Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation
Abstract
:1. Introduction
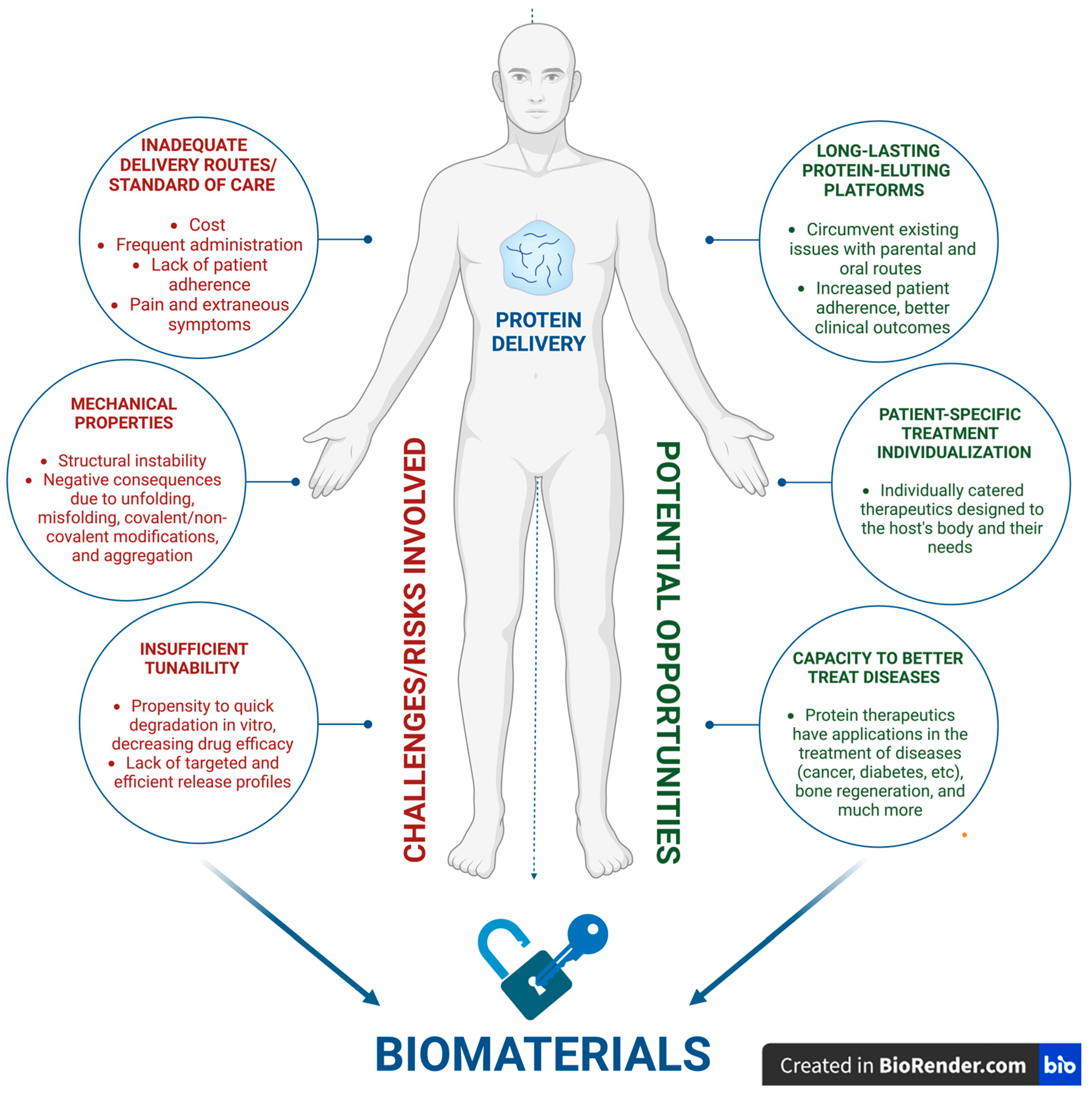
2. Opportunities and Challenges
2.1. Viable Biomaterial Platforms for Therapeutic Protein Delivery
2.1.1. Hydrogels
2.1.2. Scaffold Systems
2.1.3. Nanogels
2.1.4. Polymeric Nanoparticles
2.2. Efficient and Targeted Delivery: The Core Challenge
2.3. Addressing Immunogenicity and Biocompatibility: Safety, Toxicity, and Tolerability
2.4. Stability Issues
2.5. Production Scale-Up and Navigating Regulatory Challenges
2.6. Advancements in Biomaterial Fabrication Technologies
2.7. Nanotechnology, Hybrid Biomaterials, and Bioprinting—The Next Frontier
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abune, L.; Wang, Y. Affinity Hydrogels for Protein Delivery. Trends Pharmacol. Sci. 2021, 42, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, M.; Mo, R. Polysaccharide-based biomaterials for protein delivery. Med. Drug Discov. 2020, 7, 100031. [Google Scholar] [CrossRef]
- Ye, Q.N.; Wang, Y.; Shen, S.; Xu, C.F.; Wang, J. Biomaterials-Based Delivery of Therapeutic Antibodies for Cancer Therapy. Adv. Healthc. Mater. 2021, 10, e2002139. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Sethiya, N.K.; Gulbake, A.S.; Mehra, N.K.; Murty, U.S.N.; Gulbake, A. A review on albumin as a biomaterial for ocular drug delivery. Int. J. Biol. Macromol. 2021, 191, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Merivaara, A.; Zini, J.; Koivunotko, E.; Valkonen, S.; Korhonen, O.; Fernandes, F.M.; Yliperttula, M. Preservation of biomaterials and cells by freeze-drying: Change of paradigm. J. Control. Release 2021, 336, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, C.; Liu, Z. Protein-Engineered Biomaterials for Cancer Theranostics. Adv. Healthc. Mater. 2018, 7, e1800913. [Google Scholar] [CrossRef] [PubMed]
- Seah, I.; Zhao, X.; Lin, Q.; Liu, Z.; Su, S.Z.; Yuen, Y.S.; Hunziker, W.; Lingam, G.; Loh, X.J.; Su, X. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye 2020, 34, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Yau, A.; Lee, J.; Chen, Y. Nanomaterials for Protein Delivery in Anticancer Applications. Pharmaceutics 2021, 13, 155. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Sugandhi, V.V.; Kokare, C.R. Potential biomaterials and experimental animal models for inventing new drug delivery approaches in the neurodegenerative disorder: Multiple sclerosis. Brain Res. 2024, 1822, 148674. [Google Scholar] [CrossRef]
- Nayab, D.E.; Din, F.U.; Ali, H.; Kausar, W.A.; Urooj, S.; Zafar, M.; Khan, I.; Shabbir, K.; Khan, G.M. Nano biomaterials-based strategies for enhanced brain targeting in the treatment of neurodegenerative diseases: An up-to-date perspective. J. Nanobiotechnol. 2023, 21, 477. [Google Scholar] [CrossRef]
- Agrawal, M.; Prathyusha, E.; Ahmed, H.; Dubey, S.K.; Kesharwani, P.; Singhvi, G.; Naidu, V.G.M.; Alexander, A. Biomaterials in treatment of Alzheimer’s disease. Neurochem. Int. 2021, 145, 105008. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Cui, W. Biomaterial-based delivery of nucleic acids for tissue regeneration. Adv. Drug Deliv. Rev. 2021, 176, 113885. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Chen, S.-H. Instability Challenges and Stabilization Strategies of Pharmaceutical Proteins. Pharmaceutics 2022, 14, 2533. [Google Scholar] [CrossRef] [PubMed]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Mukunda, D.C.; Rodrigues, J.; Gail D’souza, M.; Gangadharan, G.; Pai, A.R.; Mahato, K.K. A comprehensive review of protein misfolding disorders, underlying mechanism, clinical diagnosis, and therapeutic strategies. Ageing Res. Rev. 2023, 90, 102017. [Google Scholar] [CrossRef] [PubMed]
