Approaches and Challenges of Engineering Implantable Microelectromechanical Systems (MEMS) Drug Delivery Systems for in Vitro and in Vivo Applications
Abstract
:1. Introduction
2. Drug Reservoir Size and Loading Volume
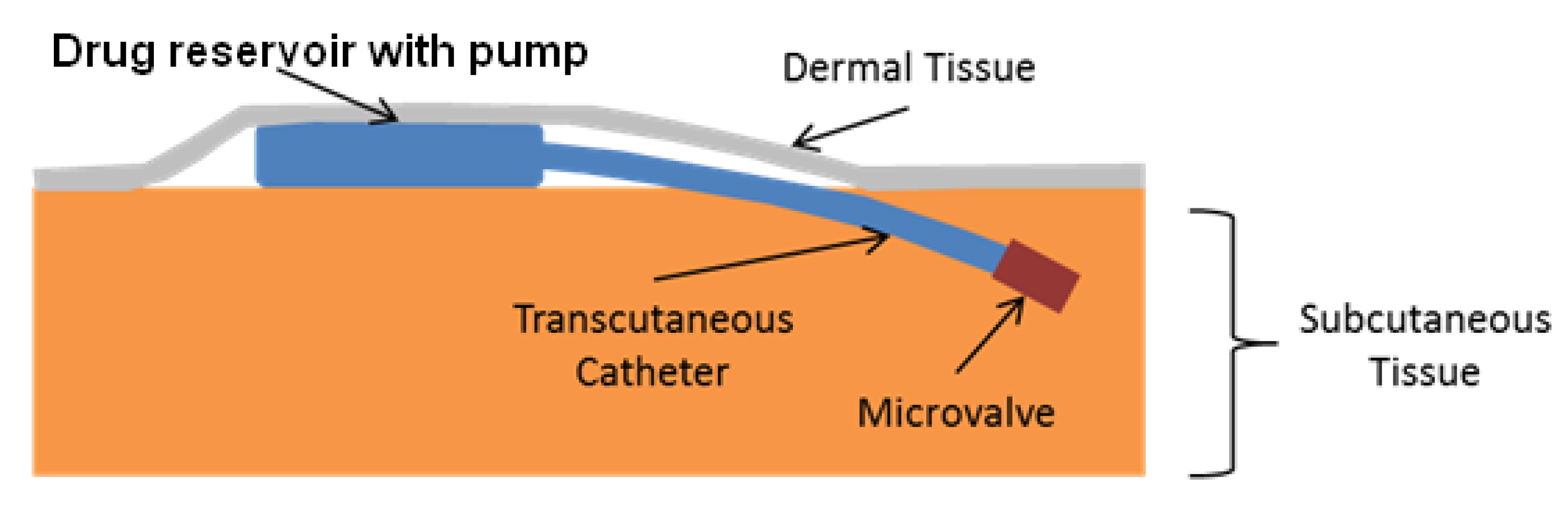
3. Effective Delivery Methods
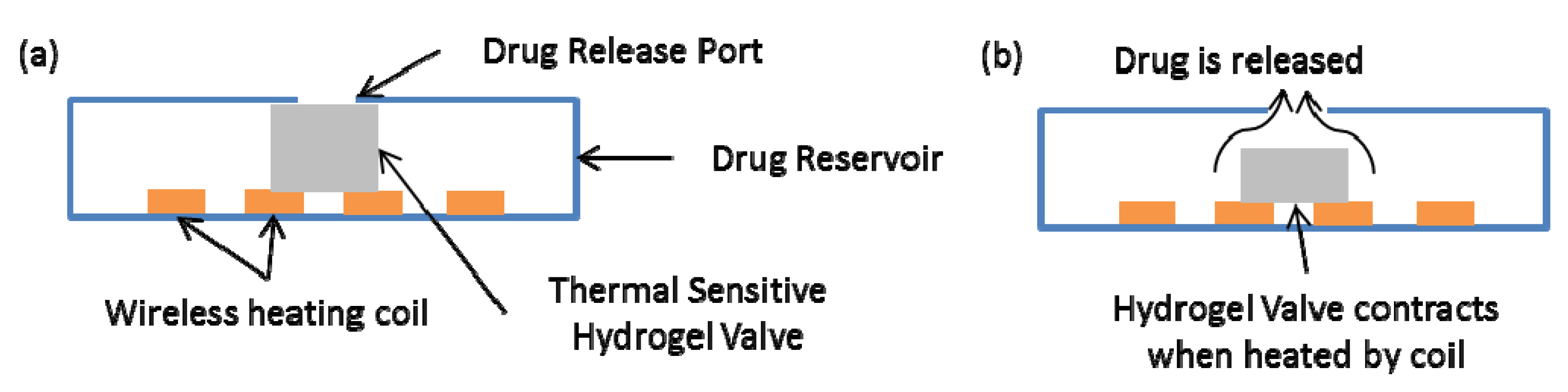
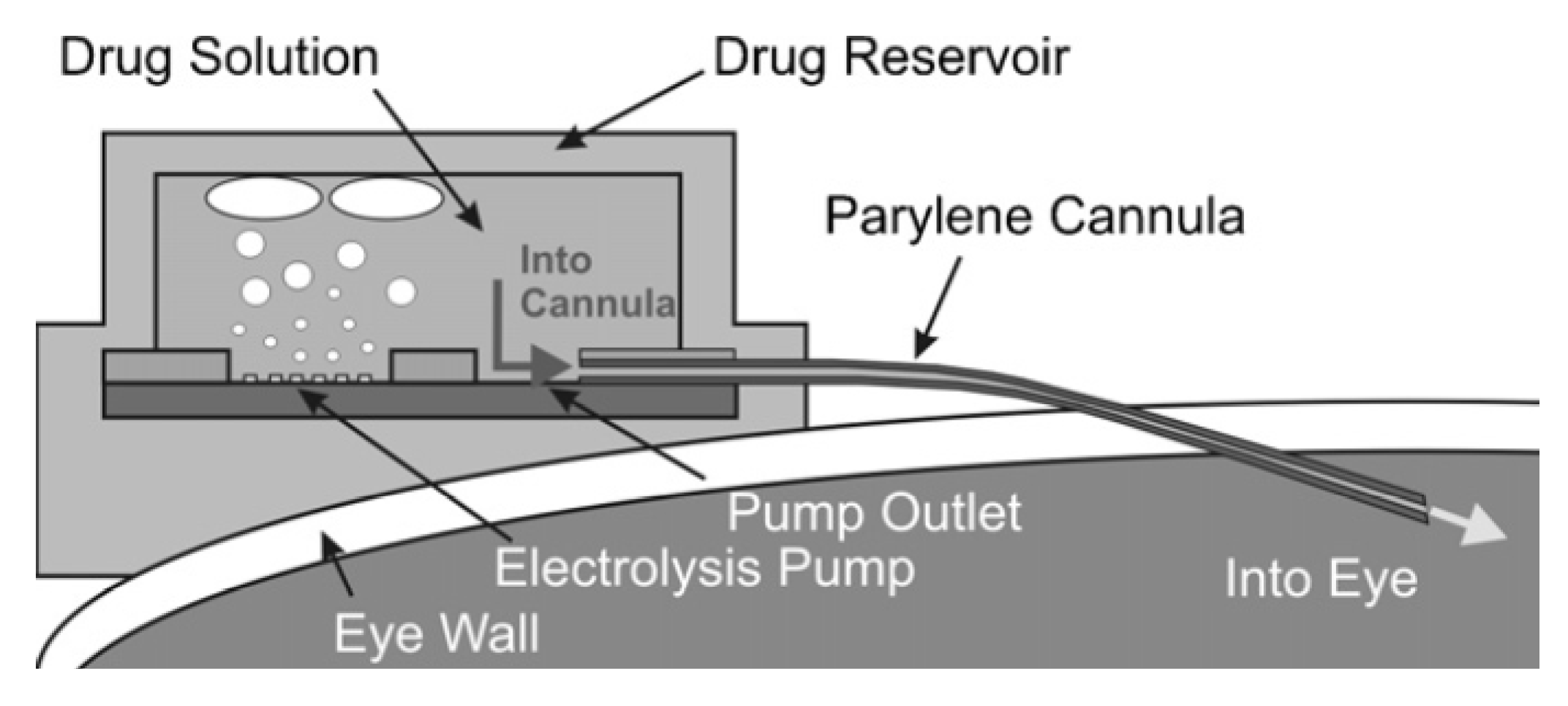
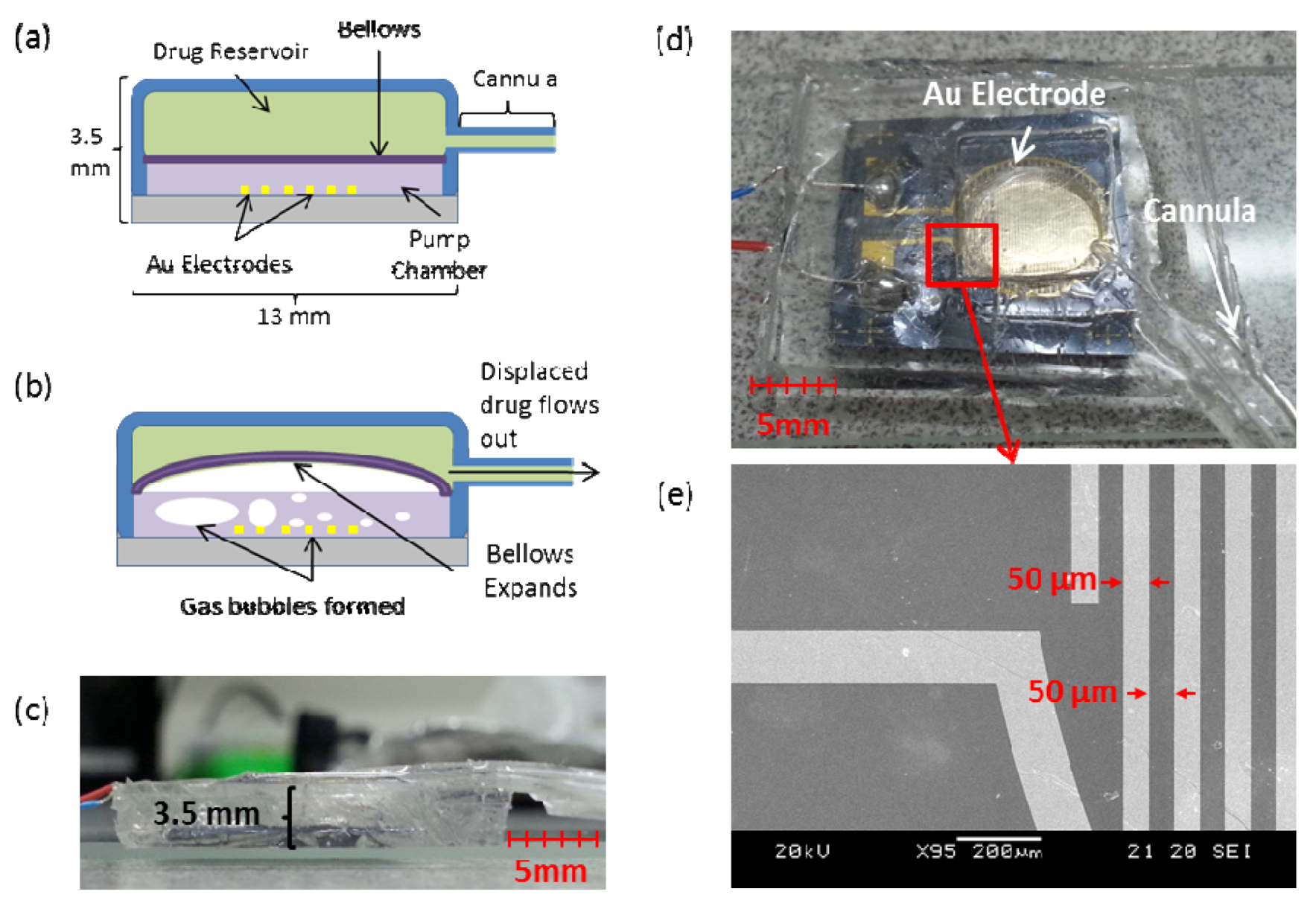
4. Long Operation Time
5. Controllability
6. Biocompatibility
7. Conclusion
Acknowledgements
References
