Ultrasonic Manipulation of Hydrodynamically Driven Microparticles in Vessel Bifurcation: Simulation, Optimization, Experimental Validation, and Potential for Targeted Drug Delivery
Abstract
:1. Introduction
2. Analytical Model
2.1. Hydrodynamic Drag Force
2.2. Force Acoustic Force
3. Results and Discussion
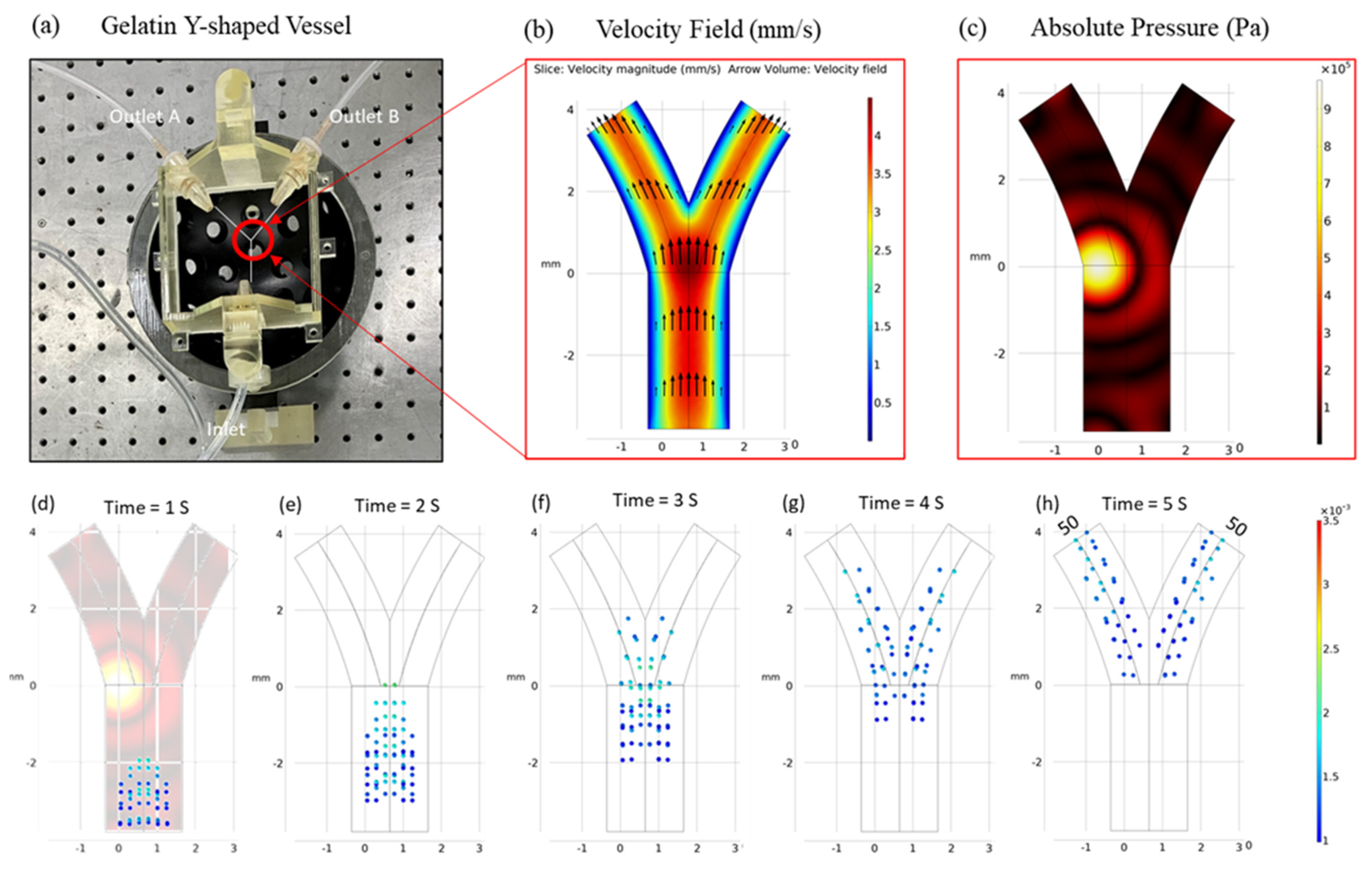
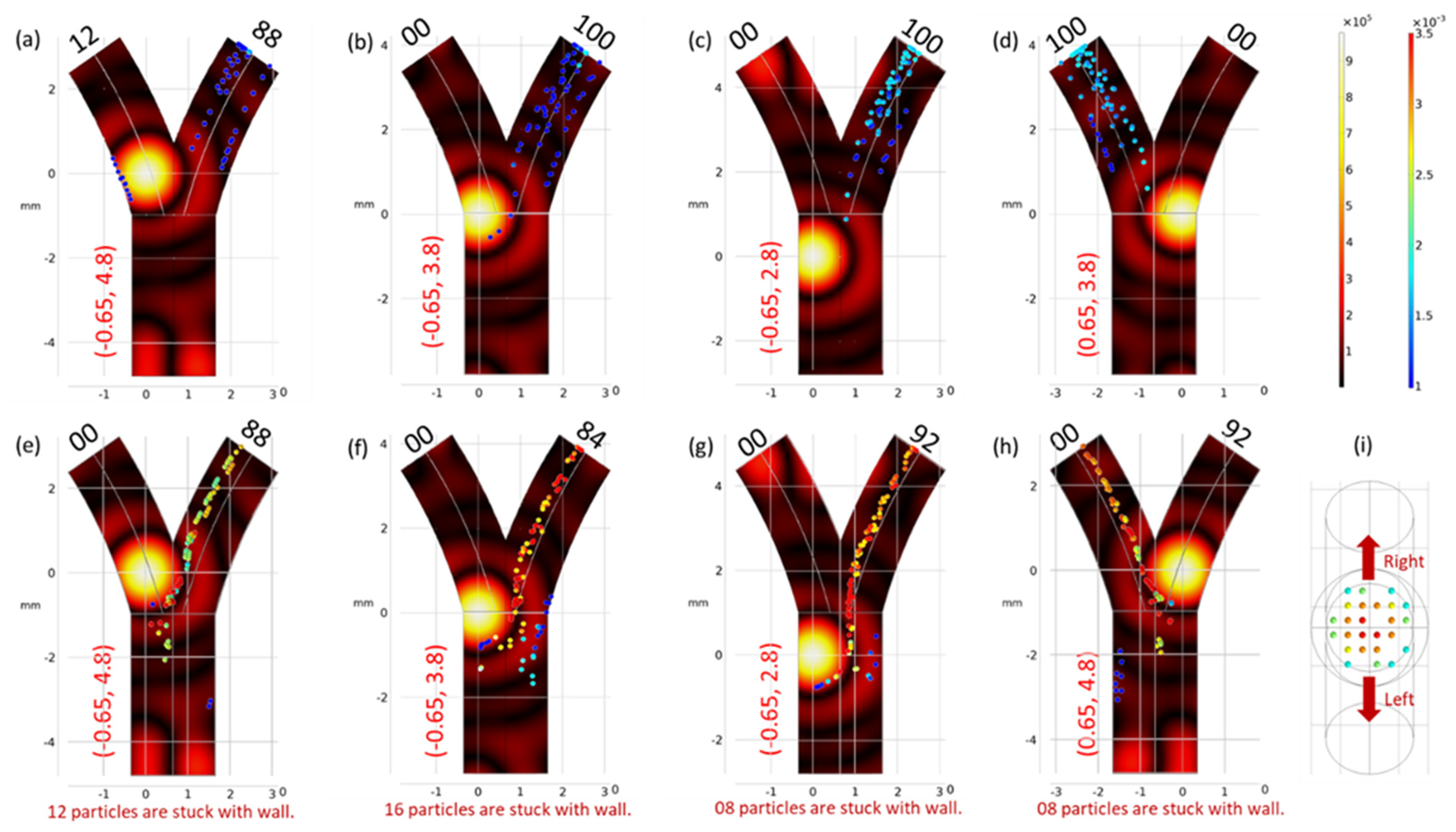
3.1. Optimized Focus Position
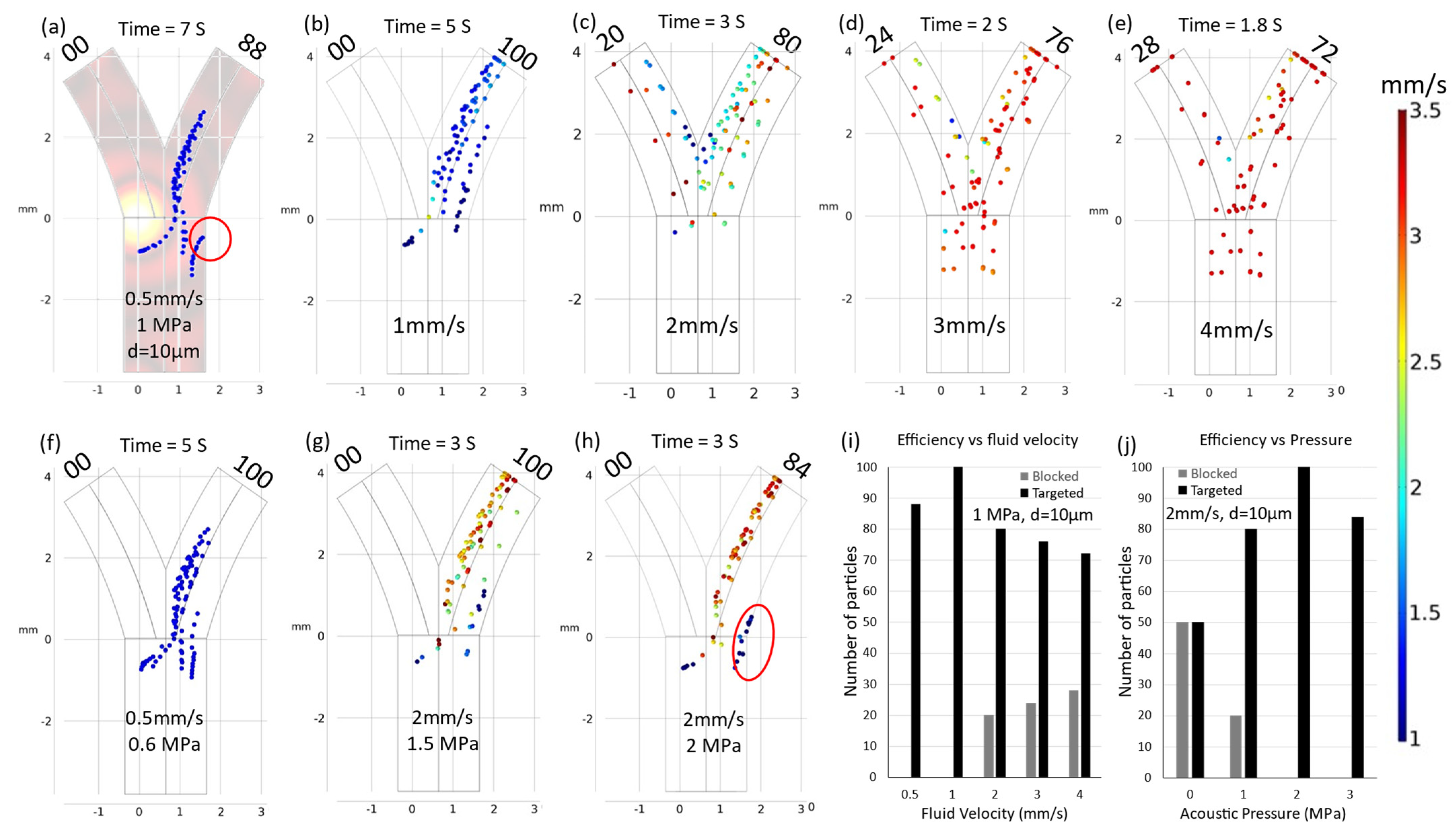
3.2. Velocity Correction
3.3. Particle Size Dependence
3.4. Experimental Validation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Pucelik, B.; Sulek, A.; Barzowska, A.; Dabrowski, J.M. Recent advances in strategies for overcoming hypoxia in photodynamic therapy of cancer. Cancer Lett. 2020, 492, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Lee, H.; Kwon, S.H.; Choi, H.; Park, S. Magnetic nano-particles retrievable biodegradable hydrogel microrobot. Sens. Actuat. B-Chem. 2019, 289, 65–77. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.H.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2020, 288, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Shaffer, S.; Sittek, M.; Alboslemy, T.; Kim, C.; Kim, M.H. Alternating Magnetic Field-Responsive Hybrid Gelatin Microgels for Controlled Drug Release. JoVE-J. Vis. Exp. 2016, e53680. [Google Scholar] [CrossRef]
- Medina-Sanchez, M.; Magdanz, V.; Guix, M.; Fomin, V.M.; Schmidt, O.G. Swimming Microrobots: Soft, Reconfigurable, and Smart. Adv. Funct. Mater. 2018, 28, 1707228. [Google Scholar] [CrossRef]
- Lu, X.L.; Zhao, K.D.; Liu, W.J.; Yang, D.X.; Shen, H.; Peng, H.M.; Guo, X.S.; Li, J.X.; Wang, J. A Human Microrobot Interface Based on Acoustic Manipulation. ACS Nano 2019, 13, 11443–11452. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Le, V.H.; Kim, C.S.; Han, J.; Park, J.O.; Choi, E. A Novel Macrophage-based Microrobot Bearing Multiple Smart Nanotherapeutics for Targeting and Drug Delivery to Solid Tumors. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob2018), Enschede, The Netherlands, 26–29 August 2018; pp. 55–60. [Google Scholar]