- Dembélé, J.; Liao, J.H.; Liu, T.P.; Chen, Y.P. Overcoming Cytosolic Delivery Barriers of Proteins Using Denatured Protein-Conjugated Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Chen, J.; Li, X.; Zhou, X.; Hu, Y.M.; Chu, S.F.; Peng, Y.; Chen, N.H. Research progress on adenosine in central nervous system diseases. CNS Neurosci. Ther. 2019, 25, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Annabi, N.; Tamayol, A.; Oklu, R.; Ghanem, A.; Khademhosseini, A. Adenosine-associated delivery systems. J. Drug Target. 2015, 23, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.-S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, G.; Yao, X.; Zhou, H.; Lyu, B.; Pei, S.; Wen, P. Microneedle-based insulin transdermal delivery system: Current status and translation challenges. Drug Deliv. Transl. Res. 2022, 12, 2403–2427. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2016, 24, 413–428. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, Z.; Wang, J.; Liu, C.; Kong, N.; Farokhzad, O.C.; Tao, W. Oral Insulin Delivery Platforms: Strategies To Address the Biological Barriers. Angew. Chem. (Int. Ed. Engl.) 2020, 59, 19787–19795. [Google Scholar] [CrossRef] [PubMed]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from Nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yuvienco, C.; Montclare, J.K. Protein based therapeutic delivery agents: Contemporary developments and challenges. Biomaterials 2017, 134, 91–116. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Niazmand, R.; Dikshit, P.K.; Kim, B.S. Recent progress in polymeric non-invasive insulin delivery. Int. J. Biol. Macromol. 2022, 203, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Asfour, M.H. Advanced trends in protein and peptide drug delivery: A special emphasis on aquasomes and microneedles techniques. Drug Deliv. Transl. Res. 2021, 11, 1–23. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Dabholkar, N.; Agrawal, M.; Singhvi, G.; Alexander, A.; Bapat, R.A.; Kesharwani, P. Oral peptide delivery: Challenges and the way ahead. Drug Discov. Today 2021, 26, 931–950. [Google Scholar] [CrossRef]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release Off. J. Control. Release Soc. 2015, 219, 2–7. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Chapa-Villarreal, F.A.; Miller, M.; Rodriguez-Cruz, J.J.; Pérez-Carlos, D.; Peppas, N.A. Self-assembled block copolymer biomaterials for oral delivery of protein therapeutics. Biomaterials 2023, 300, 122191. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Lillard, J.W., Jr. Past, present, and future technologies for oral delivery of therapeutic proteins. J. Pharm. Sci. 2008, 97, 2497–2523. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]
- “Biomaterials.” National Institute of Biomedical Imaging and Bioengineering, U.S. Department of Health and Human Services. 2017. Available online: www.nibib.nih.gov/science-education/science-topics/biomaterials (accessed on 10 February 2024).
