Application of 3D Bioprinting in Liver Diseases
Abstract
:1. Introduction
2. 3D Bioprinting Methods
| Inkjet | Extrusion | LAB | Vat Photopolymerization | References | |
|---|---|---|---|---|---|
| Speed | Fast | Slow | Medium | Fast | [51,52,53,54] |
| Cost | Low | Moderate | High | Low | [51,52,53,54] |
| Resolution | 50 μm | 100 μm | 10 μm | 1 μm | [55,56,57,58] |
| Cell viability | ~80% | >90% | <85% | >85% | [59,60,61,62] |
| Cell density | <106 cells/mL | Cell spheroids | <108 cells/mL | 108 Cells/mL | [63,64,65,66] |
| Structural Integrity | Low | High | Low | High | [67,68,69,70] |
3. Bioinks for 3D Bioprinting
3.1. Hydrogels
3.2. Cells
4. Applications of 3D Bioprinting
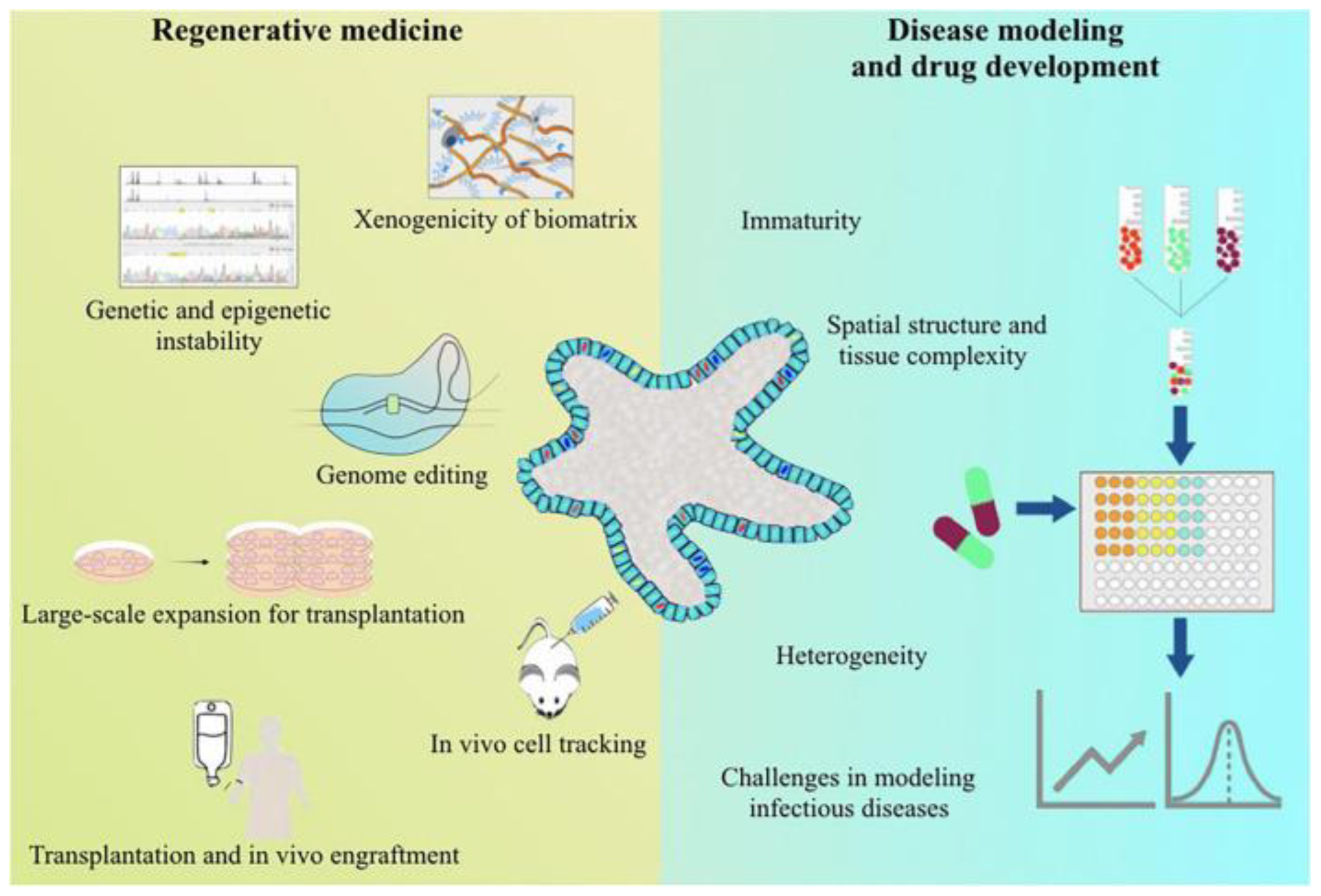
5. Discussion
6. Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Han, W.; Kim, H.; Ha, D.H.; Jang, J.; Kim, B.S.; Cho, D.W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Osna, N.A.; Kharbanda, K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J. Gastroenterol. 2017, 23, 6549–6570. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Gao, S.; Gang, J.; Yu, M.; Xin, G.; Tan, H. Computational analysis for identification of early diagnostic biomarkers and prognostic biomarkers of liver cancer based on GEO and TCGA databases and studies on pathways and biological functions affecting the survival time of liver cancer. BMC Cancer 2021, 21, 791. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A. International trends in liver cancer incidence rates. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2362–2368. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Christou, C.D.; Tsoulfas, G. Role of three-dimensional printing and artificial intelligence in the management of hepatocellular carcinoma: Challenges and opportunities. World J. Gastrointest. Oncol. 2022, 14, 765–793. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Zhu, X.; Ning, X.; Liu, W.; Wang, C.; Liu, X.; Zhao, D.; Zheng, Y.; Bao, J. Explainable liver tumor delineation in surgical specimens using hyperspectral imaging and deep learning. Biomed. Opt. Express 2021, 12, 4510–4529. [Google Scholar] [CrossRef]
- Yang, H.; Sun, L.; Pang, Y.; Hu, D.; Xu, H.; Mao, S.; Peng, W.; Wang, Y.; Xu, Y.; Zheng, Y.C.; et al. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut 2021, 70, 567–574. [Google Scholar] [CrossRef]
- Yao, L.; Hu, X.; Dai, K.; Yuan, M.; Liu, P.; Zhang, Q.; Jiang, Y. Mesenchymal stromal cells: Promising treatment for liver cirrhosis. Stem Cell Res. Ther. 2022, 13, 308. [Google Scholar] [CrossRef]
- Rifaioglu, A.S.; Nalbat, E.; Atalay, V.; Martin, M.J.; Cetin-Atalay, R.; Doğan, T. DEEPScreen: High performance drug-target interaction prediction with convolutional neural networks using 2-D structural compound representations. Chem. Sci. 2020, 11, 2531–2557. [Google Scholar] [CrossRef] [PubMed]
- Amoedo, N.D.; Obre, E.; Rossignol, R. Drug discovery strategies in the field of tumor energy metabolism: Limitations by metabolic flexibility and metabolic resistance to chemotherapy. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Feiner-Gracia, N.; Glinkowska Mares, A.; Buzhor, M.; Rodriguez-Trujillo, R.; Samitier Marti, J.; Amir, R.J.; Pujals, S.; Albertazzi, L. Real-Time Ratiometric Imaging of Micelles Assembly State in a Microfluidic Cancer-on-a-Chip. ACS Appl. Bio Mater. 2021, 4, 669–681. [Google Scholar] [CrossRef]
- Ma, L.; Wu, Y.; Li, Y.; Aazmi, A.; Zhou, H.; Zhang, B.; Yang, H. Current Advances on 3D-Bioprinted Liver Tissue Models. Adv. Healthc. Mater. 2020, 9, e2001517. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered Liver-on-a-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Nakamura, M.; Iwanaga, S.; Henmi, C.; Arai, K.; Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2010, 2, 014110. [Google Scholar] [CrossRef]
- Song, D.; Xu, Y.; Liu, S.; Wen, L.; Wang, X. Progress of 3D Bioprinting in Organ Manufacturing. Polymers 2021, 13, 3178. [Google Scholar] [CrossRef]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.X.; Watson, N.; Joh, D.Y.; Chilkoti, A.; Franklin, A.D. Aerosol jet printing of biological inks by ultrasonic delivery. Biofabrication 2020, 12, 025004. [Google Scholar] [CrossRef]