- Lee, H.S.; Go, G.; Choi, E.; Kang, B.; Park, J.O.; Kim, C.S. Medical Microrobot-Wireless Manipulation of a Drug Delivery Carrier through an External Ultrasonic Actuation: Preliminary Results. Int. J. Control Autom. 2020, 18, 175–185. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Min, H.K.; Kim, C.S.; Han, J.; Park, J.O.; Choi, E. Folate receptor-targeted liposomal nanocomplex for effective synergistic photothermal-chemotherapy of breast cancer in vivo. Colloids Surf. B Biointerfaces 2019, 173, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, B.A.; Lee, S.B.; Nguyen, V.D.; Go, G.J.; Nguyen, K.T.; Lee, H.S.; Nan, M.H.; Hong, A.Y.; Kim, C.S.; Li, H.; et al. Self-folded microrobot for active drug delivery and rapid ultrasound-triggered drug release. Sen. Actuat. B-Chem. 2020, 324, 128752. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Hoang, M.C.; Choi, E.; Kang, B.; Park, J.O.; Kim, C.S. Medical Microrobot—A Drug Delivery Capsule Endoscope with Active Locomotion and Drug Release Mechanism: Proof of Concept. Int. J. Control Autom. 2020, 18, 65–75. [Google Scholar] [CrossRef]
- Lubbe, A.S. Preclinical experiences with magnetic drug targeting: Tolerance and efficacy and clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors—Reply. Cancer Res. 1997, 57, 3064–3065. [Google Scholar]
- Ashkin, A.; Dziedzic, J.M. Optical trapping and manipulation of viruses and bacteria. Science 1987, 235, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.K. Optical tweezers for single cells. J. R. Soc. Interface 2008, 5, 671–690. [Google Scholar] [CrossRef]
- Li, J.; Alfares, A.; Zheng, Y. Optical manipulation and assembly of micro/nanoscale objects on solid substrates. iScience 2022, 25, 104035. [Google Scholar] [CrossRef]
- Alshehri, A.M.; Wilson, O.C., Jr.; Dahal, B.; Philip, J.; Luo, X.; Raub, C.B. Magnetic nanoparticle-loaded alginate beads for local micro-actuation of in vitro tissue constructs. Colloid. Surf. B Biointerfaces 2017, 159, 945–955. [Google Scholar] [CrossRef]