- Wu, J.; Sahoo, J.K.; Li, Y.; Xu, Q.; Kaplan, D.L. Challenges in delivering therapeutic peptides and proteins: A silk-based solution. J. Control. Release Off. J. Control. Release Soc. 2022, 345, 176–189. [Google Scholar] [CrossRef]
- Choi, S.M.; Chaudhry, P.; Zo, S.M.; Han, S.S. Advances in protein-based materials: From origin to novel biomaterials. Adv. Exp. Med. Biol. 2018, 1078, 161–210. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, S.; Li, S.; Pan, H. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A Review. Biomater. Sci. 2021, 9, 1583–1597. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Aktar, M.N.; Hossain, M.S.; Sarkar, N.; Islam, M.R.; Arafat, M.E.; Bhowmik, S.; Yusa, S.-I. Recent Advances in Micro- and Nano-Drug Delivery Systems Based on Natural and Synthetic Biomaterials. Polymers 2023, 15, 4563. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Agrawal, G. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Zou, Y.; Hu, J.; Li, Y.; Cheng, Y. Natural polyphenols in drug delivery systems: Current status and future challenges. Giant 2020, 3, 100022. [Google Scholar] [CrossRef]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Q.; Lin, J.; Cai, Z.; Liao, G.; Wang, K.; Bai, L.; Zhao, P.; Yu, Z. Recent Advance in Polymer Based Microspheric Systems for Controlled Protein and Peptide Delivery. Curr. Med. Chem. 2019, 26, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Aiswarya, T.T.; Mirza, I.; Saha, S. Biocompatible polymers and their applications. Encycl. Mater. Plast. Polym. 2022, 2, 796–819. [Google Scholar] [CrossRef]
- Varanko, A.; Saha, S.; Chilkoti, A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv. Drug Deliv. Rev. 2020, 156, 133–187. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Vunjak-Novakovic, G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv. Transl. Res. 2015, 5, 101–115. [Google Scholar] [CrossRef]
- Chander, S.; Kulkarni, G.T.; Dhiman, N.; Kharkwal, H. Protein-Based Nanohydrogels for Bioactive Delivery. Front. Chem. 2021, 9, 573748. [Google Scholar] [CrossRef]
- Petrak, K. Essential properties of drug-targeting delivery systems. Drug Discov. Today 2005, 10, 1667–1673. [Google Scholar] [CrossRef]
- Huang, W.; Rollett, A.; Kaplan, D.L. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin. Drug Deliv. 2015, 12, 779–791. [Google Scholar] [CrossRef]
- Nie, T.; Wang, W.; Liu, X.; Wang, Y.; Li, K.; Song, X.; Zhang, J.; Yu, L.; He, Z. Sustained Release Systems for Delivery of Therapeutic Peptide/Protein. Biomacromolecules 2021, 22, 2299–2324. [Google Scholar] [CrossRef]
- Schmalz, G.; Galler, K.M. Biocompatibility of biomaterials—Lessons learned and considerations for the design of novel materials. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017, 33, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Nagarajan, S.; Bechelany, M.; Kalkura, S.N. Collagen based biomaterials for tissue engineering applications: A Review. In Lecture Notes in Earth System Sciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–22. [Google Scholar] [CrossRef]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Khopade, S.; Gomte, S.S.; Janrao, C.; Bavaskar, A.; Agnihotri, T.G.; Jain, A.; Khatik, R. Peptide and protein delivery through cellulose, hyaluronic acid, and heparin. In Peptide and Protein Drug Delivery Using Polysaccharides; Academic Press: Cambridge, MA, USA, 2024; pp. 75–113. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, J.Z.; Fei, X.; Li, Y.; Song, Y.; Qian, Z.; Peng, Q. Can nanoparticles and nano–protein interactions bring a bright future for insulin delivery? Acta Pharm. Sin. B 2021, 11, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regen-erative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl alcohol based-drug delivery systems for cancer treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl Alcohol: A Review of Research Status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Lebeau, J.; Efromson, J.P.; Lynch, M.D. A Review of the Biotechnological Production of Methacrylic Acid. Front. Bioeng. Biotechnol. 