- Grayson, A.C.R.; Shawgo, R.S.; Johnson, A.M.; Flynn, N.T.; Yawen, L.I.; Cima, M.J.; Langer, R. A BioMEMS review: MEMS technology for physiologically integrated devices. Proc. IEEE 2004, 92, 6–21. [Google Scholar] [CrossRef]
- Elman, N.M.; Upadhyay, U.M. Medical applications of implantable drug delivery microdevices based on MEMS (Micro-Electro-Mechanical-Systems). Curr. Pharm. Biotechnol. 2010, 11, 398–403. [Google Scholar] [CrossRef]
- Nisar, A.; Afzulpurkar, N.; Mahaisavariya, B.; Tuantranont, A. MEMS-based micropumps in drug delivery and biomedical applications. Sens. Actuator. B 2008, 130, 917–942. [Google Scholar] [CrossRef]
- Staples, M.; Daniel, K.; Cima, M.; Langer, R. Application of micro- and nano-electromechanical devices to drug delivery. Pharm. Res. 2006, 23, 847–863. [Google Scholar] [CrossRef]
- Park, S.; Park, J.O. Frontier Research Program on Biomedical Microrobot for Intravascular Therapy. In 2nd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, BioRob 2008, Scottsdale, AZ, USA, 19–22 October 2008; pp. 360–365.