- Agarwal, T.; Banerjee, D.; Konwarh, R.; Esworthy, T.; Kumari, J.; Onesto, V.; Das, P.; Lee, B.H.; Wagener, F.A.D.T.G.; Makvandi, P.; et al. Recent advances in bioprinting technologies for engineering hepatic tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112013. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef]
- Arai, K.; Yoshida, T.; Okabe, M.; Goto, M.; Mir, T.A.; Soko, C.; Tsukamoto, Y.; Akaike, T.; Nikaido, T.; Zhou, K.; et al. Fabrication of 3D-culture platform with sandwich architecture for preserving liver-specific functions of hepatocytes using 3D bioprinter. J. Biomed. Mater. Res. A 2017, 105, 1583–1592. [Google Scholar] [CrossRef]
- Moya, A.; Ortega-Ribera, M.; Guimerà, X.; Sowade, E.; Zea, M.; Illa, X.; Ramon, E.; Villa, R.; Gracia-Sancho, J.; Gabriel, G. Online oxygen monitoring using integrated inkjet-printed sensors in a liver-on-a-chip system. Lab Chip 2018, 18, 2023–2035. [Google Scholar] [CrossRef]
- Jones, N. Science in three dimensions: The print revolution. Nature 2012, 487, 22–23. [Google Scholar] [CrossRef]
- Henry, S.; De Wever, L.; Vanhoorne, V.; De Beer, T.; Vervaet, C. Influence of Print Settings on the Critical Quality Attributes of Extrusion-Based 3D-Printed Caplets: A Quality-by-Design Approach. Pharmaceutics 2021, 13, 2068. [Google Scholar] [CrossRef]
- Bouwmeester, M.C.; Bernal, P.N.; Oosterhoff, L.A.; van Wolferen, M.E.; Lehmann, V.; Vermaas, M.; Buchholz, M.B.; Peiffer, Q.C.; Malda, J.; van der Laan, L.J.W.; et al. Bioprinting of Human Liver-Derived Epithelial Organoids for Toxicity Studies. Macromol. Biosci. 2021, 21, e2100327. [Google Scholar] [CrossRef] [PubMed]
- Cuvellier, M.; Ezan, F.; Oliveira, H.; Rose, S.; Fricain, J.C.; Langouët, S.; Legagneux, V.; Baffet, G. 3D Culture of HepaRG cells in GelMa and its application to bioprinting of a multicellular hepatic model. Biomaterials 2021, 269, 120611. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, S.; Khondkar, S.; Ilyas, A. Bioprinting of Stem Cells in Multimaterial Scaffolds and Their Applications in Bone Tissue Engineering. Sensors 2021, 21, 7477. [Google Scholar] [CrossRef]
- Kryou, C.; Leva, V.; Chatzipetrou, M.; Zergioti, I. Bioprinting for Liver Transplantation. Bioengineering 2019, 6, 95. [Google Scholar] [CrossRef]
- Touya, N.; Devun, M.; Handschin, C.; Casenave, S.; Ahmed Omar, N.; Gaubert, A.; Dusserre, N.; De Oliveira, H.; Kérourédan, O.; Devillard, R. In vitroandin vivocharacterization of a novel tricalcium silicate-based ink for bone regeneration using laser-assisted bioprinting. Biofabrication 2022, 14, 1758–5090. [Google Scholar] [CrossRef] [PubMed]
- Nakielski, P.; Rinoldi, C.; Pruchniewski, M.; Pawłowska, S.; Gazińska, M.; Strojny, B.; Rybak, D.; Jezierska-Woźniak, K.; Urbanek, O.; Denis, P.; et al. Laser-Assisted Fabrication of Injectable Nanofibrous Cell Carriers. Small 2022, 18, e2104971. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control Release 2021, 329, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, U.; Rossegger, E.; Schlögl, S. A Review of Multi-Material 3D Printing of Functional Materials via Vat Photopolymerization. Polymers 2022, 14, 2449. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Kumar, H.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent. Ann. Biomed. Eng. 2020, 48, 1955–1970. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Ajiteru, O.; Hong, H.; Suh, Y.J.; Sultan, M.T.; Lee, H.; Lee, J.S.; Lee, Y.J.; Lee, O.J.; Kim, S.H.; et al. A digital light processing 3D-printed artificial skin model and full-thickness wound models using silk fibroin bioink. Acta Biomater. 2023, 164, 159–174. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Shiwarski, D.J.; Hudson, A.R.; Tashman, J.W.; Feinberg, A.W. Emergence of FRESH 3D Printing as a Platform for Advanced Tissue Biofabrication. APL Bioeng. 2021, 5, 010904. [Google Scholar] [CrossRef] [PubMed]