2020, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Karnoosh-Yamchi, J.; Mobasseri, M.; Akbarzadeh, A.; Davaran, S.; Ostad-Rahimi, A.R.; Hamishehkar, H.; Salehi, R.; Bahmani, Z.; Nejati-Koshki, K.; Darbin, A.; et al. Preparation of pH sensitive insulin-loaded nano hydrogels and evaluation of insulin releasing in different pH conditions. Mol. Biol. Rep. 2014, 41, 6705–6712. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Gaur, J.K.; Bobji, M.S.; Srivastava, C. Nanoparticle-reinforced polyacrylamide hydrogel composites for clinical applications: A review. J. Mater. Sci. 2022, 57, 8041–8063. [Google Scholar] [CrossRef]
- Saptaji, K.; Iza, N.R.; Widianingrum, S.; Mulia, V.K.; Setiawan, I. Poly(2-hydroxyethyl methacrylate) hydrogels for contact lens applications–a review. Makara J. Sci. 2021, 25, 3–154. [Google Scholar] [CrossRef]
- Bhatti, S.S.; Singh, J. 3D printing of Biomaterials for Biomedical Applications: A Review. Int. J. Interact. Des. Manuf. (IJIDeM) 2023. [Google Scholar] [CrossRef]
- Davis, B.K. Control of diabetes with polyacrylamide implants containing insulin. Experientia 1972, 28, 348. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lau, L.C.; Lo, A.C.; Chau, Y. Injectable Chemically Crosslinked Hydrogel for the Controlled Release of Bevacizumab in Vitreous: A 6-Month In Vivo Study. Transl. Vis. Sci. Technol. 2015, 4, 5. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Jones, C.N.; Revzin, A.; Tae, G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials 2010, 31, 3596–3603. [Google Scholar] [CrossRef] [PubMed]
- Huerta-López, C.; Alegre-Cebollada, J. Protein Hydrogels: The Swiss Army Knife for Enhanced Mechanical and Bioactive Properties of Biomaterials. Nanomaterials 2021, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Diaferia, C.; Rosa, E.; Smaldone, G.; Morelli, G.; Accardo, A. Peptide-Based Hydrogels and Nanogels for Delivery of Doxorubicin. Int. J. Nanomed. 2021, 16, 1617–1630. [Google Scholar] [CrossRef]
- Abune, L.; Davis, B.; Wang, Y. Aptamer-functionalized hydrogels: An emerging class of biomaterials for protein delivery, cell capture, regenerative medicine, and molecular biosensing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1731. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, J.; Cai, F.; Zhang, F.; Yang, M.; Bi, S.; Gui, R.; Li, Y.; Xia, Y. Aptamer-functionalized hydrogel as effective anti-cancer drugs delivery agents. Colloids Surf. B Biointerfaces 2015, 134, 40–46. [Google Scholar] [CrossRef]
- Kang, H.; Trondoli, A.C.; Zhu, G.; Chen, Y.; Chang, Y.J.; Liu, H.; Huang, Y.F.; Zhang, X.; Tan, W. Near-infrared light-responsive core-shell nanogels for targeted drug delivery. ACS Nano 2011, 5, 5094–5099. [Google Scholar] [CrossRef]
- Bae, K.H.; Kurisawa, M. Emerging hydrogel designs for controlled protein delivery. Biomater. Sci. 2016, 4, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Heilshorn, S.C. Protein-engineered biomaterials: Highly tunable tissue engineering scaffolds. Tissue Eng. Part B Rev. 2010, 16, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.U.; Khan, R.S.; Rather, A.H.; Beigh, M.A.; Sheikh, F.A. Local dual delivery therapeutic strategies: Using biomaterials for advanced bone tissue regeneration. J. Control. Release Off. J. Control. Release Soc. 2021, 339, 143–155. [Google Scholar] [CrossRef]
