Motility Control of Bacteria-Actuated Biodegradable Polymeric Microstructures by Selective Adhesion Methods †
Abstract
:1. Introduction
2. Preparation of Microfabricated Structures and Bacteria-Tethering
2.1. Biodegradable Polymeric Microstructures

2.2. Preparation of Treated Microstructures

2.3. Bacteria Culture and Tethering


3. Motility Measurements
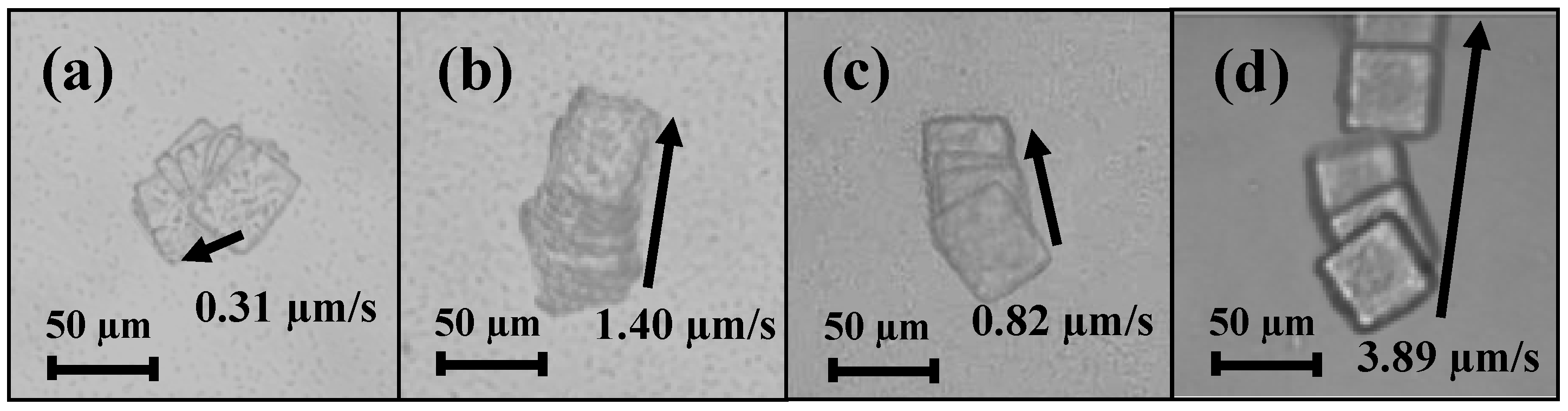
| Preferential tethering method | Total directional moving distance during 1 min (μm) | Average velocity (μm/s) |
|---|---|---|
| Untreated | 18.6 | 0.31 |
| BSA coating | 84 | 1.40 |
| X-ray irradiation treatment | 49.2 | 0.82 |
| Plasma treatment | 233.4 | 3.89 |