- Mirdamadi, E.; Tashman, J.W.; Shiwarski, D.J.; Palchesko, R.N.; Feinberg, A.W. FRESH 3D Bioprinting a Full-Size Model of the Human Heart. ACS Biomater. Sci. Eng. 2020, 6, 6453–6459. [Google Scholar] [CrossRef]
- Alison, L.; Menasce, S.; Bouville, F.; Tervoort, E.; Mattich, I.; Ofner, A. 3D printing of sacrificial templates into hierarchical porous materials. Sci. Rep. 2019, 9, 409. [Google Scholar] [CrossRef]
- Ren, B.; Song, K.; Sanikommu, A.R.; Chai, Y.; Longmire, M.A.; Chai, W. Study of sacrificial ink-assisted embedded printing for 3D perfusable channel creation for biomedical applications. Appl. Phys. Rev. 2020, 9, 011408. [Google Scholar] [CrossRef]
- Hölken, I.; Neubüser, G.; Postica, V.; Bumke, L.; Lupan, O. Sacrificial Template Synthesis and Properties of 3D Hollow-Silicon Nano- and Microstructures. ACS Appl. Mater. Interfaces 2016, 8, 20491–20498. [Google Scholar] [CrossRef]
- Cheng, F.; Cao, X.; Li, H.; Liu, T.; Xie, X.; Huang, D. Generation of Cost-Effective Paper-Based Tissue Models through Matrix-Assisted Sacrificial 3D Printing. Nano Lett. 2019, 19, 3603–3611. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; De Wijn, J.R.; Verbout, A.J.; Alblas, J.; Dhert, W.J. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng. 2008, 14, 127–133. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Yu, Y. Bioprinting Toward Organ Fabrication: Challenges and Future Trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef]
- Nahmias, Y.; Schwartz, R.E.; Verfaillie, C.M.; Odde, D.J. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol. Bioeng. 2005, 92, 129–136. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Pflaum, M.; Hess, C.; Diamantouros, S.; Schlie, S.; Deiwick, A.; Koch, L.; Wilhelmi, M.; Jockenhoevel, S.; Haverich, A.; et al. Laser printing of three-dimensional multicellular arrays for studies of cell-cell and cell-environment interactions. Tissue Eng. Part C Methods 2011, 17, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Starly, B.; Nam, J.; Darling, A. Bio-CAD modeling and its applications in computer-aided tissue engineering. Comput.-Aided Des. 2005, 37, 1097–1114. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Lanza, R.; Mikos, T.; Nerem, R. Principles of Regenerative Medicine, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 401–416. [Google Scholar]
- Zhang, A.P.; Qu, X.; Soman, P.; Hribar, K.C.; Lee, J.W.; Chen, S.; He, S. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv. Mater. 2012, 24, 4266–4270. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Kang, H.W.; Seol, Y.J.; Forsythe, S.D.; Bishop, C.; Shupe, T.; Soker, S.; Atala, A. Bioprinting Cellularized Constructs Using a Tissue-specific Hydrogel Bioink. J. Vis. Exp. JoVE 2016, 110, e53606. [Google Scholar]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Deubel, M.; von Freymann, G.; Wegener, M.; Pereira, S.; Busch, K.; Soukoulis, C.M. Direct laser writing of three-dimensional photonic-crystal templates for telecommunications. Nat. Mater. 2004, 3, 444–447. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.B.; Ahn, S.H.; Lee, J.S.; Jang, C.H.; Yoon, H.; Chun, W.; Kim, G.H. A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv. Healthc. Mater. 2015, 4, 1359–1368. [Google Scholar] [CrossRef]
- Dang, J.; He, H.; Chen, D.; Yin, L. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef]
- Lee, J.M.; Sing, S.L.; Zhou, M.; Yeong, W.Y. 3D bioprinting processes: A perspective on classification and terminology. Int. J. Bioprint. 2018, 4, 151. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q. Enzyme-Laden Bioactive Hydrogel for Biocatalytic Monitoring and Regulation. Acc. Chem. Res. 2021, 54, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Chen, G. Research progress of natural tissue-derived hydrogels for tissue repair and reconstruction. Int. J. Biol. Macromol. 2022, 214, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Muscolino, E.; Di Stefano, A.B.; Trapani, M.; Sabatino, M.A.; Giacomazza, D.; Moschella, F.; Cordova, A.; Toia, F.; Dispenza, C. Injectable xyloglucan hydrogels incorporating spheroids of adipose stem cells for bone and cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112545. [Google Scholar] [CrossRef]