- Piotrowicz, A.; Shoichet, M.S. Nerve guidance channels as drug delivery vehicles. Biomaterials 2006, 27, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Mukai, S.; Sasaki, Y.; Akiyoshi, K. Nanogel tectonics for tissue engineering: Protein delivery systems with Nanogel chaperones. Adv. Healthc. Mater. 2018, 7, e1800729. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Anooj, E.; Charumathy, M.; Sharma, V.; Vibala, B.V.; Gopukumar, S.T.; Jainab, S.I.B.; Vallinayagam, S. Nanogels: An overview of properties, biomedical applications, Future Research Trends and developments. J. Mol. Struct. 2021, 1239, 130446. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Research 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation Therapeutic Delivery Platform: A Review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Lin, C.C.; Metters, A.T. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvi-ronments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric System for Dual Growth Factor Delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and Biocompatibility of PLA and PLGA Micro-spheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in Biomaterials for Tissue Engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and Design of Polymeric Surfactant-Based Drug Delivery Systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active Targeting Schemes for Nanoparticle Systems in Cancer Therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Owens III, D.E.; Peppas, N.A. Opsonization, Biodistribution, and Pharmacokinetics of Poly-meric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jain, R.A. The Manufacturing Techniques of Various Drug Loaded Biodegradable Poly(Lactide-Co-Glycolide) (PLGA) Devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G. Degradation of Poly(Lactic-Co-Glycolic Acid) Microspheres: Effect of Copolymer Composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Con-trolled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug Deliv. Rev. 2012, 64 (Suppl. S1), 37–48. [Google Scholar] [CrossRef]
- Lukyanov, A.N.; Torchilin, V.P. Micelles from Lipid Derivatives of Water-Soluble Polymers as Delivery Systems for Poorly Soluble Drugs. Adv. Drug Deliv. Rev. 2004, 56, 1273–1289. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Block Copolymer Micelles as Long-Circulating Drug Vehicles. Adv. Drug Deliv. Rev. 1996, 16, 295–309. [Google Scholar] [CrossRef]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for Peptide and Protein PEGylation. Adv. Drug Deliv. Rev. 2012, 64, 116–127. [Google Scholar] [CrossRef]
- Mahler, H.C.; Friess, W.; Grauschopf, U.; Kiese, S. Protein Aggregation: Pathways, Induction Factors and Analysis. J. Pharm. Sci. 2009, 98, 2909–2934. [Google Scholar] [CrossRef]
- Ratanji, K.D.; Derrick, J.P.; Dearman, R.J.; Kimber, I. Immunogenicity of Therapeutic Proteins: Influence of Aggregation. J. Immunotoxicol. 2014, 11, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, M.E.M.; Hilario, E.; Jacobson, F. Protein Aggregation and Bioprocessing. AAPS J. 2006, 8, E572–E579. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwan, M.R.; Schwartz, A.G.; Xia, Y. Electrospun nanofibers for neural tissue engineering. Nanoscale 2010, 2, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, Y.; Li, Y.; Zhao, P.; Zhu, K.; Chen, W. A Facile Technique to Prepare Biodegrada-ble Coaxial Electrospun Nanofibers for Controlled Release of Bioactive Agents. J. Control. Release 2005, 108, 237–243. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of Tetracycline Hydrochloride from Electrospun Poly(Ethylene-Co-Vinylacetate), Poly(Lactic Acid), and a Blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun Poly(ε-Caprolactone) Microfiber and Mul-tilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Narang, N.; Sharma, J. Sublingual mucosa as a route for systemic drug delivery. Int. J. Pharm. Pharm. Sci. 2011, 3 (Suppl. S2), 18–22. [Google Scholar]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar] [PubMed]
