 81, 1653–1658. [Google Scholar] [CrossRef]
- Gharibshahi, E.; Saion, E. Influence of dose on particle size and optical properties of colloidal platinum nanoparticles. Int. J. Mol. Sci. 2012, 13, 14723–14741. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, K.H. Synthesis and spectroscopic investigation of cobalt oxide nanoparticles. Polym. Compos. 2016, 37, 1881–1885. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
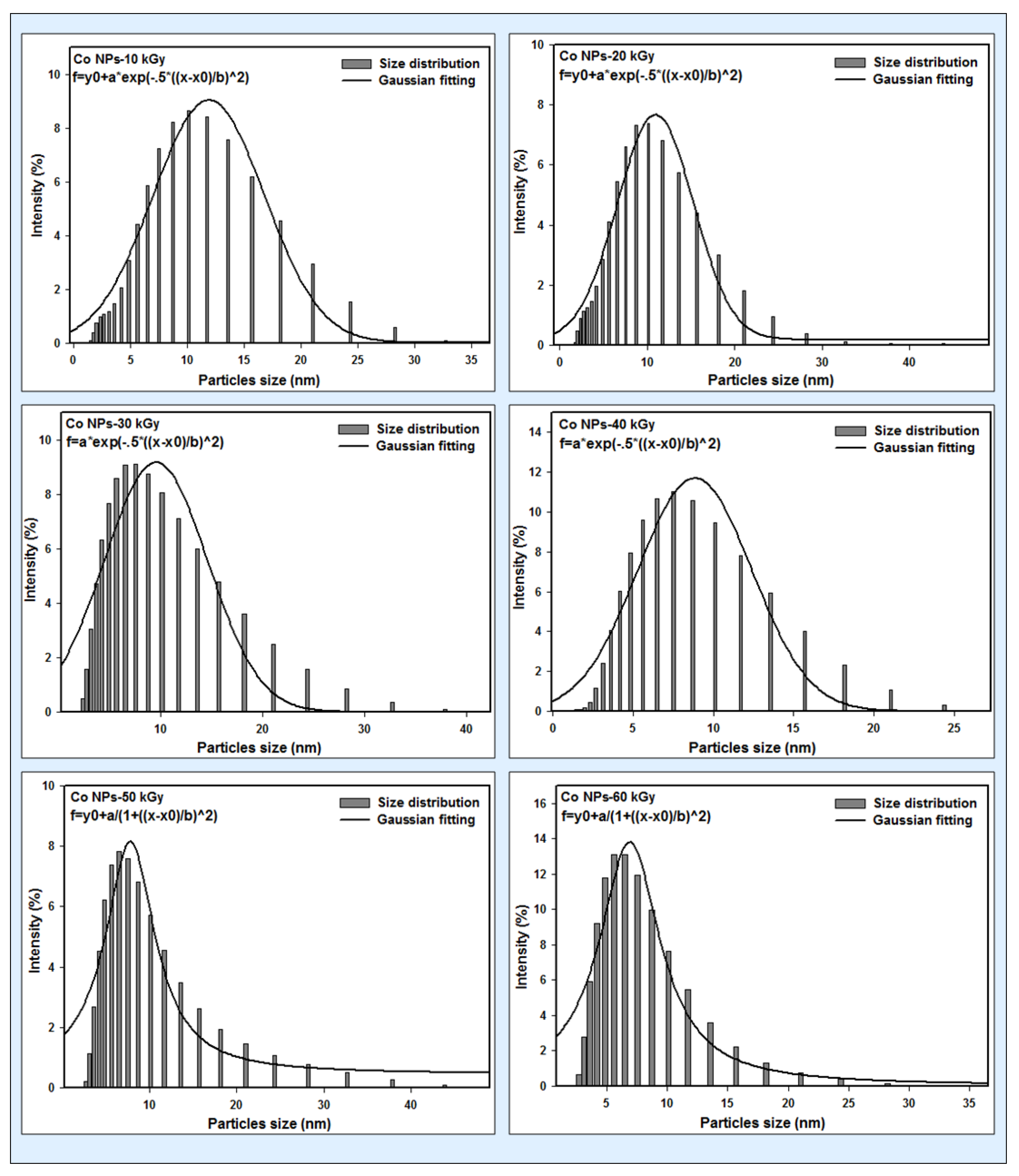

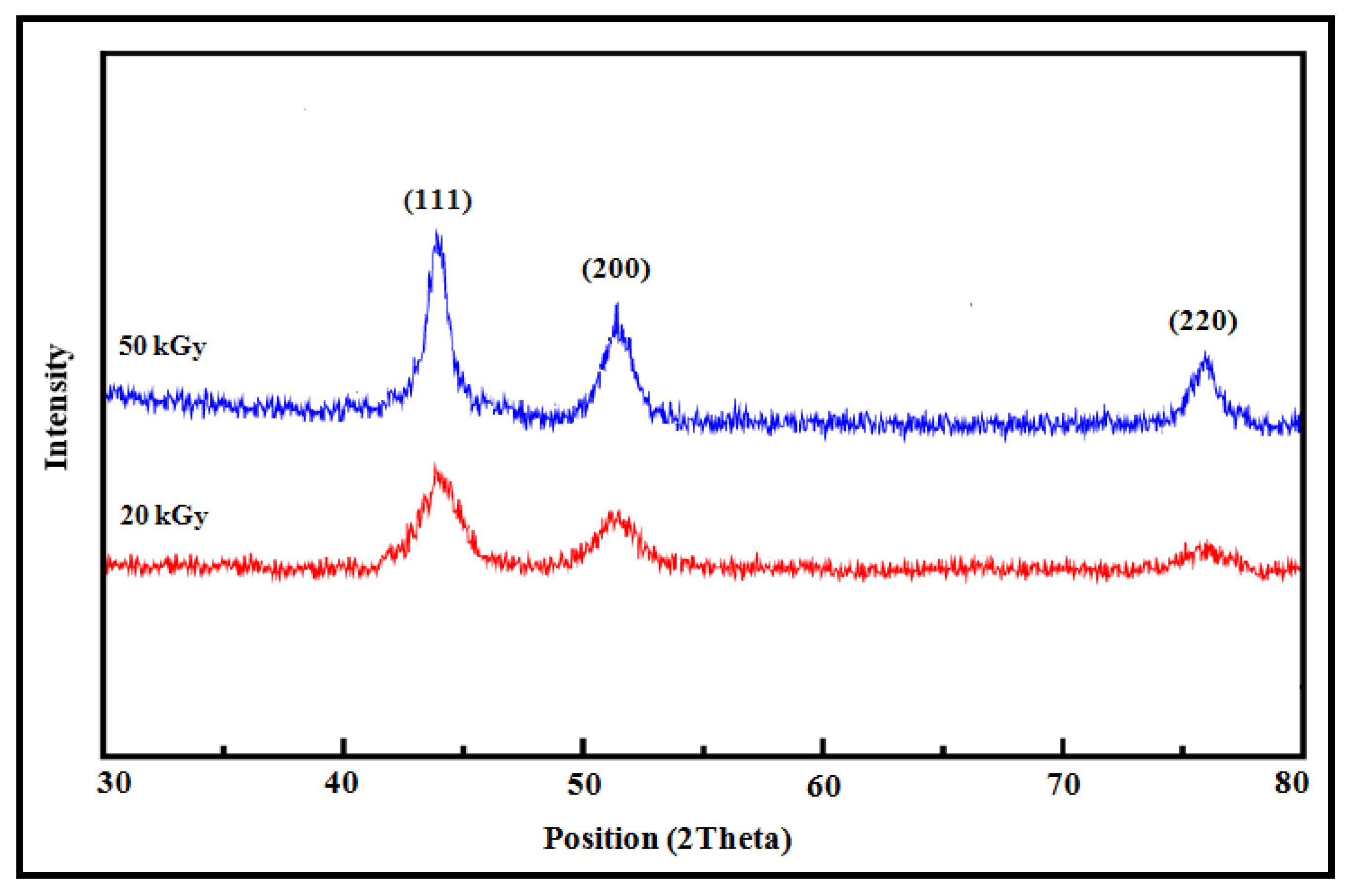
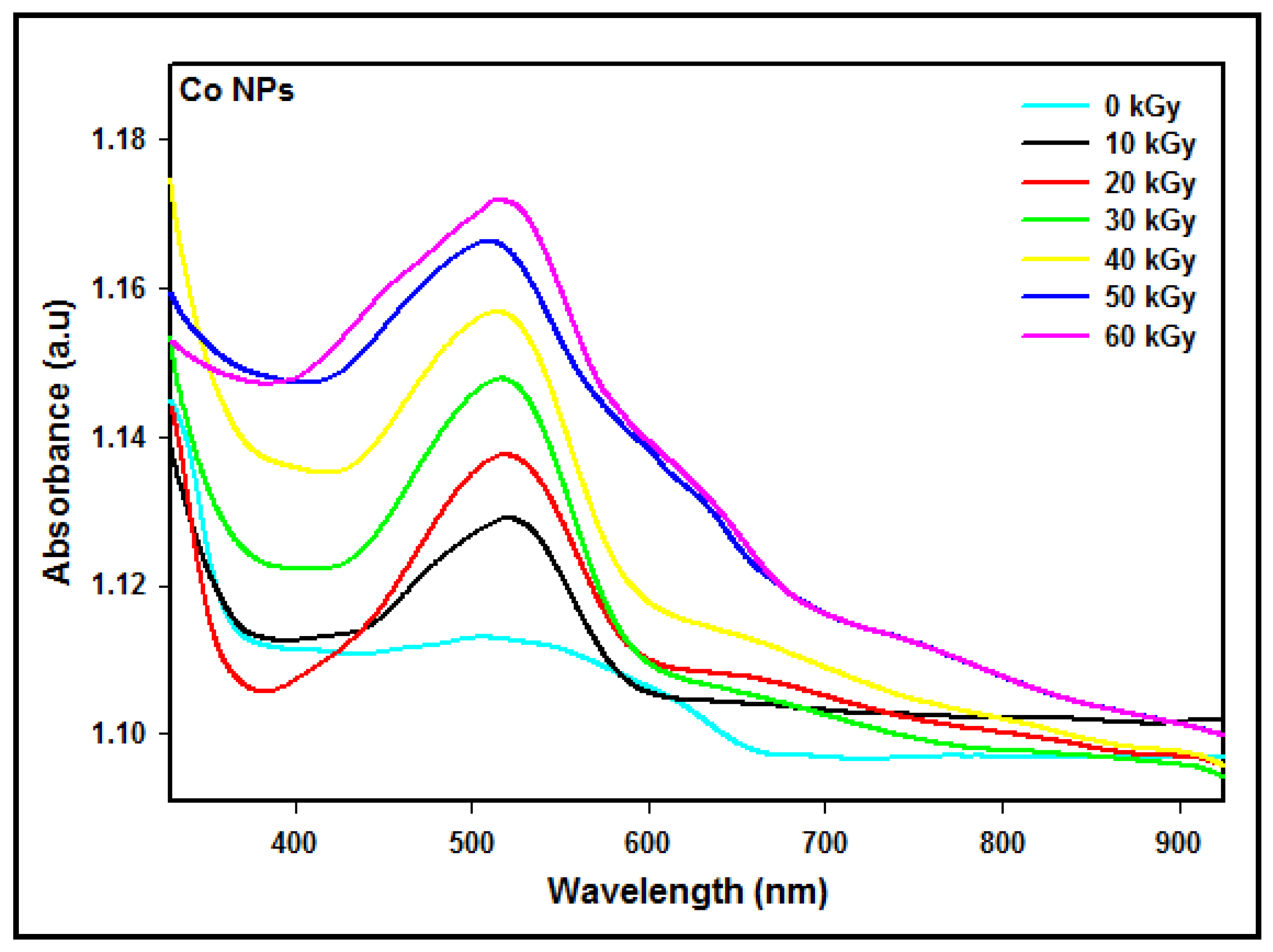

