Continuous Jetting of Alginate Microfiber in Atmosphere Based on a Microfluidic Chip
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Principle and Structure Design
2.3. Microfluidic Device Fabrication
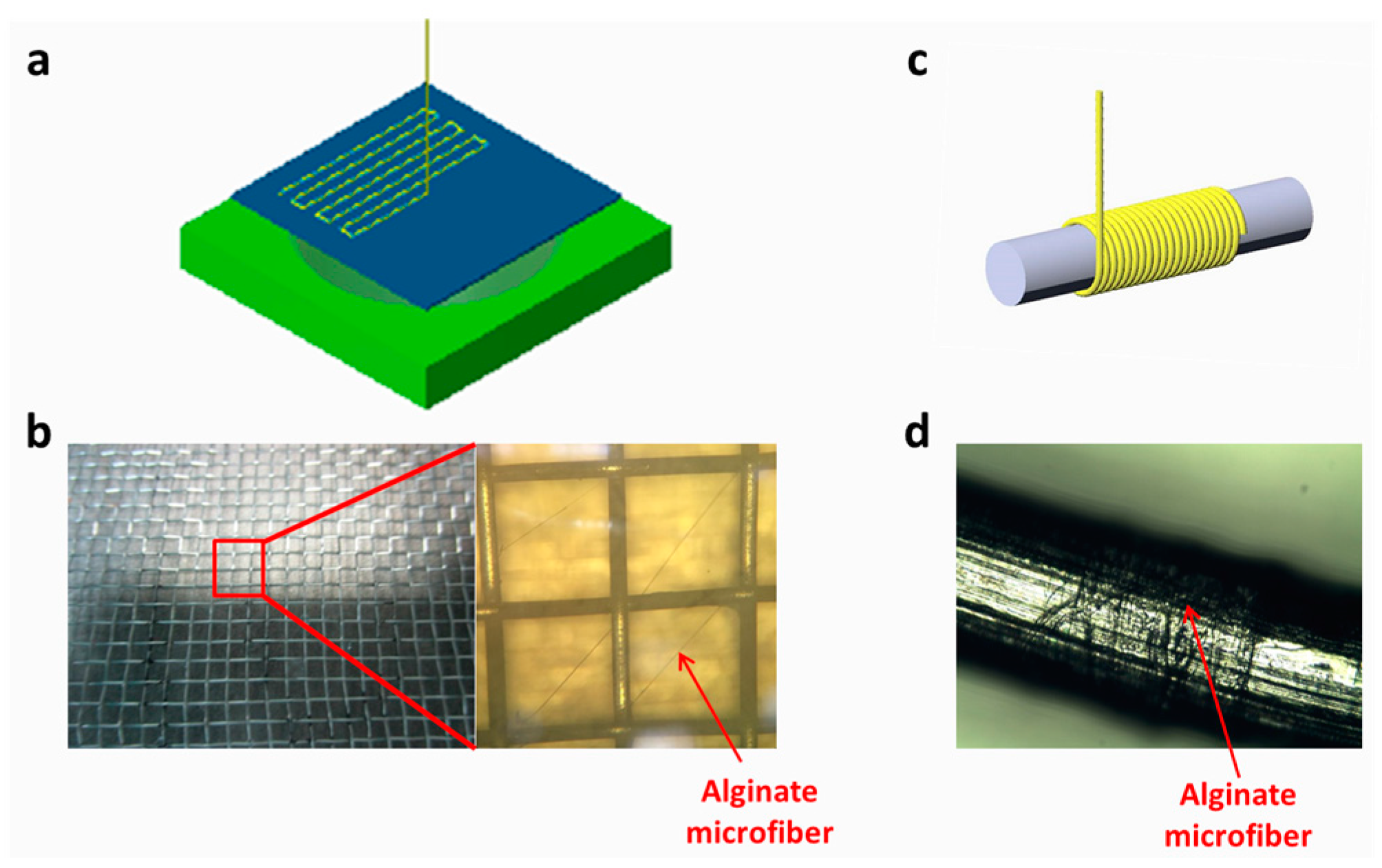
2.4. Fabrication and Characterization of Microfiber
3. Results and Discussion
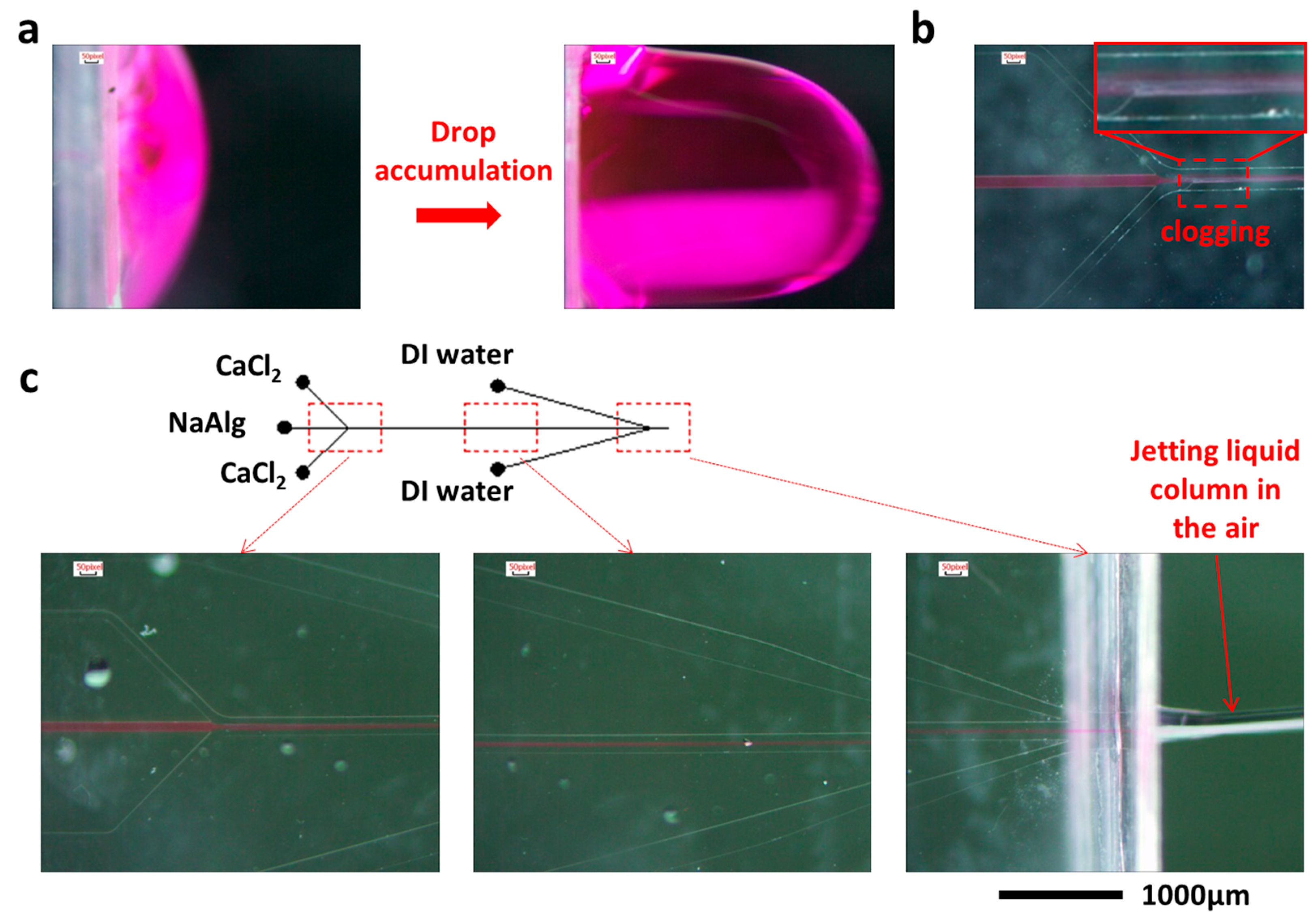
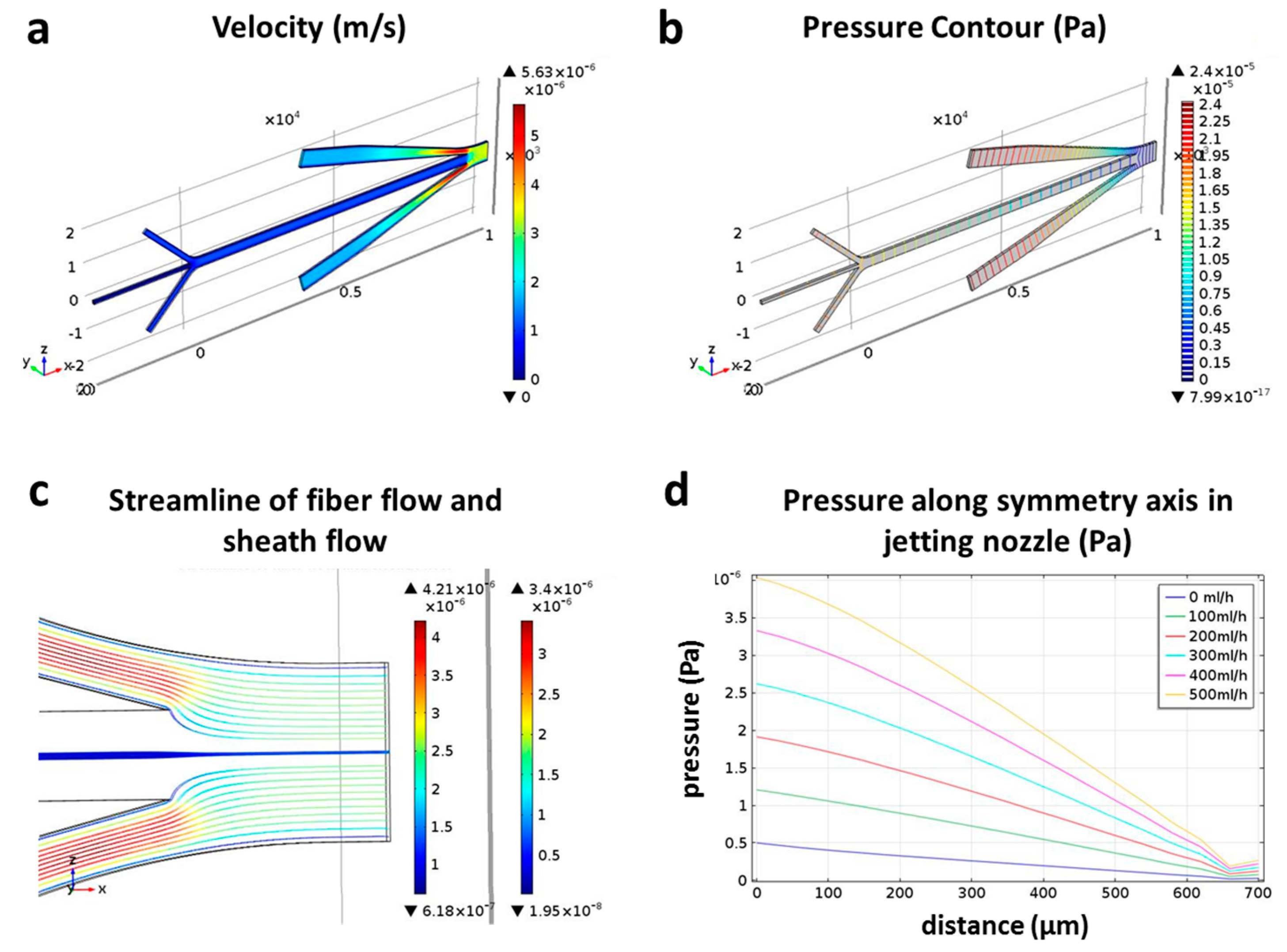
3.1. Continuous Jetting of Microfiber
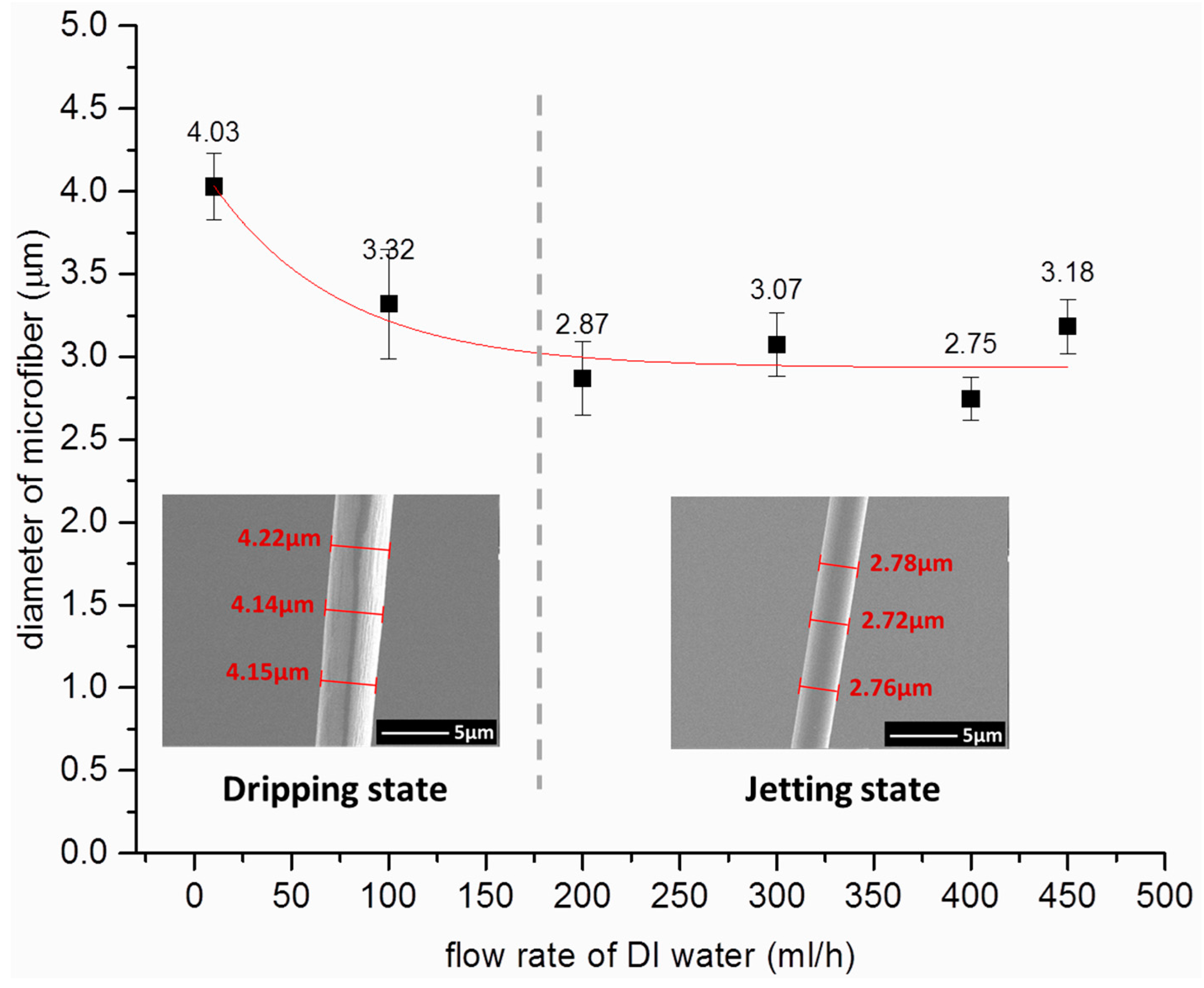
3.2. Effect of Sheath Flow on the Diameter of Microfiber
3.3. Microfiber Collection and Assembly
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Onoe, H.; Takeuchi, S. Cell-laden microfibers for bottom-up tissue engineering. Drug Discov. Today 2015, 20, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.G.; Lee, K.-H.; Khademhosseini, A.; Lee, S.-H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip 2012, 12, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Pham, U.H.; Hanif, M.; Asthana, A.; Iqbal, S.M. A microfluidic device approach to generate hollow alginate microfibers with controlled wall thickness and inner diameter. J. Appl. Phys. 2015, 117, 214703. [Google Scholar] [CrossRef]