- Nelson, B.J. Towards Nanorobots. In International Conference of Solid-State Sensors, Actuators and Microsystems, 2009, TRANSDUCERS 2009, Denver, CO, USA, 21–25 June 2009; pp. 2155–2159.
- Shuxiang, G.; Sawamoto, J.; Qingxue, P. A Novel Type of Microrobot for Biomedical Application. In 2005 IEEE/RSJ International Conference on Intelligent Robots and Systems, IROS 2005, Edmonton, Alberta, Canada, 2–6 August 2005; pp. 1047–1052.
- LaVan, D.A.; McGuire, T.; Langer, R. Small-scale systems for in vivo drug delivery. Nat. Biotech. 2003, 21, 1184–1191. [Google Scholar] [CrossRef]
- Gensler, H.; Sheybani, R.; Po-Ying, L.; Lo, R.; Zhu, S.; Ken-Tye, Y.; Roy, I.; Prasad, P.N.; Masood, R.; Sinha, U.K.; Meng, E. Implantable MEMS Drug Delivery Device for Cancer Radiation Reduction. In IEEE 23rd International Conference on Micro Electro Mechanical Systems (MEMS), Wanchai, Hong Kong, 24–28 January 2010; pp. 23–26.
- Li, Y.; Hong Linh Ho, D.; Tyler, B.; Williams, T.; Tupper, M.; Langer, R.; Brem, H.; Cima, M.J. In vivo delivery of BCNU from a MEMS device to a tumor model. J. Control. Release 2005, 106, 138–145. [Google Scholar] [CrossRef]
- Li, Y.; Shawgo, R.S.; Tyler, B.; Henderson, P.T.; Vogel, J.S.; Rosenberg, A.; Storm, P.B.; Langer, R.; Brem, H.; Cima, M.J. In vivo release from a drug delivery MEMS device. J. Control. Release 2004, 100, 211–219. [Google Scholar] [CrossRef]
- Pirmoradi, F.N.; Jackson, J.; Burt, H.; Chiao, M. Delivery of an Anti-Cancer Drug from a Magnetically Controlled MEMS Device Show Cytotoxicity in PC3 and HUVEC Cells. In the 16th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS 2011), Beijing, China, 5–9 June 2011; pp. 2831–2834.
- Lo, R.; Li, P.-Y.; Saati, S.; Agrawal, R.; Humayun, M.; Meng, E. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed. Microdevices 2009, 11, 959–970. [Google Scholar] [CrossRef]
- Cao, L.; Mantell, S.; Polla, D. Design and simulation of an implantable medical drug delivery system using microelectromechanical systems technology. Sens. Actuator. A 2001, 94, 117–125. [Google Scholar]
- Simons, R.N.; Hall, D.G.; Miranda, F.A. RF Telemetry System for an Implantable Bio-MEMS Sensor. In IEEE MTT-S International Microwave Symposium Digest, Forth Worth, TX, USA, 6–11 June 2004; 1433, pp. 1433–1436.