- Chen, L.; Offenhausser, A.; Krause, H.J. Magnetic tweezers with high permeability electromagnets for fast actuation of magnetic beads. Rev. Sci. Instrum. 2015, 86, 044701. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Hoang, M.C.; Go, G.; Kang, B.; Choi, E.; Park, J.O.; Kim, C.S. Regularization-based independent control of an external electromagnetic actuator to avoid singularity in the spatial manipulation of a microrobot. Control Eng. Pract. 2020, 97, 104340. [Google Scholar] [CrossRef]
- Kummer, M.P.; Abbott, J.J.; Kratochvil, B.E.; Borer, R.; Sengul, A.; Nelson, B.J. OctoMag: An Electromagnetic System for 5-DOF Wireless Micromanipulation. IEEE Trans. Robot. 2010, 26, 1006–1017. [Google Scholar] [CrossRef]
- Go, G.; Nguyen, V.D.; Jin, Z.; Park, J.O.; Park, S. A Thermo-electromagnetically Actuated Microrobot for the Targeted Transport of Therapeutic Agents. Int. J. Control Autom. 2018, 16, 1341–1354. [Google Scholar] [CrossRef]
- Soffe, R.; Tang, S.Y.; Baratchi, S.; Nahavandi, S.; Nasabi, M.; Cooper, J.M.; Mitchell, A.; Khoshmanesh, K. Controlled Rotation and Vibration of Patterned Cell Clusters Using Dielectrophoresis. Anal. Chem. 2015, 87, 2389–2395. [Google Scholar] [CrossRef]
- Probst, R.; Shapiro, B. Three-dimensional electrokinetic tweezing: Device design, modeling, and control algorithms. J. Micromech. Microeng. 2011, 21, 027004. [Google Scholar] [CrossRef]
- Cohen, A.E.; Moerner, W.E. Method for trapping and manipulating nanoscale objects in solution. Appl. Phys. Lett. 2005, 86, 093109. [Google Scholar] [CrossRef]
- Franzl, M.; Cichos, F. Hydrodynamic manipulation of nano-objects by optically induced thermo-osmotic flows. Nat. Commun. 2022, 13, 656. [Google Scholar] [CrossRef]
- Yalikun, Y.; Kanda, Y.; Morishima, K. A Method of Three-Dimensional Micro-Rotational Flow Generation for Biological Applications. Micromachines 2016, 7, 140. [Google Scholar] [CrossRef]
- Martinez-Pedrero, F.; Tierno, P. Advances in colloidal manipulation and transport via hydrodynamic interactions. J. Colloid. Interface Sci. 2018, 519, 296–311. [Google Scholar] [CrossRef]