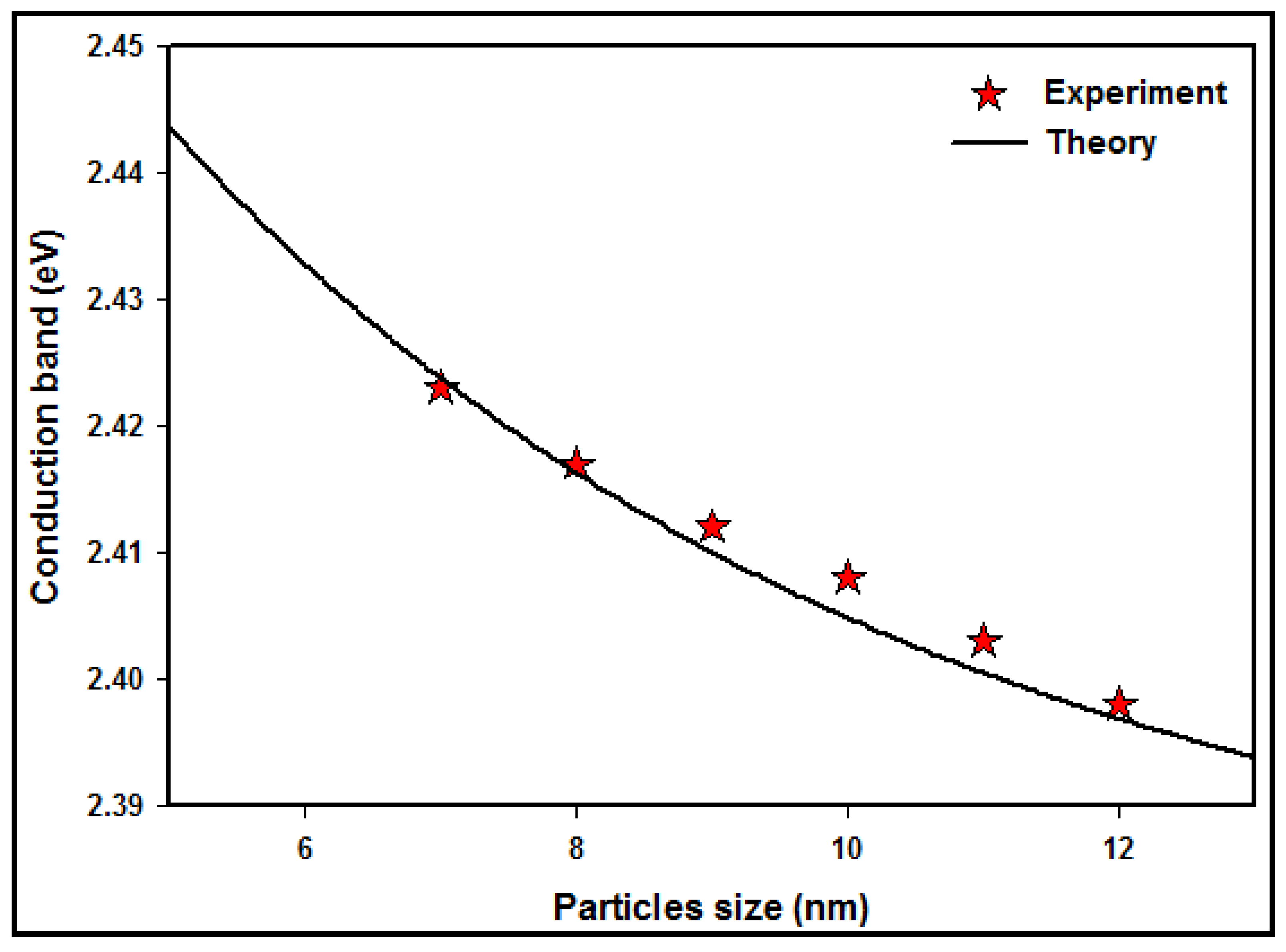
| Dose (kGy) | Particle Size (nm) | Absorption Peak λmax (nm) | Conduction Energy (eV) |
|---|---|---|---|
| 10 | 517 | 2.398 | |
| 20 | 516 | 2.403 | |
| 30 | 515 | 2.408 | |
| 40 | 514 | 2.412 | |
| 50 | 513 | 2.417 | |
| 60 | 512 | 2.423 |
| Particle Size (nm) | Absorption Peak λmax (nm) | Conduction Energy (eV) |
|---|---|---|
| 6 | 509.63 | 2.433 |
| 7 | 511.52 | 2.424 |
| 8 | 513.52 | 2.415 |
| 9 | 514.47 | 2.410 |
| 10 | 515.41 | 2.406 |
| 11 | 516.42 | 2.401 |
| 12 | 517.43 | 2.396 |