- Jackson, N.; Muthuswamy, J. Flexible chip-scale package and interconnect for implantable MEMS movable microelectrodes for the brain. J. Microelectromechanical. Syst. 2009, 18, 396–404. [Google Scholar] [CrossRef]
- Abel, P.U.; von Woedtke, T. Biosensors for in vivo glucose measurement: Can we cross the experimental stage. Biosens. Bioelectron. 2002, 17, 1059–1070. [Google Scholar] [CrossRef]
- Elman, N.; Ho Duc, H.; Cima, M. An implantable MEMS drug delivery device for rapid delivery in ambulatory emergency care. Biomed. Microdevices 2009, 11, 625–631. [Google Scholar] [CrossRef]
- Evans, A.; Park, J.; Chiravuri, S.; Gianchandani, Y. A low power, microvalve regulated architecture for drug delivery systems. Biomed. Microdevices 2010, 12, 159–168. [Google Scholar] [CrossRef]
- Staples, M. Microchips and controlled-release drug reservoirs. WIRs Nanomed. Nanobiotechnol. 2010, 2, 400–417. [Google Scholar]
- Kotzar, G.; Freas, M.; Abel, P.; Fleischman, A.; Roy, S.; Zorman, C.; Moran, J.M.; Melzak, J. Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials 2002, 23, 2737–2750. [Google Scholar] [CrossRef]
- Richards Grayson, A.C.; Scheidt Shawgo, R.; Li, Y.; Cima, M.J. Electronic MEMS for triggered delivery. Advan. Drug Delivery Rev. 2004, 56, 173–184. [Google Scholar] [CrossRef]
- Hanaire, H.; Lassmann-Vague, V.; Jeandidier, N.; Renard, E.; Tubiana-Rufi, N.; Vambergue, A.; Raccah, D.; Pinget, M.; Guerci, B. Treatment of diabetes mellitus using an external insulin pump: The state of the art. Diabetes Metab. 2008, 34, 401–423. [Google Scholar] [CrossRef]
- Lenhard, M.J.; Reeves, G.D. Continuous subcutaneous insulin infusion: A comprehensive review of insulin pump therapy. Arch. Intern. Med. 2001, 161, 2293–2300. [Google Scholar]
- Park, J.-H.; Allen, M.; Prausnitz, M. Polymer microneedles for controlled-release drug delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef]
- Mark, R.P. Microneedles for transdermal drug delivery. Advan. Drug Delivery Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles for gene and drug delivery. Annu. Rev. Biomed. Eng. 2000, 2, 289–313. [Google Scholar]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Evans, A.; Chiravuri, S.; Gianchandani, Y. Transdermal power transfer for recharging implanted drug delivery devices via the refill port. Biomed. Microdevices 2010, 12, 179–185. [Google Scholar] [CrossRef]
- Li, P.-Y.; Shih, J.; Lo, R.; Saati, S.; Agrawal, R.; Humayun, M.S.; Tai, Y.-C.; Meng, E. An electrochemical intraocular drug delivery device. Sens. Actuator. A 2008, 143, 41–48. [Google Scholar]
- Nathan, M. Microbattery technologies for miniaturized implantable medical devices. Curr. Pharm. Biotechnol. 2010, 11, 404–410. [Google Scholar] [CrossRef]
- Smitha, M.N.; Rao, A.M.; Popa, D.O.; Chiao, J.C.; Ativanichayaphong, T.; Sin, J.; Stephanou, H.E. MEMS-based implantable drug delivery system. In VII International Conference on Micro electro Mechanical Systems, 2005 TexMEMS, El Paso, TX, USA, 21–22 September 2005.
