A Reduced Graphene Oxide Based Radio Frequency Glucose Sensing Device Using Multi-Dimensional Parameters
Abstract
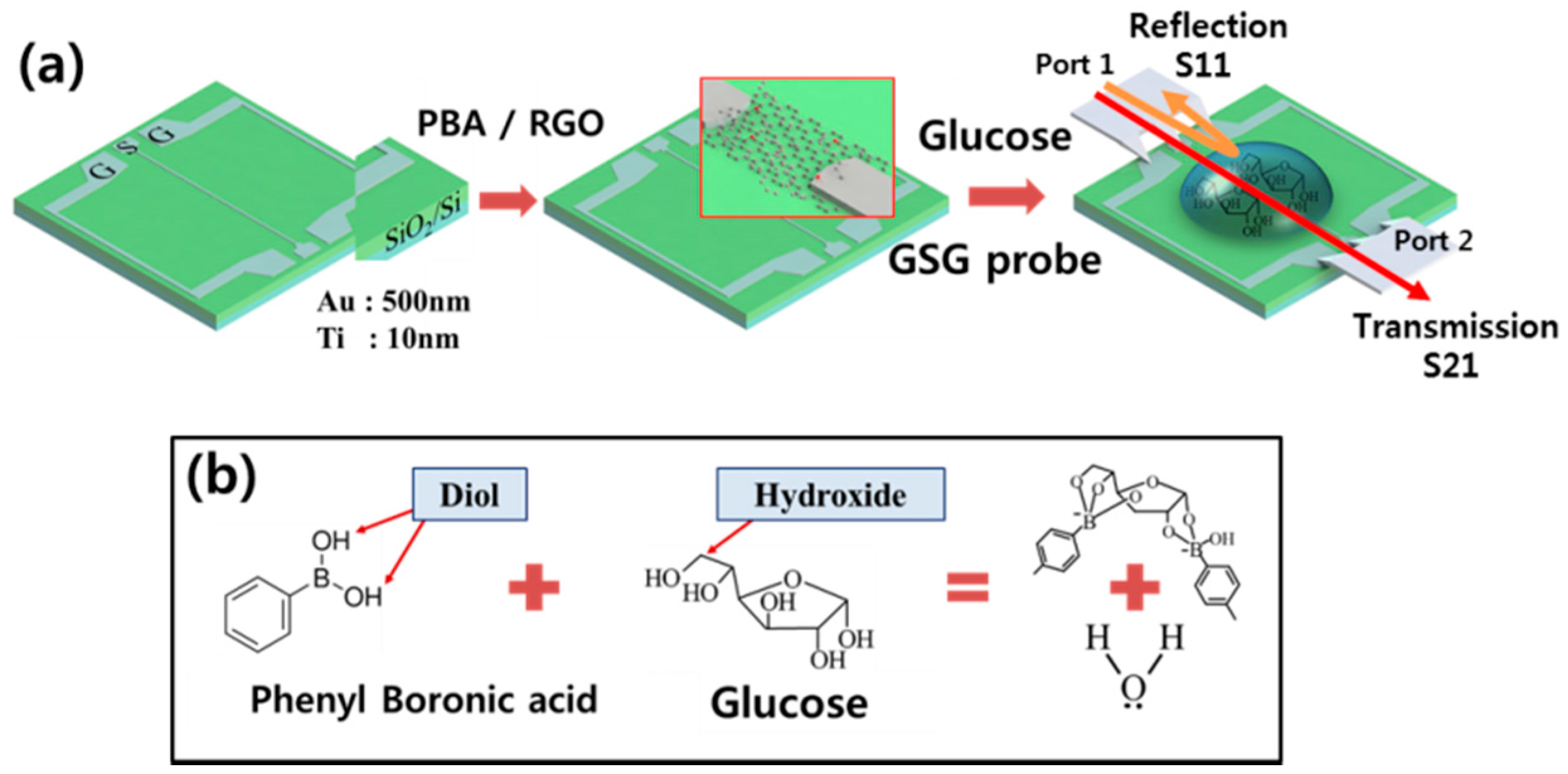
:1. Introduction
2. Experimental Section
3. Results and Discussion
4. Electrical Signal of RLGC
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harsányi, G. Sensors in Biomedical Applications: Fundamentals, Technology and Applications; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Khan, F.; Saxl, T.E.; Pickup, J.C. Fluorescence intensity-and lifetime-based glucose sensing using an engineered high-K d mutant of glucose/galactose-binding protein. Anal. Biochem. 2010, 399, 39–43. [Google Scholar] [PubMed]
- Wang, S.; Zhang, Q.; Wang, R.; Yoon, S. A novel multi-walled carbon nanotube-based biosensor for glucose detection. Biochem. Biophys. Res. Commun. 2003, 311, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Shafer-Peltier, K.E.; Haynes, C.L.; Glucksberg, M.R.; Van Duyne, R.P. Toward a glucose biosensor based on surface-enhanced raman scattering. J. Am. Chem. Soc. 2003, 125, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Moschou, E.A.; Sharma, B.V.; Deo, S.K.; Daunert, S. Fluorescence glucose detection: Advances toward the ideal in vivo biosensor. J. Fluorescence 2004, 14, 535–547. [Google Scholar] [CrossRef]
- Cella, L.N.; Chen, W.; Myung, N.V.; Mulchandani, A. Single-walled carbon nanotube-based chemiresistive affinity biosensors for small molecules: Ultrasensitive glucose detection. J. Am. Chem. Soc. 2010, 132, 5024–5026. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Jia, D.; He, Y.; Miao, Y.; Wu, H.-L. Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens. Bioelectron. 2011, 26, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Navarro, C.; Meyer, J.C.; Sundaram, R.S.; Chuvilin, A.; Kurasch, S.; Burghard, M.; Kern, K.; Kaiser, U. Atomic structure of reduced graphene oxide. Nano Lett. 2010, 10, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Morozov, S.; Novoselov, K.; Katsnelson, M.; Schedin, F.; Elias, D.; Jaszczak, J.; Geim, A. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.; Geim, A.K.; Morozov, S.; Jiang, D.; Katsnelson, M.; Grigorieva, I.; Dubonos, S.; Firsov, A. Two-dimensional gas of massless dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-M.; Li, H.-B.; Qu, F.; Zhang, X.-B.; Shen, G.-L.; Yu, R.-Q. In situ synthesis of palladium nanoparticle-graphene nanohybrids and their application in nonenzymatic glucose biosensors. Biosens. Bioelectron. 2011, 26, 3500–3504. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jiang, S.; Zhang, H.; Jiang, J.; Liu, X. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta 2012, 709, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.E.; Chen, J. Gas detection using low-temperature reduced graphene oxide sheets. Appl. Phys. Lett. 2009, 94, 083111. [Google Scholar] [CrossRef]