- Ji, X.; Guo, S.; Zeng, C.; Wang, C.; Zhang, L. Continuous generation of alginate microfibers with spindle-knots by using a simple microfluidic device. RSC Adv. 2015, 5, 2517–2522. [Google Scholar] [CrossRef]
- Bonhomme, O.; Leng, J.; Colin, A. Microfluidic wet-spinning of alginate microfibers: A theoretical analysis of fiber formation. Soft Matter 2012, 8, 10641–10649. [Google Scholar] [CrossRef]
- Shin, S.-J.; Park, J.-Y.; Lee, J.-Y.; Park, H.; Park, Y.-D.; Lee, K.-B.; Whang, C.-M.; Lee, S.-H. “On the fly” continuous generation of alginate fibers using a microfluidic device. Langmuir 2007, 23, 9104–9108. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Shin, S.J.; Kim, C.B.; Kim, J.K.; Cho, Y.W.; Chung, B.G.; Lee, S.H. Microfluidic synthesis of pure chitosan microfibers for bio-artificial liver chip. Lab Chip 2010, 10, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-H.; Lin, P.-W.; Lin, Y.-C. Chitosan microfiber fabrication using a microfluidic chip and its application to cell cultures. Microfluid. Nanofluid. 2010, 8, 115–121. [Google Scholar] [CrossRef]
- He, X.-H.; Wang, W.; Deng, K.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Microfluidic fabrication of chitosan microfibers with controllable internals from tubular to peapod-like structures. RSC Adv. 2015, 5, 928–936. [Google Scholar] [CrossRef]