- Marzo, A.; Seah, S.A.; Drinkwater, B.W.; Sahoo, D.R.; Long, B.; Subramanian, S. Holographic acoustic elements for manipulation of levitated objects. Nat. Commun. 2015, 6, 8661. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, A.; Rufo, J.; Guo, F.; Gu, Y.Y.; Li, P.; Lata, J.; Huang, T.J. Acoustic tweezers for the life sciences. Nat. Methods 2018, 15, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.X.; Jung, D.; Lee, H.S.; Nguyen, V.D.; Choi, E.; Kang, B.; Park, J.O.; Kim, C.S. Holographic Acoustic Tweezers for 5-DoF Manipulation of Nanocarrier Clusters toward Targeted Drug Delivery. Pharmaceutics 2022, 14, 1490. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.Q.; Nama, N.; McNeill, J.M.; Soto, F.; Yan, Z.F.; Liu, W.; Wang, W.; Wang, J.; Mallouk, T.E. 3D steerable, acoustically powered microswimmers for single-particle manipulation. Sci. Adv. 2019, 5, eaax3084. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, M.; Thomas, J.L.; Al Sahely, R.; Gerbedoen, J.C.; Gong, Z.X.; Sivery, A.; Matar, O.B.; Smagin, N.; Favreau, P.; Vlandas, A. Spatially selective manipulation of cells with single-beam acoustical tweezers. Nat. Commun. 2020, 11, 4244. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, H.W.; Yu, P.P.; Zhang, S.H.; Zhou, J.H.; Li, Y.M.; Gong, L. Trapping and Manipulation of Single Cells in Crowded Environments. Front. Bioeng. Biotechnol. 2020, 8, 422. [Google Scholar] [CrossRef]
- Xu, T.; Soto, F.; Gao, W.; Garcia-Gradilla, V.; Li, J.; Zhang, X.; Wang, J. Ultrasound-modulated bubble propulsion of chemically powered microengines. J. Am. Chem. Soc. 2014, 136, 8552–8555. [Google Scholar] [CrossRef]
- Marzo, A.; Barnes, A.; Drinkwater, B.W. TinyLev: A multi-emitter single-axis acoustic levitator. Rev. Sci. Instrum. 2017, 88, 085105. [Google Scholar] [CrossRef]
- O’Neil, H.T. Theory of Focusing Radiators. J. Acoust. Soc. Am. 2005, 21, 516–526. [Google Scholar] [CrossRef]