- Jose, G.; Shalumon, K.T.; Chen, J.P. Natural Polymers Based Hydrogels for Cell Culture Applications. Curr. Med. Chem. 2020, 27, 2734–2776. [Google Scholar] [CrossRef] [PubMed]
- Turković, E.; Vasiljević, I.; Drašković, M.; Obradović, N.; Vasiljević, D.; Parojčić, J. An Investigation into Mechanical Properties and Printability of Potential Substrates for Inkjet Printing of Orodispersible Films. Pharmaceutics. 2021, 13, 468. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Cao, D.; Xie, Y.; Song, J. DNA Hydrogels in the Perspective of Mechanical Properties. Macromol. Rapid Commun. 2022, 43, e2200281. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nagaki, A. Anionic Polymerization Using Flow Microreactors. Molecules 2019, 24, 1532. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Tabasum, S.; Younas, M.; Zaeem, M.A.; Majeed, I.; Majeed, M.; Noreen, A.; Iqbal, M.N.; Zia, K.M. A review on blending of corn starch with natural and synthetic polymers, and inorganic nanoparticles with mathematical modeling. Int. J. Biol. Macromol. 2019, 122, 969–996. [Google Scholar] [CrossRef]
- Guagliano, G.; Volpini, C.; Briatico-Vangosa, F.; Cornaglia, A.I.; Visai, L.; Petrini, P. Toward 3D-Bioprinted Models of the Liver to Boost Drug Development. Macromol. Biosci. 2022, 22, e2200264. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and its applications in tissue engineering and regenera tive medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, X.; Chen, J.L.; Shen, W.L.; Hieu Nguyen, T.M.; Gao, L.; Ouyang, H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 2010, 31, 2163–2175. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, H.; Gao, G.; Jang, J.; Cho, D.W. Decellularized extracellular matrix: A step towards the next generation source for bioink manufacturing. Biofabrication 2017, 9, 034104. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.C.; Al-Zeer, M.A.; Kurtz, A.; Hocke, A.C.; et al. Generation of a 3D Liver Model Comprising Human Extracellular Matrix in an Alginate/Gelatin-Based Bioink by Extrusion Bioprinting for Infection and Transduction Studies. Int. J. Mol. Sci. 2018, 19, 3129. [Google Scholar] [CrossRef]
- Xu, J.; Yang, S.; Su, Y.; Hu, X.; Xi, Y.; Cheng, Y.Y.; Kang, Y.; Nie, Y.; Pan, B.; Song, K. A 3D bioprinted tumor model fabricated with gelatin/sodium alginate/decellularized extracellular matrix bioink. Int. J. Bioprint. 2023, 9, 630. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, Y.J.; Yong, W.J.; Pati, F.; Shim, J.H.; Kang, K.S.; Kang, I.H.; Park, J.; Cho, D.W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2016, 8, 015007. [Google Scholar] [CrossRef]
- Kim, M.K.; Jeong, W.; Lee, S.M.; Kim, J.B.; Jin, S.; Kang, H.W. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication 2020, 12, 025003. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Gao, Q.; He, Y.; Fu, J.Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutriaents delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Wang, Y.; Li, Y.; Juengpanich, S.; Li, W.; Chen, M.; Yin, J.; Fu, J.; Cai, X. Fabrication of liver mi crotissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater. Sci. Eng. C 2020, 109, 110625. [Google Scholar] [CrossRef] [PubMed]
- Khati, V.; Ramachandraiah, H.; Pati, F.; Svahn, H.A.; Gaudenzi, G.; Russom, A. 3D Bioprinting of Multi-Material Decellularized Liver Matrix Hydrogel at Physiological Temperatures. Biosensors 2022, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wenger, A.; Golzar, H.; Tang, X.S. 3D bioprinting of bicellular liver lobule-mimetic structures via microextrusion of cellulose nanocrystal-incorporated shear-thinning bioink. Sci. Rep. 2020, 10, 20648. [Google Scholar] [CrossRef]
- Janani, G.; Priya, S.; Dey, S.; Mandal, B.B. Mimicking Native Liver Lobule Microarchitecture In Vitro with Parenchymal and Non-parenchymal Cells Using 3D Bioprinting for Drug Toxicity and Drug Screening Applications. ACS Appl. Mater. Interfaces 2022, 14, 10167–10186. [Google Scholar] [CrossRef]