- Dixit, R.P.; Puthli, S.P. Oral Strip Technology: Overview and Future Potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Appli-cations. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applica-tions. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The Development of Micro-gels/Nanogels for Drug Delivery Applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Vinogradov, S.V. Nanogels in the race for drug delivery. Nanomedicine 2010, 5, 165–168. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Wang, A.L.; Bhattacharya, A.; Montclare, J.K. Protein-Based Biomaterials for Thera-peutic and Diagnostic Applications. Prog. Biomed. Eng. 2022, 4, 012003. [Google Scholar] [CrossRef] [PubMed]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Gagner, J.E.; Kim, W.; Chaikof, E.L. Designing protein-based biomaterials for medical applications. Acta Biomater. 2014, 10, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yang, L.; Cheng, X.; Li, Y.; Cheng, Y. Aminoglycoside-Based Biomaterials: From Mate-rial Design to Antibacterial and Gene Delivery Applications. Adv. Funct. Mater. 2021, 31, 2103718. [Google Scholar] [CrossRef]
- Chambre, L.; Martín-Moldes, Z.; Parker, R.N.; Kaplan, D.L. Bioengineered elastin- and silk-biomaterials for drug and gene delivery. Adv. Drug Deliv. Rev. 2020, 160, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.P.; Reynolds, H.M.; Lumicisi, B.; Bryson, C.J. Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self/nonself 2010, 1, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.; Arkin, S.; Cocea, L.; Devanarayan, V.; Kirshner, S.; Kromminga, A.; Quarmby, V.; Richards, S.; Schneider, C.K.; Subramanyam, M.; et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 2014, 16, 658–673. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Turner, M.R.; Balu-Iyer, S.V. Challenges and Opportunities for the Subcutaneous Delivery of Therapeutic Proteins. J. Pharm. Sci. 2018, 107, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, N.L.; Balu-Iyer, S.V. Immunogenicity Challenges Associated with Subcutaneous Delivery of Therapeutic Proteins. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2021, 35, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers 2022, 14, 986. [Google Scholar] [CrossRef]
- Nasiri, H.; Valedkarimi, Z.; Aghebati-Maleki, L.; Majidi, J. Antibody-drug conjugates: Promising and efficient tools for targeted cancer therapy. J. Cell. Physiol. 2018, 233, 6441–6457. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Zhu, Y.; Qi, C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv. Mater. Interfaces 2020, 7, 2000819. [Google Scholar] [CrossRef]
- Heymann, D.; Pradal, G.; Benahmed, M. Cellular mechanisms of calcium phosphate ceramic degradation. Histol. Histopathol. 1999, 14, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Diez-Escudero, A.; Espanol, M.; Beats, S.; Ginebra, M.P. In vitro degradation of calcium phosphates: Effect of multiscale porosity, textural properties and composition. Acta Biomater. 2017, 60, 81–92. [Google Scholar] [CrossRef]
- Schaefer, S.; Detsch, R.; Uhl, F.; Deisinger, U.; Ziegler, G. How degradation of calcium phosphate bone substitute materials is influenced by phase composition and porosity. Adv. Eng. Mater. 2011, 13, 342–350. [Google Scholar] [CrossRef]
- Andrée, L.; Yang, F.; Brock, R.; Leeuwenburgh, S.C.G. Designing biomaterials for the delivery of RNA therapeutics to stimulate bone healing. Mater. Today Bio 2021, 10, 100105. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.P. Protein Adsorption on Biomaterial Surfaces: Subsequent Conformational and Biological Consequences—A Review. J. Surf. Sci. Technol. 2020, 36, 7–38. [Google Scholar] [CrossRef]
- Wei, Q.; Becherer, T.; Angioletti-Uberti, S.; Dzubiella, J.; Wischke, C.; Neffe, A.T.; Lendlein, A.; Ballauff, M.; Haag, R. Protein interactions with polymer coatings and biomaterials. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 8004–8031. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Khurana, V.; Patel, S.; Mitra, A.K. Long-term delivery of protein therapeutics. Expert Opin. Drug Deliv. 2015, 12, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Hamid Akash, M.S.; Rehman, K.; Chen, S. Natural and synthetic polymers as drug carriers for delivery of therapeutic proteins. Polym. Rev. 2015, 55, 371–406. [Google Scholar] [CrossRef]