- Hwang, C.; Park, Y.; Park, J.; Lee, K.; Sun, K.; Khademhosseini, A.; Lee, S.H. Controlled cellular orientation on PLGA microfibers with defined diameters. Biomed. Microdevices 2009, 11, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.M.; Khademhosseini, A.; Park, Y.; Sun, K.; Lee, S.-H. Microfluidic chip-based fabrication of PLGA microfiber scaffolds for tissue engineering. Langmuir 2008, 24, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
- Attalla, R.; Ling, C.; Selvaganapathy, P. Fabrication and characterization of gels with integrated channels using 3D printing with microfluidic nozzle for tissue engineering applications. Biomed. Microdevices 2016, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Choi, Y.Y.; Chae, S.K.; Moon, J.H.; Chang, J.Y.; Lee, S.H. Microfluidic spinning of flat alginate fibers with grooves for cell-aligning scaffolds. Adv. Mater. 2012, 24, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Thangawng, A.L.; Howell, P.B., Jr.; Spillmann, C.M.; Naciri, J.; Ligler, F.S. UV polymerization of hydrodynamically shaped fibers. Lab Chip 2011, 11, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yu, Y.; Fu, F.; Wang, J.; Shang, L.; Gu, Z.; Zhao, Y. Controlled fabrication of bioactive microfibers for creating tissue constructs using microfluidic techniques. ACS Appl. Mater. Interfaces 2016, 8, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.-J.; Wang, W.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Microfluidic generation of hollow Ca-alginate microfibers. Lab Chip 2016, 16, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; He, X.; Yang, Y.; Wei, D.; Sun, J.; Zhong, M.; Xie, R.; Fan, H.; Zhang, X. Microfluidic-based generation of functional microfibers for biomimetic complex tissue construction. Acta Biomater. 2016, 38, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Choi, C.-H.; Chung, S.; Chung, Y.-M.; Lee, C.-S. Microfluidic synthesis of a cell adhesive Janus polyurethane microfiber. Lab Chip 2009, 9, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zheng, F.; Lu, J.; Shang, L.; Xie, Z.; Zhao, Y.; Chen, Y.; Gu, Z. Bioinspired multicompartmental microfibers from microfluidics. Adv. Mater. 2014, 26, 5184–5190. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jeong, G.S.; Choi, Y.Y.; Lee, K.H.; Khademhosseini, A.; Lee, S.H. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater. 2011, 10, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Kim, M.J.; Hwang, Y.H.; Jeon, E.A.; Kang, A.R.; Lee, S.H.; Lee, D.Y. Microfluidics-generated pancreatic islet microfibers for enhanced immunoprotection. Biomaterials 2013, 34, 8122–8130. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Utoh, R.; Ohashi, K.; Tatsumi, K.; Yamato, M.; Okano, T.; Seki, M. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials 2012, 33, 8304–8315. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Sugaya, S.; Naganuma, Y.; Seki, M. Microfluidic synthesis of chemically and physically anisotropic hydrogel microfibers for guided cell growth and networking. Soft Matter 2012, 8, 3122. [Google Scholar] [CrossRef]
- Leong, M.F.; Toh, J.K.; Du, C.; Narayanan, K.; Lu, H.F.; Lim, T.C.; Wan, A.C.; Ying, J.Y. Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres. Nat. Commun. 2013, 4, 2353. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Mun, C.H.; Kang, E.; No, D.Y.; Ju, J.; Lee, S.H. One-stop microfiber spinning and fabrication of a fibrous cell-encapsulated scaffold on a single microfluidic platform. Biofabrication 2014, 6, 024108. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Nam, J.; Sun, W. Direct cell writing of 3D microorgan for in vitro pharmacokinetic model. Tissue Eng. Part C: Methods 2008, 14, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Kang, A.R.; Lee, J.S.; Park, S.