- Taymour, R.; Kilian, D.; Ahlfeld, T.; Gelinsky, M.; Lode, A. 3D bioprinting of hepatocytes: Core-shell structured co-cultures with fibroblasts for enhanced functionality. Sci. Rep. 2021, 11, 5130. [Google Scholar] [CrossRef]
- Taymour, R.; Chicaiza-Cabezas, N.A.; Gelinsky, M.; Lode, A. Core-shell bioprinting of vascularizedin vitroliver si-nusoid models. Biofabrication 2022, 14, 1758–5090. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Pang, Y.; Yu, H.; Qin, X.; Su, K.; Xu, T.; Ren, H. Bioprinting of a Hepatic Tissue Model Using Human-Induced Pluripotent Stem Cell-derived Hepatocytes for Drug-Induced Hepatotoxicity Evaluation. Int. J. Bioprint. 2022, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Berg, J.; Roehrs, V.; Kurreck, J.; Al-Zeer, M.A. 3D-bioprinted HepaRG cultures as a model for testing long term aflatoxin B1 toxicity in vitro. Toxicol. Rep. 2020, 7, 1578–1587. [Google Scholar] [CrossRef]
- Sun, L.; Yang, H.; Wang, Y.; Zhang, X.; Jin, B.; Xie, F.; Jin, Y.; Pang, Y.; Zhao, H.; Lu, X.; et al. Application of a 3D Bioprinted Hepatocellular Carcinoma Cell Model in Antitumor Drug Research. Front. Oncol. 2020, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Grix, T.; Ruppelt, A.; Thomas, A.; Amler, A.K.; Noichl, B.P.; Lauster, R.; Kloke, L. Bioprinting Perfusion-Enabled Liver Equivalents for Advanced Organ-on-a-Chip Applications. Genes 2018, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, C.; Wang, P.; Xu, W.; Wan, X.; Lai, C.S.E.; Liu, J.; Koroleva-Maharajh, A.; Chen, S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials. 2018, 185, 310–321. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.; Gou, M.; Xu, Y.; et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, K.; Yoon, S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Lee, S.B.; Ryu, K.Y.; Jeong, J.; Choi, D. Prolongation of liver-specific function for primary hepatocytes maintenance in 3D printed architectures. Organogenesis 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.G.; Funk, J.; Robbins, J.B.; Crogan-Grundy, C.; Presnell, S.C.; Singer, T.; Roth, A.B. Bioprinted 3D Primary Liver Tissues Allow Assessment of Organ-Level Response to Clinical Drug Induced Toxicity In Vitro. PLoS ONE 2016, 11, e0158674. [Google Scholar] [CrossRef]
- Chen, Y.; Wong, P.P.; Sjeklocha, L.; Steer, C.J.; Sahin, M.B. Mature hepatocytes exhibit unexpected plasticity by direct dedifferentiation into liver progenitor cells in culture. Hepatology 2012, 55, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yoon, H.; Yoon, D.; Kim, G.H.; Yang, H.T.; Chun, W. Development of hepatic blocks using human adipose tissue-derived stem cells through three-dimensional cell printing techniques. J. Mater. Chem. B 2017, 5, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Kim, Y.; Jeon, H.; Lee, S.B.; Kim, J.S.; Park, S.A.; Kim, W.D.; Yang, H.M.; Kim, S.J.; Jeong, J.; et al. Three-Dimensional Bioprinting of Hepatic Structures with Directly Converted Hepatocyte-Like Cells. Tissue Eng. Part A 2018, 24, 576–583. [Google Scholar] [CrossRef]
- Higuchi, Y.; Kawai, K.; Kanaki, T.; Yamazaki, H.; Chesné, C.; Guguen-Guillouzo, C.; Suemizu, H. Functional polymer-dependent 3D culture accelerates the differentiation of HepaRG cells into mature hepatocytes. Hepatol. Res. 2016, 46, 1045–1057. [Google Scholar] [CrossRef]
- Feng, L.; Liang, S.; Zhou, Y.; Luo, Y.; Chen, R.; Huang, Y.; Chen, Y.; Xu, M.; Yao, R. Three-Dimensional Printing of Hydrogel Scaffolds with Hierarchical Structure for Scalable Stem Cell Culture. ACS Biomater. Sci. Eng. 2020, 6, 2995–3004. [Google Scholar] [CrossRef]
- Ide, I.; Nagao, E.; Kajiyama, S.; Mizoguchi, N. A novel evaluation method for determining drug-induced hepatotoxicity using 3D bio-printed human liver tissue. Toxicol. Mech. Methods 2020, 30, 189–196. [Google Scholar] [CrossRef]
- Pandey, E.; Nour, A.S.; Harris, E.N. Prominent Receptors of Liver Sinusoidal Endothelial Cells in Liver Homeostasis and Disease. Front. Physiol. 2020, 11, 873. [Google Scholar] [CrossRef]