- Inke, J.; Lucas, R.; Leonard, H.; Hedley, H.; Vijay, V.; Chris, B.; Simon, M.; Stefan, E.; David, S.; Said, A.-S.; Derek, A. Wireless RF communication in biomedical applications. Smart Mater. Struct. 2008, 17. [Google Scholar]
- Studer, V.; Hang, G.; Pandolfi, A.; Ortiz, M.; Anderson, W.F.; Quake, S.R. Scaling properties of a low-actuation pressure microfluidic valve. J. Appl. Phys. 2004, 95, 393–398. [Google Scholar] [CrossRef]
- Rahimi, S.; Sarraf, E.; Wong, G.; Takahata, K. Implantable drug delivery device using frequency-controlled wireless hydrogel microvalves. Biomed. Microdevices 2011, 13, 267–277. [Google Scholar] [CrossRef]
- Po-Ying, L.; Sheybani, R.; Gutierrez, C.A.; Kuo, J.T.W.; Meng, E. A parylene bellows electrochemical actuator. J. Microelectromechanical. Syst. 2010, 19, 215–228. [Google Scholar] [CrossRef]
- Chung, A.; Huh, Y.; Erickson, D. A robust, electrochemically driven microwell drug delivery system for controlled vasopressin release. Biomed. Microdevices 2009, 11, 861–867. [Google Scholar] [CrossRef]
- Shawgo, R.S.; Richards Grayson, A.C.; Li, Y.; Cima, M.J. BioMEMS for drug delivery. Curr. Opin. Solid State Mat. Sci. 2002, 6, 329–334. [Google Scholar] [CrossRef]
- Tsai, N.C.; Sue, C.Y. Review of MEMS-based drug delivery and dosing systems. Sens. Actuator. A 2007, 134, 555–564. [Google Scholar] [CrossRef]
- Gensler, H.; Sheybani, R.; Li, P.Y.; Lo Mann, R.; Meng, E. An implantable MEMS micropump system for drug delivery in small animals. Biomed. Microdevices 2012, 14, 483–496. [Google Scholar]
- Tsai, N.-C.; Sue, C.-Y. Review of MEMS-based drug delivery and dosing systems. Sens. Actuator. A 2007, 134, 555–564. [Google Scholar] [CrossRef]
- Humble, P.H.; Harb, J.N.; LaFollette, R. Microscopic nickel-zinc batteries for use in autonomous microsystems. J. Electrochem. Soc. 2001, 148, A1357–A1361. [Google Scholar] [CrossRef]
- Nathan, M.; Golodnitsky, D.; Yufit, V.; Strauss, E.; Ripenbein, T.; Shechtman, I.; Menkin, S.; Peled, E. Three-dimensional thin-film Li-ion microbatteries for autonomous MEMS. J. Microelectromechanical. Syst. 2005, 14, 879–885. [Google Scholar] [CrossRef]
- Goto, K.; Nakagawa, T.; Nakamura, O.; Kawata, S. An implantable power supply with an optically rechargeable lithium battery. IEEE Trans. Biomed. Eng. 2001, 48, 830–833. [Google Scholar] [CrossRef]
- Tang, T.B.; Smith, S.; Flynn, B.W.; Stevenson, J.T.M.; Gundlach, A.M.; Reekie, H.M.; Murray, A.F.; Renshaw, D.; Dhillon, B.; Ohtori, A.; Inoue, Y.; Terry, J.G.; Walton, A.J. Implementation of wireless power transfer and communications for an implantable ocular drug delivery system. IET Nanobiotechnol. 2008, 2, 72–79. [Google Scholar] [CrossRef]
- Geipel, A.; Goldschmidtboeing, F.; Jantscheff, P.; Esser, N.; Massing, U.; Woias, P. Design of an implantable active microport system for patient specific drug release. Biomed. Microdevices 2008, 10, 469–478. [Google Scholar] [CrossRef]
- Don, W.D.; Said, A.-S.; Derek, A. Modelling and simulation of wirelessly and securely interrogated low-powered actuators for bio-MEMS. Smart Mat. Struct. 2011, 20, 015025. [Google Scholar]
- Defrère, S.; Mestagdt, M.; Riva, R.; Krier, F.; van Langendonckt, A.; Drion, P.; Jérôme, C.; Evrard, B.; Dehoux, J.-P.; Foidart, J.-M.; Donnez, J. In vivo biocompatibility of three potential intraperitoneal implants. Macromol. Biosci. 2011, 11, 1336–1345. [Google Scholar] [CrossRef]
- Smith, S.; Tang, T.B.; Terry, J.G.; Stevenson, J.T.M.; Flynn, B.W.; Reekie, H.M.; Murray, A.F.; Gundlach, A.M.; Renshaw, D.; Dhillon, B.; Ohtori, A.; Inoue, Y.; Walton, A.J. Development of a miniaturised drug delivery system with wireless power transfer and communication. IET Nanobiotechnol. 2007, 1, 80–86. [Google Scholar] [CrossRef]
- Najafi, N.; Ludomirsky, A. Initial animal studies of a wireless, batteryless, MEMS implant for cardiovascular applications. Biomed. Microdevices 2004, 6, 61–65. [Google Scholar] [CrossRef]
- Vaillancourt, P.; Djemouai, A.; Harvey, J.F.; Sawan, M. EM Radiation Behavior upon Biological Tissues in a Radio-Frequency Power Transfer Link for a Cortical Visual Implant. In Proceedings of the 19th Annual International Conference of the IEEE on Engineering in Medicine and Biology Society, Chicago, IL, USA, 30 October–2 November 1997; 2496, pp. 2499–2502.