- Park, H.G.; Hwang, S.; Lim, J.; Kim, D.-H.; Song, I.S.; Kim, J.H.; Woo, D.H.; Lee, S.; Jun, S.C. Comparison of chemical vapor sensing properties between graphene and carbon nanotubes. Jpn. J. Appl. Phys. 2012, 51, 045101. [Google Scholar] [CrossRef]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, Y.; Wu, J.; Zhang, D. Graphene oxide sheet-mediated silver enhancement for application to electrochemical biosensors. Anal. Chem. 2010, 83, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Zhang, Y.; Wang, D.; Dai, H. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 2001, 123, 3838–3839. [Google Scholar] [CrossRef] [PubMed]
- Zayats, M.; Katz, E.; Willner, I. Electrical contacting of flavoenzymes and nad (p)+-dependent enzymes by reconstitution and affinity interactions on phenylboronic acid monolayers associated with au-electrodes. J. Am. Chem. Soc. 2002, 124, 14724–14735. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Davis, E.N.; Anderson, J.; Lin, Q.; Wang, Q. Development of boronic acid grafted random copolymer sensing fluid for continuous glucose monitoring. Biomacromolecules 2008, 10, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Lee, J.-H.; Jung, H.-I. A symmetric metamaterial element-based RF biosensor for rapid and label-free detection. Appl. Phys. Lett. 2011, 99, 163703. [Google Scholar] [CrossRef]
- Kim, W.K.; Jung, Y.M.; Cho, J.H.; Kang, J.Y.; Oh, J.Y.; Kang, H.; Lee, H.-J.; Kim, J.H.; Lee, S.; Shin, H. Radio-frequency characteristics of graphene oxide. Appl. Phys. Lett. 2010, 97, 193103. [Google Scholar] [CrossRef]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Kim, S.J.; Lim, J.; Some, S.; Park, J.-E.; Kim, S.-J.; Kim, C.; Lee, T.J.; Jun, S.C. Tunable wide blue photoluminescence with europium decorated graphene. J. Mater. Chem. C 2015, 3, 4030–4038. [Google Scholar] [CrossRef]
- Rani, J.; Oh, J.; Park, J.-E.; Lim, J.; Park, B.; Kim, K.; Kim, S.-J.; Jun, S.C. Controlling the luminescence emission from palladium grafted graphene oxide thin films via reduction. Nanoscale 2013, 5, 5620–5627. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Liu, B.; Jiang, Y.-B. An extremely sensitive monoboronic acid based fluorescent sensor for glucose. Anal. Chim. Acta 2004, 515, 285–290. [Google Scholar] [CrossRef]
- Stephenson-Brown, A.; Wang, H.-C.; Iqbal, P.; Preece, J.A.; Long, Y.; Fossey, J.S.; James, T.D.; Mendes, P.M. Glucose selective surface plasmon resonance-based bis-boronic acid sensor. Analyst 2013, 138, 7140–7145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Losego, M.D.; Braun, P.V. Hydrogel-based glucose sensors: Effects of phenylboronic acid chemical structure on response. Chem. Mater. 2013, 25, 3239–3250. [Google Scholar] [CrossRef]