- Massa, S.; Sakr, M.A.; Seo, J.; Bandaru, P.; Arneri, A.; Bersini, S.; Zare-Eelanjegh, E.; Jalilian, E.; Cha, B.H.; Antona, S.; et al. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 2017, 11, 044109. [Google Scholar] [CrossRef]
- Okamoto, N.; Ohama, H.; Matsui, M.; Fukunishi, S.; Higuchi, K.; Asai, A. Hepatic F4/80 (+) CD11b (+) CD68 (−) cells influence the antibacterial response in irradiated mice with sepsis by Enterococcus faecalis. J. Leukoc. Biol. 2021, 109, 943–952. [Google Scholar] [CrossRef]
- Scott-Conner, C.E.; Grogan, J.B. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J. Surg. Res. 1994, 57, 316–336. [Google Scholar] [CrossRef] [PubMed]
- Norona, L.M.; Nguyen, D.G.; Gerber, D.A.; Presnell, S.C.; Mosedale, M.; Watkins, P.B. Bioprinted liver provides early insight into the role of Kupffer cells in TGF-β1 and methotrexate-induced fibrogenesis. PLoS ONE 2019, 14, e0208958. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Zhang, Y.; Lv, M.; Zang, G.; Ng, S.S.; Chen, X. Advances in 3D cell culture for liver preclinical studies. Acta Biochim. Biophys. Sin. 2021, 53, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Moghaddam, K.M.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Guvendiren, M. Complex 3D bioprinting methods. APL Bioeng. 2021, 5, 011508. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cotton, K.; Samarasekera, D.; Khetani, S.R. Engineered Platforms for Maturing Pluripotent Stem Cell-Derived Liver Cells for Disease Modeling. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 1147–1160. [Google Scholar] [CrossRef]
- Satpathy, A.; Datta, P.; Wu, Y.; Ayan, B.; Bayram, E.; Ozbolat, I.T. Developments with 3D bioprinting for novel drug discovery. Expert Opin. Drug Discov. 2018, 13, 1115–1129. [Google Scholar] [CrossRef]
- Yang, W.; Xia, R.; Zhang, Y.; Zhang, H.; Bai, L. Decellularized Liver Scaffold for Liver Regeneration. Methods Mol. Biol. 2018, 1577, 11–23. [Google Scholar]
- Kamimura, H.; Ito, S. Assessment of chimeric mice with humanized livers in new drug development: Generation of pharmacokinetics, metabolism and toxicity data for selecting the final candidate compound. Xenobiotica 2016, 46, 557–569. [Google Scholar] [CrossRef]
- van Grunsven, L.A. 3D in vitro models of liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Sun, L.; Pang, Y.; Xu, G.; Jin, B.; Xu, H.; Lu, X.; Xu, Y.; Du, S.; Wang, Y.; et al. Three-dimensional bio-printing of primary human hepatocellular carcinoma for personalized medicine. Biomaterials 2021, 265, 120416. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, Z.; Lu, X.; Dai, J.; Zhou, C.; Yan, J.; Wang, L.; Wang, Z.; Zang, J. Minimally invasive bioprinting for in situ liver regeneration. Bioact. Mater. 2023, 26, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Li, X.; Dong, Q.; Tian, S.; Bai, S. In vitro construction of liver organoids with biomimetic lobule structure by a multicellular 3D bioprinting strategy. Cell Prolif. 2023, 56, e13465. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.G.; Choi, Y.M.; Jang, J. 3D Bioprinting-Based Vascularized Tissue Models Mimicking Tissue-Specific Architecture and Pathophysiology for in vitro Studies. Front. Bioeng. Biotechnol. 2021, 9, 685507. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, X.; Ma, Z.; Sun, X.; Zhang, Y.; Li, W.; Yang, H.; Qiang, L.; Yang, Z.; Liu, Y.; et al. Developments and Opportunities for 3D Bioprinted Organoids. Int. J. Bioprint. 2021, 7, 364. [Google Scholar] [CrossRef]
- Wang, J.Z.; Xiong, N.Y.; Zhao, L.Z.; Hu, J.T.; Kong, D.C. Review fantastic medical implications of 3D-printing in liver surgeries, liver regeneration, liver transplantation and drug hepatotoxicity testing: A review. Int. J. Surg. 2018, 56, 1–6. [Google Scholar] [CrossRef]
- Miri, A.K.; Nieto, D.; Iglesias, L.; Goodarzi Hosseinabadi, H.; Maharjan, S.; Ruiz-Esparza, G.U.; Khoshakhlagh, P.; Manbachi, A.; Dokmeci, M.R.; Chen, S.; et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv. Mater. 2018, 30, e1800242. [Google Scholar] [CrossRef]
- Robbins, J.B.; Gorgen, V.; Min, P.; Shepherd, B.R.; Presnell, S.C. A novel in vitro three-dimensional bioprinted liver tissue system for drug development. FASEB J. 2013, 27, 872812. [Google Scholar] [CrossRef]