- Prescott, J.H.; Lipka, S.; Baldwin, S.; Sheppard, N.F.; Maloney, J.M.; Coppeta, J.; Yomtov, B.; Staples, M.A.; Santini, J.T. Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat. Biotech. 2006, 24, 437–438. [Google Scholar] [CrossRef]
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely triggerable drug delivery systems. Adv. Mat. 2010, 22, 4925–4943. [Google Scholar] [CrossRef]
- Grayson, A.C.R.; Choi, I.S.; Tyler, B.M.; Wang, P.P.; Brem, H.; Cima, M.J.; Langer, R. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat. Mater. 2003, 2, 767–772. [Google Scholar]
- Desai, T.A.; Hansford, D.; Ferrari, M. Characterization of micromachined silicon membranes for immunoisolation and bioseparation applications. J. Membrane Sci. 1999, 159, 221–231. [Google Scholar] [CrossRef]
- Deo, S.K.; Moschou, E.A.; Peteu, S.F.; Bachas, L.G.; Daunert, S.; Eisenhardt, P.E.; Madou, M.J. Peer reviewed: Responsive drug delivery systems. Anal. Chem. 2003, 75, 206A–213A. [Google Scholar]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Delivery Rev. 2012. in press. Available online: http://dx.doi.org/10.1016/j.addr.2012.09.024.
- Miyata, T.; Asami, N.; Uragami, T. A reversibly antigen-responsive hydrogel. Nature 1999, 399, 766–769. [Google Scholar]
- Eddington, D.T.; Beebe, D.J. Flow control with hydrogels. Adv. Drug Delivery Rev. 2004, 56, 199–210. [Google Scholar]
- Chen, J.; Chu, M.; Koulajian, K.; Wu, X.; Giacca, A.; Sun, Y. A monolithic polymeric microdevice for pH-responsive drug delivery. Biomed. Microdevices 2009, 11, 1251–1257. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Delivery Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Park, H.; Park, K. Biocompatibility issues of implantable drug delivery systems. Pharm. Res. 1996, 13, 1770–1776. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Tseng, H.-J. In vitro biocompatibility of PTMO-based polyurethanes and those containing PDMS blocks. J. Biomat. Appl. 2004, 19, 135–146. [Google Scholar] [CrossRef]
- Kurian, P.; Kasibhatla, B.; Daum, J.; Burns, C.A.; Moosa, M.; Rosenthal, K.S.; Kennedy, J.P. Synthesis, permeability and biocompatibility of tricomponent membranes containing polyethylene glycol, polydimethylsiloxane and polypentamethylcyclopentasiloxane domains. Biomaterials 2003, 24, 3493–3503. [Google Scholar] [CrossRef]
- Sanders, R.S.; Lee, M.T. Implantable pacemakers. Proc. IEEE 1996, 84, 480–486. [Google Scholar] [CrossRef]
- Kane, M.J.; Breen, P.P.; Quondamatteo, F.; ÓLaighin, G. BION microstimulators: A case study in the engineering of an electronic implantable medical device. Med. Eng. Phys. 2011, 33, 7–16. [Google Scholar] [CrossRef]
- Ferrara, L.; Fleischman, A.; Dunning, J.; Zorman, C.; Roy, S. Effects of biomedical sterilization processes on performance characteristics of MEMS pressure sensors. Biomed. Microdevices 2007, 9, 809–814. [Google Scholar] [CrossRef]
- Voskerician, G.; Shive, M.S.; Shawgo, R.S.; Recum, H.V.; Anderson, J.M.; Cima, M.J.; Langer, R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials 2003, 24, 1959–1967. [Google Scholar] [CrossRef]
- Wisniewski, N.; Moussy, F.; Reichert, W.M. Characterization of implantable biosensor membrane biofouling. Fresenius J. Anal. Chem. 2000, 366, 611–621. [Google Scholar] [CrossRef]
- Reuben, B.G.; Perl, O.; Morgan, N.L.; Stratford, P.; Dudley, L.Y.; Hawes, C. Phospholipid coatings for the prevention of membrane fouling. J. Chem. Technol. Biotechnol. 1995, 63, 85–91. [Google Scholar] [CrossRef]
- Clausen, I.; Seeberg, T.M.; Gheorghe, C.; Wang, D.T. Biofouling on Protective Coatings for Implantable MEMS. In IEEE Sensors, Kona, HI, USA, 1–4 November 2010; pp. 751–754.