- Shiino, D.; Murata, Y.; Kataoka, K.; Koyama, Y.; Yokoyama, M.; Okano, T.; Sakurai, Y. Preparation and characterization of a glucose-responsive insulin-releasing polymer device. Biomaterials 1994, 15, 121–128. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Guo, Y.; Cheng, C.; Yang, L.; Jiang, L.; Yu, G.; Hu, W.; Liu, Y.; Zhu, D. Reduction of graphene oxide to highly conductive graphene by lawesson’s reagent and its electrical applications. J. Mater. Chem. C 2013, 1, 3104–3109. [Google Scholar] [CrossRef]
- Dworsky, L.N. Modern Transmission Line Theory and Applications; RE Krieger Pub. Co.: Malabar, FL, USA, 1979. [Google Scholar]
- Eisenstadt, W.R.; Eo, Y. S-parameter-based IC interconnect transmission line characterization. IEEE Trans. Compon. Hybrids Manuf. Technol. 1992, 15, 483–490. [Google Scholar] [CrossRef]
- Jun, S.C.; Choi, J.; Cha, S.; Baik, C.; Lee, S.; Kim, H.J.; Hone, J.; Kim, J. Radio-frequency transmission characteristics of a multi-walled carbon nanotube. Nanotechnology 2007, 18, 255701. [Google Scholar] [CrossRef]
- Craddock, G.; Diamond, P. Theory of shear suppression of edge turbulence by externally driven radio-frequency waves. Phys. Rev. Lett. 1991, 67, 1535. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, E.F.; Berry, L.A.; Batchelor, D.B. Second-order radio frequency kinetic theory with applications to flow drive and heating in tokamak plasmas. Phys. Plasmas 2000, 7, 641–656. [Google Scholar] [CrossRef]
- Parks, G.S.; Barton, L.E.; Spaght, M.E.; Richardson, J.W. The viscosity of undercooled liquid glucose. J. Appl. Phys. 1934, 5, 193–199. [Google Scholar] [CrossRef]
- Harper, A.; Anderson, M.R. Electrochemical glucose sensors—Developments using electrostatic assembly and carbon nanotubes for biosensor construction. Sensors 2010, 10, 8248–8274. [Google Scholar] [CrossRef] [PubMed]





| Range of Glucose Concentration (mM) | Linearity (R-Square, %) | |||
|---|---|---|---|---|
| R | L | G | C | |
| 0.1–0.4 | 83.15 ± 4.22 | 69.04 ± 31.41 | 23.80 ± 2.28 | 21.51 ± 6.15 |
| 1–4 | 95.45 ± 9.60 | 93.23 ± 29.37 | 69.28 ± 42.54 | 86.49 ± 19.82 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.; Park, H.G.; Ji, J.-h.; Cho, J.; Jun, S.C. A Reduced Graphene Oxide Based Radio Frequency Glucose Sensing Device Using Multi-Dimensional Parameters. Micromachines 2016, 7, 136. https://doi.org/10.3390/mi7080136
Park B, Park HG, Ji J-h, Cho J, Jun SC. A Reduced Graphene Oxide Based Radio Frequency Glucose Sensing Device Using Multi-Dimensional Parameters. Micromachines. 2016; 7(8):136. https://doi.org/10.3390/mi7080136
Chicago/Turabian StylePark, Byeongho, Hyung Goo Park, Jae-hoon Ji, Jinsoo Cho, and Seong Chan Jun. 2016. "A Reduced Graphene Oxide Based Radio Frequency Glucose Sensing Device Using Multi-Dimensional Parameters" Micromachines 7, no. 8: 136. https://doi.org/10.3390/mi7080136