- Gor’kov, L.P. On the forces acting on a small particle in an acoustical field in an ideal fluid. Sov. Phys.-Dokl. 1962, 6, 773–775. [Google Scholar]
- Bruus, H. Acoustofluidics 7: The acoustic radiation force on small particles. Lab Chip 2012, 12, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Godin, M.; Bryan, A.K.; Burg, T.P.; Babcock, K.; Manalis, S.R. Measuring the mass, density, and size of particles and cells using a suspended microchannel resonator. Appl. Phys. Lett. 2007, 91, 123121. [Google Scholar] [CrossRef]
- Richardson, N.D.; Williams, K.L.; Briggs, K.B.; Thorsos, E.I. Dynamic measurement of sediment grain compressibility at atmospheric pressure: Acoustic applications. IEEE J. Ocean. Eng. 2002, 27, 593–601. [Google Scholar] [CrossRef]
- Hidalgo Baltasar, E.; Taravillo, M.; Baonza, V.G.; Sanz, P.D.; Guignon, B. Speed of Sound in Liquid Water from (253.15 to 348.15) K and Pressures from (0.1 to 700) MPa. J. Chem. Eng. Data 2011, 56, 4800–4807. [Google Scholar]
- Cao, H.X.; Nguyen, V.D.; Jung, D.; Choi, E.; Kim, C.S.; Park, J.O.; Kang, B. Acoustically Driven Cell-Based Microrobots for Targeted Tumor Therapy. Pharmaceutics 2022, 14, 2143. [Google Scholar] [CrossRef]



| Actuation Power Platform | Particle Size (μm) | Input Power | Control Force 1 | Limitations |
|---|---|---|---|---|
| Optical field [17,18,19,20] | 0.1–100 | 106–107 (W/cm2) | Trapping force and torque (pN) | Limited skin depth, high-powered laser system. |
| Magnetic field [21,22,23,24,25] | 0.1–10 | 1–10 (Tesla) | Magnetic gradient field force (nN to μN) | Particle size limitations, only works with magnetized particles. |
| Electric field [26,27,28] | 0.001–1000 | 104–107 (V/m) | Dielectrophoresis force (pN to μN) | Low-conductivity media, ionization. |
| Hydrodynamic field [29,30,31] | 0.1–1100 | N/A | Hydrodynamic effects (pN to μN) | Flow control limitation, specific platform. |
| Acoustic field [32,33,34,35,36,37,38,39] | 0.1–1000 | 10−2–10 (W/cm2) | Axial acoustic force (μN) | Low acoustic impedance media, trap is too weak in strong flows. |
| Name | Expression | Value |
|---|---|---|