- Schmehl, J.M.; Harder, C.; Wendel, H.P.; Claussen, C.D.; Tepe, G. Silicon carbide coating of nitinol stents to increase antithrombogenic properties and reduce nickel release. Cardiovasc. Revascularization Medicine 2008, 9, 255–262. [Google Scholar] [CrossRef]
- Singh, R.A.; Siyuan, L.; Satyanarayana, N.; Kustandi, T.S.; Sinha, S.K. Bio-inspired polymeric patterns with enhanced wear durability for microsystem applications. Mat. Sci. Eng. C 2011, 31, 1577–1583. [Google Scholar] [CrossRef]
- Subhash, G.; Corwin, A.; de Boer, M. Evolution of wear characteristics and frictional behavior in MEMS devices. Tribology. Lett. 2011, 41, 177–189. [Google Scholar] [CrossRef]
- Hongbin, Y.; Guangya, Z.; Sinha, S.K.; Leong, J.Y.; Fook Siong, C. Characterization and reduction of MEMS sidewall friction using novel microtribometer and localized lubrication method. J. Microelectromechanical. Syst. 2011, 20, 991–1000. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Xue, Q.; Yuan, N.; Ding, J. A facile method to improve tribological properties of silicon surface by combining nanogrooves patterning and thin film lubrication. Colloid. Surface. A 2010, 372, 139–145. [Google Scholar] [CrossRef]
- Mercanzini, A.; Cheung, K.; Buhl, D.L.; Boers, M.; Maillard, A.; Colin, P.; Bensadoun, J.-C.; Bertsch, A.; Renaud, P. Demonstration of cortical recording using novel flexible polymer neural probes. Sens. Actuator. A: 2008, 143, 90–96. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Meth. 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Lee, K.; Singh, A.; He, J.; Massia, S.; Kim, B.; Raupp, G. Polyimide based neural implants with stiffness improvement. Sens. Actuator. B 2004, 102, 67–72. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef]
- Yuli, W.; Jeng-Hao, P.; Hsuan-Hong, L.; Christopher, E.S.; Mark, B.; Li, G.P.; Nancy, L.A. Surface graft polymerization of SU-8 for bio-MEMS applications. J. Micromechanic. Microengineer. 2007, 17, 1371–1380. [Google Scholar]
- Bouaidat, S.; Winther-Jensen, B.; Christensen, S.F.; Jonsmann, J. Plasma-polymerized coatings for bio-MEMS applications. Sens. Actuator. A 2004, 110, 390–394. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tng, D.J.H.; Hu, R.; Song, P.; Roy, I.; Yong, K.-T. Approaches and Challenges of Engineering Implantable Microelectromechanical Systems (MEMS) Drug Delivery Systems for in Vitro and in Vivo Applications. Micromachines 2012, 3, 615-631. https://doi.org/10.3390/mi3040615
Tng DJH, Hu R, Song P, Roy I, Yong K-T. Approaches and Challenges of Engineering Implantable Microelectromechanical Systems (MEMS) Drug Delivery Systems for in Vitro and in Vivo Applications. Micromachines. 2012; 3(4):615-631. https://doi.org/10.3390/mi3040615
Chicago/Turabian StyleTng, Danny Jian Hang, Rui Hu, Peiyi Song, Indrajit Roy, and Ken-Tye Yong. 2012. "Approaches and Challenges of Engineering Implantable Microelectromechanical Systems (MEMS) Drug Delivery Systems for in Vitro and in Vivo Applications" Micromachines 3, no. 4: 615-631. https://doi.org/10.3390/mi3040615