4. Conclusions
Acknowledgments
Author Contributions
Supplementary Materials
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug delivery systems: entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [PubMed]
- Zafar Razzacki, S.; Thwar, P.K.; Yang, M.; Ugaz, V.M.; Burns, M.A. Integrated microsystems for controlled drug delivery. Adv. Drug Delivery Rev. 2004, 56, 185–198. [Google Scholar]
- Jain, R.; Shah, N.H.; Malick, A.W.; Rhodes, C.T. Controlled drug delivery by biodegradable poly (ester) devices: Different preparative approaches. Drug Dev. Ind. Pharm. 1998, 24, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cho, S.K. Mini and micro propulsion for medical swimmers. Micromachines 2014, 5, 97–113. [Google Scholar] [CrossRef]
- Bogue, R. The development of medical microrobots: A review of progress. Ind. Robot. 2008, 35, 294–299. [Google Scholar] [CrossRef]
- Sowa, Y.; Berry, R.M. Bacterial flagellar motor. Q. Rev. Biophys. 2008, 41, 103–132. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Imada, K.; Namba, K. Molecular motors of the bacterial flagella. Curr. Opin. Struc. Biol. 2008, 18, 693–701. [Google Scholar] [CrossRef]
- Copeland, M.F.; Weibel, D.B. Bacterial swarming: A model system for studying dynamic self-assembly. Soft Matter 2009, 5, 1174–1187. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.C.; Robert, A.A. Bacteria swim by rotating their flagellar filaments. Nature 1973, 380–382. [Google Scholar]
- Forbes, N.S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 2010, 10, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Darnton, N.; Turner, L.; Breuer, K.; Berg, H.C. Moving fluid with bacterial carpets. Biophys. J. 2004, 86, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Le, U.N.; Kim, H.S.; Kwon, J.S.; Kim, M.Y.; Nguyen, V.H.; Jiang, S.N.; Lee, B.I.; Hong, Y.; Shin, M.G.; Rhee, J.H.; Bom, H.S.; Ahn, Y.; Gambhir, S.S.; Choy, H.E.; Min, J.J. Engineering and visualization of bacteria for targeting infarcted myocardium. Mol. Ther. 2011, 19, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Patyar, S.; Joshi, R.; Byrav, D.P.; Prakash, A.; Medhi, B.; Das, B.K. Bacteria in cancer therapy: A novel experimental strategy. J. Biomed. Sci. 2010, 17, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Park, S.H.; Cho, S.; Kim, D.M.; Lee, Y.; Ko, S.Y.; Hong, Y.; Choy, H.E.; Min, J.J.; Park, J.O.; Park, S. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci. Rep. 2013, 3, 3394. [Google Scholar] [PubMed]
- Taherkhani, S.; Mohammadi, M.; Daoud, J.; Martel, S.; Tabrizian, M. Covalent binding of nanoliposomes to the surface of magnetotactic bacteria for the synthesis of self-propelled therapeutic agents. ACS Nano 2014, 8, 5049–5060. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Liu, A.; Diller, E.; Sitti, M. Chemotactic steering of bacteria propelled microbeads. Biomed. Microdevices 2012, 14, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.L.; Zhulin, I.B.; Johnson, M.S. Aerotaxis and other energy-sensing behavior in bacteria. Annu. Rev. Microbiol. 1999, 53, 103–128. [Google Scholar] [CrossRef] [PubMed]
- Steager, E.B.; Sakar, M.S.; Kim, D.H.; Kumar, V.; Pappas, G.J.; Kim, M.J. Electrokinetic and optical control of bacterial microrobots. J. Micromech. Microeng. 2011, 21, 035001. [Google Scholar] [CrossRef]
- Frankel, R.B.; Bazylinski, D.A.; Johnson, M.S.; Taylor, B.L. Magneto-aerotaxis in marine coccoid bacteria. Biophys. J. 1997, 73, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Magdanz, V.; Sanchez, S.; Schmidt, O.G. Development of a sperm-flagella driven micro-bio-robot. Adv. Mater. 2013, 25, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.S.; Magdanz, V.; Sanchez, S.; Schmidt, O.G.; Misra, S. Biocompatible, accurate, and fully autonomous: A sperm-driven micro-bio-robot. J. Micro Bio Robot. 2014, 9, 79–86. [Google Scholar] [CrossRef]
- Dmochowski, R.R.; Staskin, D.R. Advances in drug delivery: Improved bioavailability and drug effect. Curr. Urol. Rep. 2002, 3, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Bae, H.; Kim, J.; Lim, B.; Park, J.O.; Park, S. Motility enhancement of bacteria actuated microstructures using selective bacteria adhesion. Lab Chip 2010, 10, 1706–1711. [Google Scholar] [PubMed]
- Cho, S.; Park, S.J.; Ko, S.Y.; Park, J.O.; Park, S. Development of bacteria-based microrobot using biocompatible poly(ethylene glycol). Biomed. Microdevices 2012, 14, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Koo, K.I.; Park, S.G.; Jeong, M.J.; Kim, G.S.; Park, J.H.; Lim, J.M.; Chung, W.J.; Lee, S.H.; Jin, S.W.; Lee, Y.S.; Park, T.H.; Yoo, J.Y.; Cho, D.I. Improvement of bacterial tethering using both physical and chemical surface modification for flagella spin actuators. Sens. Actuators B Chem. 2007, 123, 269–276. [Google Scholar] [CrossRef]
- Lee, S.; Hong, S.J.; Yoo, H.J.; Cho, D.I. Surface energy modification of biodegradable polymeric surfaces. In Proceedings of the International Symposium on Microchemistry and Microsystems 2011 in Conjunction with the Korean BioChip Society Spring Meeting, Seoul, Korea, 2–4 June 2011.
- Yoo, H.J.; Lee, S.M.; Ahn, J.H.; Oh, S.J.; Song, B.H.; Kim, S.J.; Seo, J.M.; Kim, T.Y.; Cho, D.I. Surface energy modification method using X-ray synchrotron irradiation for controlling bacterial adhesion on biodegradable-polymer structures for bacteria-flagellated microrobots. In Proceedings of the 2013 International Conference on Control, Automation and Systems, Gwangju, Korea, 20–23 October 2013.
- Yoo, H.J.; Park, J.H.; Lee, S.M.; Ahn, J.H.; Oh, S.J.; Song, B.H.; Shin, J.Y.; Cho, D.I. Motility of bacteria-attached microstructures: Bovine serum albumin coating and plasma treatment cases. In Proceedings of the 7th Asia-Pacific Conference of Transducers and Micro-Nano Technology, Daegu, Korea, 29 June–2 July 2014.
- Cho, D.I.; Yoo, H.J.; Lee, S.; Hong, S.J.; Seo, J.M.; Kim, T.Y.; Kim, S.J. Trends in drug-delivery-system microfabrication and a new scalable technology using X-ray synchrotron irradiation. In Proceedings of the 17th International Conference on Solid-State Sensors, Actuators and Microsystems, Barcelona, Spain, 16–20 June 2013.
- Yoo, H.J.; Lee, S.M.; Ahn, J.H.; Cho, D.I. Drug-loaded cubic micro-chamber made of a biodegradable polymer for bacteria-based drug delivery. In Proceedings of the 8th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013.
- Pohang Accelerator laboratory. Available online: http://pal.postech.ac.kr (accessed on 24 November 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.J.; Lee, S.; Cho, D.-i.D. Motility Control of Bacteria-Actuated Biodegradable Polymeric Microstructures by Selective Adhesion Methods. Micromachines 2014, 5, 1287-1295. https://doi.org/10.3390/mi5041287
Yoo HJ, Lee S, Cho D-iD. Motility Control of Bacteria-Actuated Biodegradable Polymeric Microstructures by Selective Adhesion Methods. Micromachines. 2014; 5(4):1287-1295. https://doi.org/10.3390/mi5041287
Chicago/Turabian StyleYoo, Hyung Jung, Sangmin Lee, and Dong-il Dan Cho. 2014. "Motility Control of Bacteria-Actuated Biodegradable Polymeric Microstructures by Selective Adhesion Methods" Micromachines 5, no. 4: 1287-1295. https://doi.org/10.3390/mi5041287
APA StyleYoo, H. J., Lee, S., & Cho, D.-i. D. (2014). Motility Control of Bacteria-Actuated Biodegradable Polymeric Microstructures by Selective Adhesion Methods. Micromachines, 5(4), 1287-1295. https://doi.org/10.3390/mi5041287