- Robbins, J.B.; O’Neill, C.M.; Gorgen, V.; Shepherd, B.R.; Presnell, S.C. Bioprinted three dimensional (3D) human liver constructs provide a model for interrogating liver biology. Mol. Biol. Cell. 2013, 24, 2176. [Google Scholar]
- Visk, D. Will advances in Preclinical In Vitro Models lower the costs of drug development? Appl. In Vitro Toxicol. 2015, 1, 79–82. [Google Scholar] [CrossRef]
- Ribeiro, A.J.S.; Yang, X.; Patel, V.; Madabushi, R.; Strauss, D.G. Liver Microphysiological Systems for Predicting and Evaluating Drug Effects. Clin. Pharmacol. Ther. 2019, 106, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, J.; Lee, J.S.; Min, S.; Kim, S.; Ahn, D.H. Vascularized liver organoids generated using induced hepatic tissue and dynamic liver-specific microenvironment as a drug testing platform. Adv. Funct. Mater. 2018, 28, 1801954. [Google Scholar] [CrossRef]
- Chang, M.; Bogacheva, M.S.; Lou, Y.R. Challenges for the Applications of Human Pluripotent Stem Cell-Derived Liver Organoids. Front. Cell Dev. Biol. 2021, 9, 748576. [Google Scholar] [CrossRef] [PubMed]
- Underhill, G.H.; Khetani, S.R. Bioengineered Liver Models for Drug Testing and Cell Differentiation Studies. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 426–439. [Google Scholar] [CrossRef]
- Chen, L.; Wei, X.; Gu, D.; Xu, Y.; Zhou, H. Human liver cancer organoids: Biological applications, current challenges, and prospects in hepatoma therapy. Cancer Lett. 2023, 555, 216048. [Google Scholar] [CrossRef]
- Hong, G.; Kim, J.; Oh, H.; Yun, S.; Kim, C.M.; Jeong, Y.M.; Yun, W.S.; Shim, J.H.; Jang, I.; Kim, C.Y.; et al. Production of Multiple Cell-Laden Microtissue Spheroids with a Biomimetic Hepatic-Lobule-Like Structure. Adv. Mater. 2021, 33, e2102624. [Google Scholar] [CrossRef] [PubMed]
- Kizawa, H.; Nagao, E.; Shimamura, M.; Zhang, G.; Torii, H. Scaffold-free 3D bio-printed human liver tissue stably maintains metabolic functions useful for drug discovery. Biochem. Biophys. Rep. 2017, 10, 186–191. [Google Scholar] [CrossRef]
- Kang, H.K.; Sarsenova, M.; Kim, D.H.; Kim, M.S.; Lee, J.Y.; Sung, E.A.; Kook, M.G.; Kim, N.G.; Choi, S.W.; Ogay, V.; et al. Establishing a 3D In Vitro Hepatic Model Mimicking Physiologically Relevant to In Vivo State. Cells 2021, 10, 1268. [Google Scholar] [CrossRef]
- Kang, D.; Hong, G.; An, S.; Jang, I.; Yun, W.S.; Shim, J.H.; Jin, S. Bioprinting of Multiscaled Hepatic Lobules within a Highly Vascularized Construct. Small 2020, 16, e1905505. [Google Scholar] [CrossRef]
- Tasnim, N.; De la Vega, L.; Anil Kumar, S.; Abelseth, L.; Alonzo, M.; Amereh, M.; Joddar, B.; Willerth, S.M. 3D Bioprinting Stem Cell Derived Tissues. Cell Mol. Bioeng. 2018, 11, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Cuvellier, M.; Rose, S.; Ezan, F.; Jarry, U.; de Oliveira, H.; Bruyère, A.; Drieu La Rochelle, C.; Legagneux, V.; Langouët, S.; Baffet, G. In vitrolong term differentiation and functionality of three-dimensional bioprinted primary human hepatocytes: Application forin vivoengraftment. Biofabrication 2022, 14, 035021. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Zhang, L.; Sun, L.; Wang, H.; Zhao, H.; Zhang, Z.; Liu, W.; Huang, Y.; Ji, S.; et al. 3D Liver Tissue Model with Branched Vascular Networks by Multimaterial Bioprinting. Adv. Healthc. Mater. 2021, 10, e2101405. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.B., Jr.; Steffick, D.E.; Guidinger, M.K.; Farmer, D.G.; Berg, C.L.; Merion, R.M. Liver and intestine transplantation in the United States, 1997–2006. Am. J. Transplant. 2008, 8, 958–976. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D Bioprinting of Human Tissues: Biofabrication, Bioinks, and Bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- You, S.; Xiang, Y.; Hwang, H.H.; Berry, D.B.; Kiratitanaporn, W.; Guan, J.; Yao, E.; Tang, M.; Zhong, Z.; Ma, X. High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 2023, 9, eade7923. [Google Scholar] [CrossRef]