| Fluid viscosity | ||
| Fluid density | ||
| Particle density | ||
| Particle diameter | ||
| Vessel diameter | ||
| Average fluid velocity | ||
| Number of UT array elements | UT | 30 |
| Resonance frequency | ||
| Controllable voltage | ||
| UT array diameter | ||
| The distance from array surface to focal point |
| No External Acoustic Pressure | BLOCKING-A | ||||||
|---|---|---|---|---|---|---|---|
| Sample-1 | Sample-2 | Sample-1 | Sample-2 | ||||
| Channel_A | Channel_B | Channel_A | Channel_B | Channel_A | Channel_B | Channel_A | Channel_B |
| 47 | 48 | 42 | 56 | 23 | 88 | 19 | 68 |
| 53 | 50 | 54 | 43 | 16 | 44 | 14 | 79 |
| 40 | 47 | 62 | 58 | 16 | 64 | 20 | 85 |
| 49 | 57 | 45 | 63 | 17 | 72 | 31 | 73 |
| 472,500 | 505,000 | 507,500 | 550,000 | 180,000 | 670,000 | 210,000 | 762,500 |
| 48.34 | 51.66 | 47.99 | 52.01 | 21.18 | 78.82 | 21.59 | 78.41 |
| 48.16 | 0.17 | 51.84 | 0.17 | 21.39 | 0.21 | 78.61 | 0.21 |
| CH_A: 48.16% ± 0.17% | CH_B: 51.84% ± 0.17% | CH_A: 21.39% ± 0.21% | CH_B: 78.61% ± 0.21% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, S.; Jung, D.; Cao, H.X.; Park, J.-O.; Kang, B.; Choi, E. Ultrasonic Manipulation of Hydrodynamically Driven Microparticles in Vessel Bifurcation: Simulation, Optimization, Experimental Validation, and Potential for Targeted Drug Delivery. Micromachines 2024, 15, 13. https://doi.org/10.3390/mi15010013
Sharif S, Jung D, Cao HX, Park J-O, Kang B, Choi E. Ultrasonic Manipulation of Hydrodynamically Driven Microparticles in Vessel Bifurcation: Simulation, Optimization, Experimental Validation, and Potential for Targeted Drug Delivery. Micromachines. 2024; 15(1):13. https://doi.org/10.3390/mi15010013
Chicago/Turabian StyleSharif, Saqib, Daewon Jung, Hiep Xuan Cao, Jong-Oh Park, Byungjeon Kang, and Eunpyo Choi. 2024. "Ultrasonic Manipulation of Hydrodynamically Driven Microparticles in Vessel Bifurcation: Simulation, Optimization, Experimental Validation, and Potential for Targeted Drug Delivery" Micromachines 15, no. 1: 13. https://doi.org/10.3390/mi15010013