-J.; Lee, D.Y.; Moon, S.-H.; Lee, S.-H. Microchip-based engineering of super-pancreatic islets supported by adipose-derived stem cells. Biomaterials 2014, 35, 4815–4826. [Google Scholar] [CrossRef] [PubMed]
- Trebbin, M.; Krüger, K.; DePonte, D.; Roth, S.V.; Chapman, H.N.; Förster, S. Microfluidic liquid jet system with compatibility for atmospheric and high-vacuum conditions. Lab Chip 2014, 14, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Huang, Z.-X.; Yang, R.-J. Three-dimensional hydrodynamic focusing in two-layer polydimethylsiloxane (PDMS) microchannels. J. Micromech. Microeng. 2007, 17, 1479. [Google Scholar] [CrossRef]
- Zhao, J.; You, Z. Microfluidic hydrodynamic focusing for high-throughput applications. J. Micromech. Microeng. 2015, 25, 125006. [Google Scholar] [CrossRef]
- Ambravaneswaran, B.; Subramani, H.J.; Phillips, S.D.; Basaran, O.A. Dripping-jetting transitions in a dripping faucet. Phys. Rev. Lett. 2004, 93, 034501. [Google Scholar] [CrossRef] [PubMed]
- Clanet, C.; Lasheras, J.C. Transition from dripping to jetting. J. Fluid Mech. 1999, 383, 307–326. [Google Scholar] [CrossRef]