- Lee, V.K.; Dai, G. Printing of Three-Dimensional Tissue Analogs for Regenerative Medicine. Ann. Biomed. Eng. 2017, 45, 115–131. [Google Scholar] [CrossRef]
- Bertassoni, L.E. Bioprinting of complex multicellular organs with advanced functionality—Recent progress and challenges ahead. Adv. Mater. 2022, 34, 2101321. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Mistewicz, K.; Nowacki, B.; In-Na, P.; Krushynska, A.; Mishra, Y.K.; Kim, H.J. A focused review on three-dimensional bioprinting technology for artificial organ fabrication. Biomater. Sci. 2022, 10, 5054–5080. [Google Scholar] [CrossRef]
- Pati, F.; Gantelius, J.; Svahn, H.A. 3D bioprinting of tissue/organ models. Angew. Chem. Int. Ed. 2016, 55, 4650–4665. [Google Scholar] [CrossRef]
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater. 2018, 69, 63–70. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Bernal, P.N.; Bouwmeester, M.; Madrid-Wolff, J.; Falandt, M.; Florczak, S.; Rodriguez, N.G.; Li, Y.; Größbacher, G. Volumetric Bioprinting of Organoids and Optically Tuned Hydrogels to Build Liver-Like Metabolic Biofactories. Adv. Mater. 2022, 34, e2110054. [Google Scholar] [CrossRef]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.L.; Shah, R.N. 3D Printing for Liver Tissue Engineering: Current Approaches and Future Challenges. Curr. Transplant. Rep. 2016, 3, 100–108. [Google Scholar] [CrossRef]
- Carvalho, V.; Gonçalves, I.; Lage, T.; Rodrigues, R.O.; Minas, G.; Teixeira, S.F.C.F.; Moita, A.S. 3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms: A Systematic Review. Sensors 2021, 21, 3304. [Google Scholar] [CrossRef] [PubMed]
- Fetah, K.; Tebon, P.J.; Goudie, M.J.; Eichenbaum, J.V.; Ren, L.; Barros, N.R.; Nasiri, R.; Ahadian, S.; Ashammakhi, N. The emergence of 3D bioprinting in organ-on-chip systems. Prog. Biomed. Eng. 2019, 1, 012001. [Google Scholar] [CrossRef]


| Cell Sources | Biomaterials | Techniques | References |
|---|---|---|---|
| HepG2 cells | Decellularized liver matrix, gelatin and polyethylene glycol | Extrusion | [99] |
| HepG2 and NIH/3T3 cells | Alginate, GelMa, cellulose nanocrystal | Extrusion | [100] |
| HepaRG cells | Gelatin | Extrusion | [11] |
| Huh7 and HepaRG cells | Methacrylated gelatin | Extrusion | [35] |
| Human adipose mesenchymal stem cell-derived hepatocyte-like cells, human umbilical vein endothelial cells, and human hepatic stellate cells | Liver ECM | Extrusion | [101] |
| Fibroblasts and hepatocytes | Alginate and methylcellulose | Extrusion | [102] |
| HepG2 cells, HUVECs and NHDFs | alginate and methyl-cellulose (algMC) | Extrusion | [103] |
| Huh7 cells | Decellularized liver matrix, silk fibroin, and gelatin | Extrusion | [103] |
| Human-induced pluripotent stem cells-derived hepatocytes | Gelatin/alginate | Extrusion | [104] |
| HepaRG cells | Alginate-gelatin | Extrusion | [105] |
| HepG2 cells | Alginate, gelatin | Extrusion | [106] |
| Human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes and hepatocytes | Liver decellularized extracellular matrix (dECM) bioink | DLP | [107] |
| HepaRG and human HSCs | Gelatin and PEG | DLP | [108] |
| human-induced hepatocytes | GelMA/dECM | DLP | [109] |
| human induced pluripotent stem cells (hiPSC)-hematopoietic progenitor cells (HPCs), human umbilical vein endothelial cells (HUVECs), and adipose-derived stem cells | GelMA | DLP | [110] |
| HUVECs and HepG2 | Glycidal methacrylate-hyaluronic acid (GM-HA) | DLP | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Liu, Z.; Tang, F.; Jiang, H.; Zhou, Z.; Hao, X.; Zhang, J.M. Application of 3D Bioprinting in Liver Diseases. Micromachines 2023, 14, 1648. https://doi.org/10.3390/mi14081648
Li W, Liu Z, Tang F, Jiang H, Zhou Z, Hao X, Zhang JM. Application of 3D Bioprinting in Liver Diseases. Micromachines. 2023; 14(8):1648. https://doi.org/10.3390/mi14081648
Chicago/Turabian StyleLi, Wenhui, Zhaoyue Liu, Fengwei Tang, Hao Jiang, Zhengyuan Zhou, Xiuqing Hao, and Jia Ming Zhang. 2023. "Application of 3D Bioprinting in Liver Diseases" Micromachines 14, no. 8: 1648. https://doi.org/10.3390/mi14081648