| Particle Size (nm) | Absorption Peak λmax (nm) | ||
|---|---|---|---|
| Experiment | Theory | Difference (%) | |
| 12 | 517 | 517.43 | 0.083 |
| 11 | 516 | 516.42 | 0.081 |
| 10 | 515 | 515.41 | 0.080 |
| 9 | 514 | 514.47 | 0.091 |
| 8 | 513 | 513.52 | 0.101 |
| 7 | 512 | 511.52 | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharibshahi, E.; Radiman, S.; Ashraf, A.; Saion, E.; Gharibshahi, L.; Ashraf, S. Simulation and Synthesis of Cobalt (Co) Nanoparticles by Gamma Radiation Technique. Micromachines 2023, 14, 1383. https://doi.org/10.3390/mi14071383
Gharibshahi E, Radiman S, Ashraf A, Saion E, Gharibshahi L, Ashraf S. Simulation and Synthesis of Cobalt (Co) Nanoparticles by Gamma Radiation Technique. Micromachines. 2023; 14(7):1383. https://doi.org/10.3390/mi14071383
Chicago/Turabian StyleGharibshahi, Elham, Shahidan Radiman, Ahmadreza Ashraf, Elias Saion, Leila Gharibshahi, and Sina Ashraf. 2023. "Simulation and Synthesis of Cobalt (Co) Nanoparticles by Gamma Radiation Technique" Micromachines 14, no. 7: 1383. https://doi.org/10.3390/mi14071383