- Agnieray, H.; Glasson, J.L.; Chen, Q.; Kaur, M.; Domigan, L.J. Recent developments in sustainably sourced protein-based biomaterials. Biochem. Soc. Trans. 2021, 49, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Wren, S.; Minelli, C.; Pei, Y.; Akhtar, N. Evaluation of particle size techniques to support the development of manufacturing scale nanoparticles for application in pharmaceuticals. J. Pharm. Sci. 2020, 109, 2284–2293. [Google Scholar] [CrossRef]
- Cooper, R.C.; Yang, H. Hydrogel-based Ocular Drug Delivery Systems: Emerging Fabrication Strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Alu, A.; Liu, H.; Shi, Y.; Wei, X.; Cai, L.; Wei, Y. Biomaterial-assisted biotherapy: A brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact. Mater. 2022, 17, 29–48. [Google Scholar] [CrossRef]
- Haq-Siddiqi, N.A.; Britton, D.; Kim Montclare, J. Protein-engineered biomaterials for cartilage therapeutics and repair. Adv. Drug Deliv. Rev. 2023, 192, 114647. [Google Scholar] [CrossRef]
- Mirzaei, M.; Okoro, O.V.; Nie, L.; Petri, D.F.S.; Shavandi, A. Protein-Based 3D Biofabrication of Biomaterials. Bioengineering 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Uzunalli, G.; Guler, M.O. Peptide gels for controlled release of proteins. Ther. Deliv. 2020, 11, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A., IV; Acharya, S.; Gadodia, T.; Shukla, S.; Harshita, J.; Akre, C.; Khare, M.; Huse, S. A Review on Techniques and Biomaterials Used in 3D Bioprinting. Cureus 2022, 14, e28463. [Google Scholar] [CrossRef] [PubMed]
- Dorogin, J.; Townsend, J.M.; Hettiaratchi, M.H. Biomaterials for protein delivery for complex tissue healing responses. Biomater. Sci. 2021, 9, 2339–2361. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D Printing Technology in Bone Tissue Engineering: A Review. Curr. Drug Deliv. 2021, 18, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Oliva, N.; Almquist, B.D. Spatiotemporal delivery of bioactive molecules for wound healing using stimuli-responsive biomaterials. Adv. Drug Deliv. Rev. 2020, 161–162, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, M.; Forootanfar, H.; Ramakrishna, S. Regenerative medicine and drug delivery: Progress via electrospun biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110521. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadehmoghadam, S.; Dong, Y.; Davies, I.J. Modeling Electrospun nanofibers: An overview from theoretical, empirical, and numerical approaches. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 901–915. [Google Scholar] [CrossRef]
- Komatsu, T. Protein-based smart microtubes and nanotubes as ultrasmall biomaterials. Chem. Lett. 2020, 49, 1245–1255. [Google Scholar] [CrossRef]
- Yang, C.; Blum, N.T.; Lin, J.; Qu, J.; Huang, P. Biomaterial scaffold-based local drug delivery systems for cancer immunotherapy. Sci. Bull. 2020, 65, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef] [PubMed]
- Teal, C.J.; Lu, S.P.; Shoichet, M.S. Engineering hydrogels for affinity-based release of therapeutic proteins. Chem. Mater. 2024, 36, 614–641. [Google Scholar] [CrossRef]
- Ji, X.; Li, Q.; Song, H.; Fan, C. Protein-mimicking nanoparticles in Biosystems. Adv. Mater. 2022, 34, 2201562. [Google Scholar] [CrossRef] [PubMed]
- Farhat, W.; Yeung, V.; Ross, A.; Kahale, F.; Boychev, N.; Kuang, L.; Chen, L.; Ciolino, J.B. Advances in biomaterials for the treatment of retinoblastoma. Biomater. Sci. 2022, 10, 5391–5429. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, Y.; Mitragotri, S.; Rai, B. Permeation of nanoparticles across the intestinal lipid membrane: Dependence on shape and surface chemistry studied through molecular simulations. Nanoscale 2020, 12, 6318–6333. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Rehman, K.; Xia, J.; Shabbir, M.; Zaman, M.; Liang, Y.; Duan, L. Biomaterial-assisted targeted and controlled delivery of CRISPR/Cas9 for precise gene editing. Biomater. Sci. 2023, 11, 3762–3783. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Shahrivarkevishahi, A.; Herbert, F.C.; Wijesundara, Y.H.; Gassensmith, J.J. Biomaterials and nanomaterials for sustained release vaccine delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2021, 13, e1735. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of Anticancer Therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Quadros, M.; Momin, M.; Verma, G. Design strategies and evolving role of biomaterial assisted treatment of osteosarcoma. Mater. Sci. Eng. C 2021, 121, 111875. [Google Scholar] [CrossRef] [PubMed]
- Um, W.; Gupta, A.; Song, S.H.; Kim, C.H.; Park, J.H. Biomaterials as antigen delivery carrier for cancer immunotherapy. Macromol. Res. 2021, 29, 834–842. [Google Scholar] [CrossRef]
- Kumar, A.; Zhou, L.; Zhi, K.; Raji, B.; Pernell, S.; Tadrous, E.; Kodidela, S.; Nookala, A.; Kochat, H.; Kumar, S. Challenges in Biomaterial-Based Drug Delivery Approach for the Treatment of Neurodegenerative Diseases: Opportunities for Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhen, X.; Wu, W.; Jiang, X. Responsive boron biomaterials and their biomedical applications. Sci. China Chem. 2020, 63, 648–664. [Google Scholar] [CrossRef]
- Xu, N.; Peng, X.L.; Li, H.R.; Liu, J.X.; Cheng, J.S.; Qi, X.Y.; Ye, S.J.; Gong, H.L.; Zhao, X.H.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 702108. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A.L. Recent advances in silk sericin/calcium phosphate biomaterials. Front. Mater. 2020, 7, 24. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate-Containing Biocomposites and Hybrid Biomaterials for Biomedical Applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef]
- Erezuma, I.; Eufrasio-da-Silva, T.; Golafshan, N.; Deo, K.; Mishra, Y.K.; Castilho, M.; Gaharwar, A.K.; Leeuwenburgh, S.; Dolatshahi-Pirouz, A.; Orive, G. Nanoclay Reinforced Biomaterials for Mending Musculoskeletal Tissue Disorders. Adv. Healthc. Mater. 2021, 10, e2100217. [Google Scholar] [CrossRef]
- Cao, D.; Ding, J. Recent advances in regenerative biomaterials. Regen. Biomater. 2022, 9, rbac098. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Ouyang, L.; Xu, R.; Yang, Y.; Sun, W. Responsive biomaterials for 3D bioprinting: A Review. Mater. Today 2022, 52, 112–132. [Google Scholar] [CrossRef]
- Carnes, M.E.; Pins, G.D. Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss. Bioengineering 2020, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S. Therapeutic proteins. Methods Mol. Biol. 2012, 899, 1–26. [Google Scholar] [CrossRef] [PubMed]
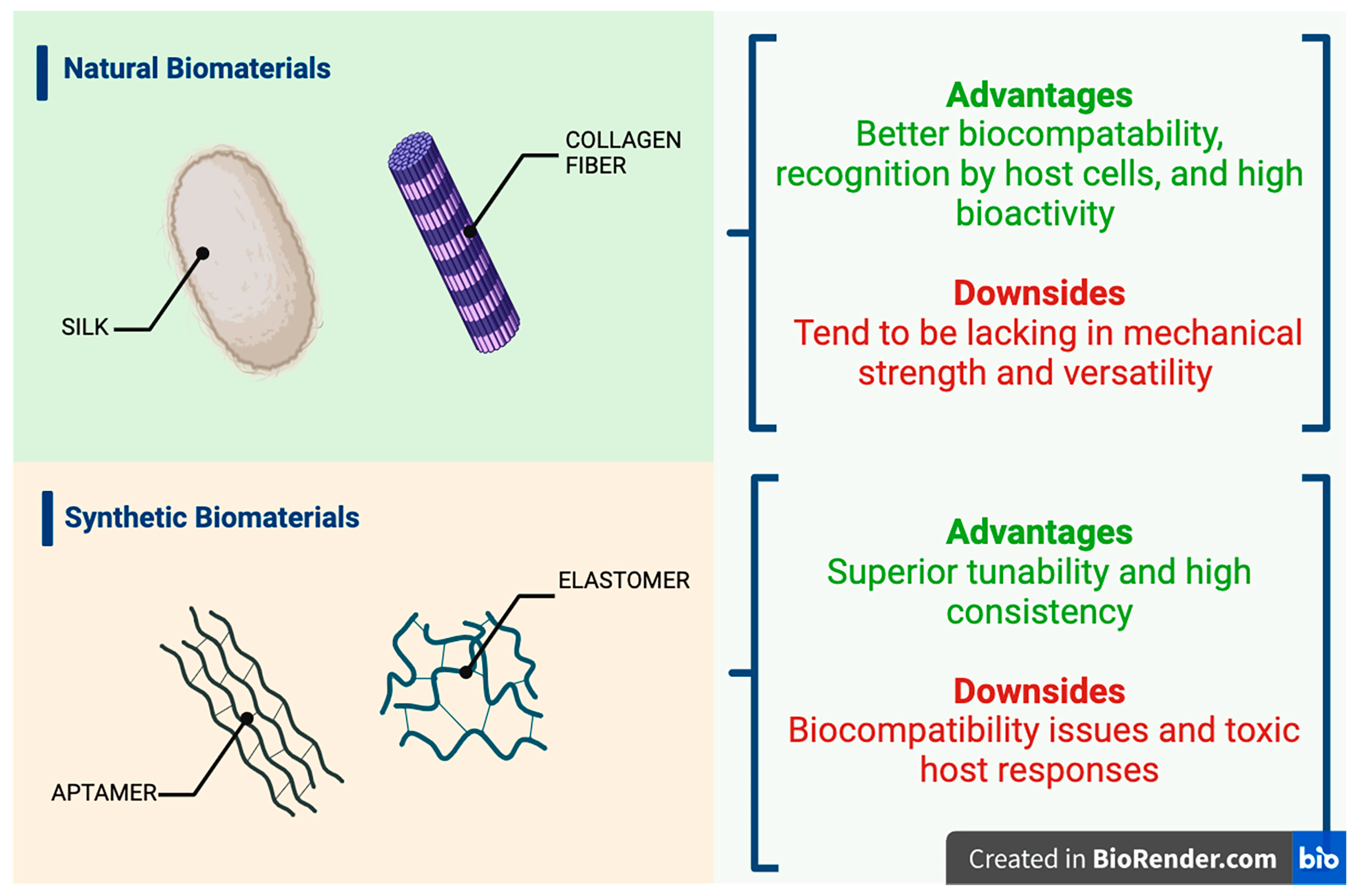
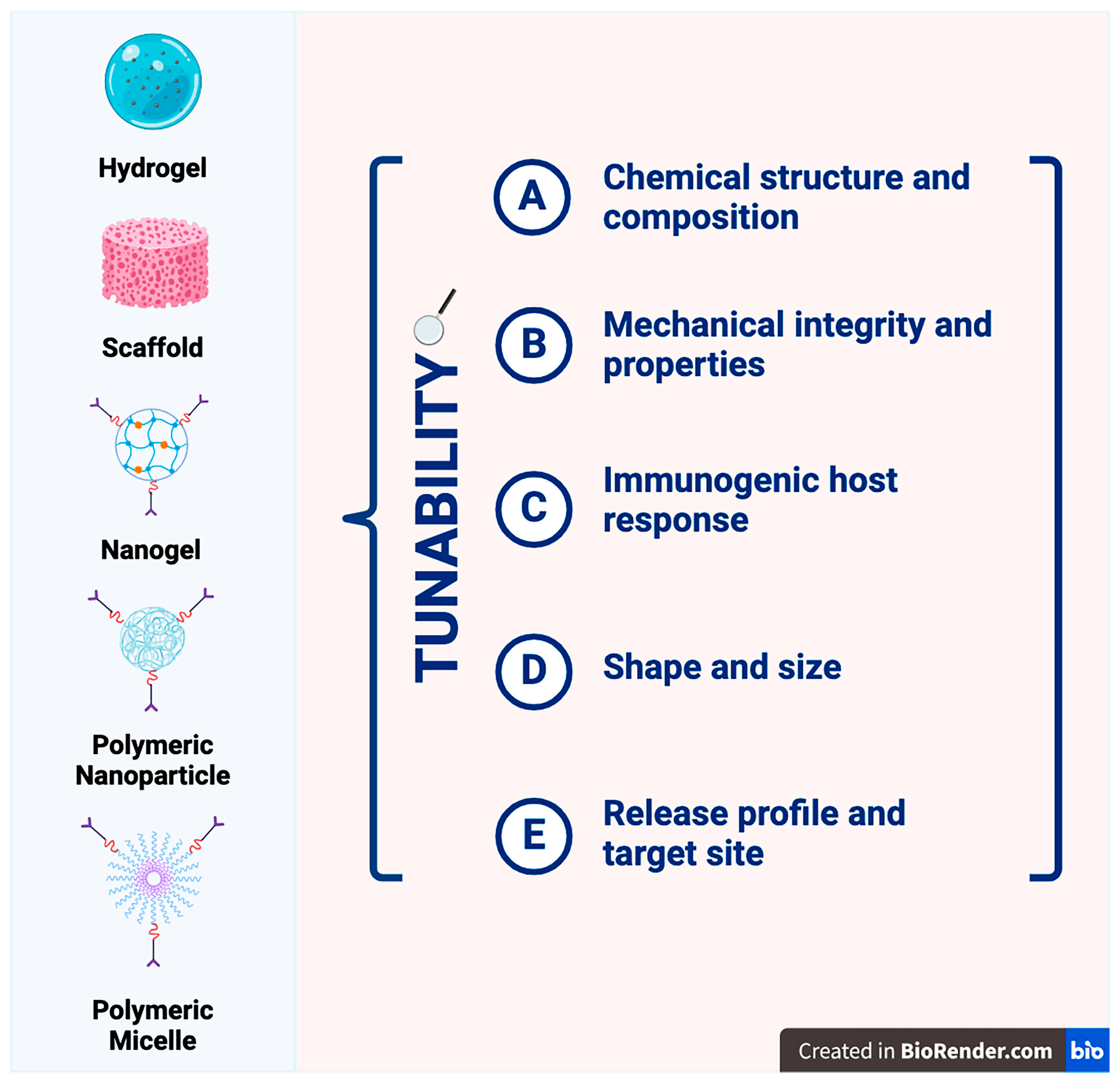
| Natural Carriers | Results Based on Chemical Structure | Synthetic Carriers | Results Based on Chemical Structure |
| Silk Fibroin |
| Poly (ethylene glycol) (PEG) |
|
| Cellulose |
| Poly (vinyl alcohol) (PVA) |
|
| Heparin |
| Methacrylic acid (MAA), |
|
| Hyaluronic Acid |
| N-isopropyl acrylamide (NIPAAm) |
|
| Starch |
| Polyacrylamide (PAA) |
|
| Chitosan |
| 2-hydroxyethyl methacrylate (HEMA) |
|
| Biomaterials | Advantages | Disadvantages |
|---|---|---|
| Traditional Hydrogels | ||
| Affinity Hydrogels | ||
| Scaffolds | ||
| Nanoparticles | ||
| Microparticles | ||
| Micelles | ||
| Aggregates | ||
| Electrospun Fibers | ||
| Buccal/Sublingual Films | ||
| Liposomes | ||
| Nanogels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorantla, A.; Hall, J.T.V.E.; Troidle, A.; Janjic, J.M. Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation. Micromachines 2024, 15, 533. https://doi.org/10.3390/mi15040533
Gorantla A, Hall JTVE, Troidle A, Janjic JM. Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation. Micromachines. 2024; 15(4):533. https://doi.org/10.3390/mi15040533
Chicago/Turabian StyleGorantla, Amogh, Jacques T. V. E. Hall, Anneliese Troidle, and Jelena M. Janjic. 2024. "Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation" Micromachines 15, no. 4: 533. https://doi.org/10.3390/mi15040533