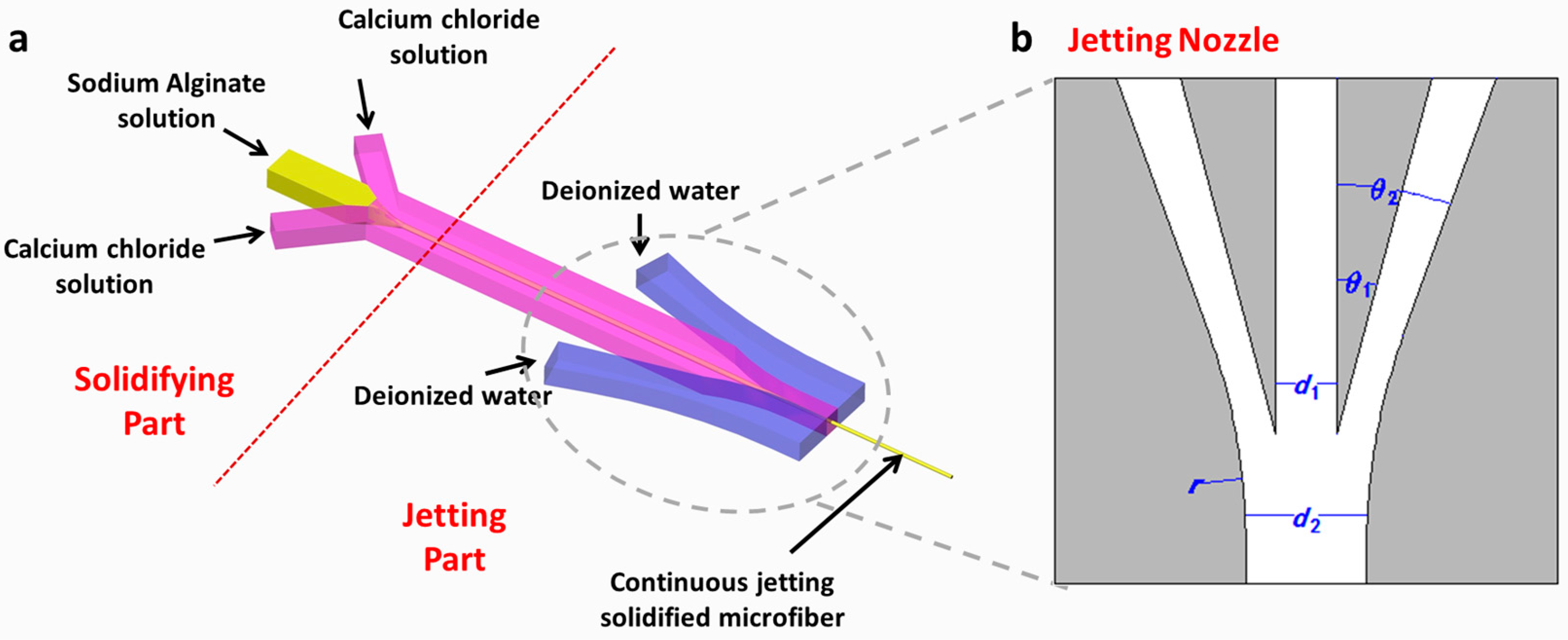

| Parameters | Definition | Values |
|---|---|---|
| Angle between the inner edge curve of sheath channel and the edge line of the central channel | 15° | |
| Angle between the outer edge curve of sheath channel and the edge line of the central channel | 20° | |
| d1 | Width of central channel | 300 μm |
| d2 | Width at the outlet | 600 μm |
| r | Radius of nozzle | 3000 μm |
| h | Height of microfluidic channel | 80 μm |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Xiong, W.; Yu, N.; Yang, X. Continuous Jetting of Alginate Microfiber in Atmosphere Based on a Microfluidic Chip. Micromachines 2017, 8, 8. https://doi.org/10.3390/mi8010008
Zhao J, Xiong W, Yu N, Yang X. Continuous Jetting of Alginate Microfiber in Atmosphere Based on a Microfluidic Chip. Micromachines. 2017; 8(1):8. https://doi.org/10.3390/mi8010008
Chicago/Turabian StyleZhao, Junyi, Wei Xiong, Ning Yu, and Xing Yang. 2017. "Continuous Jetting of Alginate Microfiber in Atmosphere Based on a Microfluidic Chip" Micromachines 8, no. 1: 8. https://doi.org/10.3390/mi8010008
APA StyleZhao, J., Xiong, W., Yu, N., & Yang, X. (2017). Continuous Jetting of Alginate Microfiber in Atmosphere Based on a Microfluidic Chip. Micromachines, 8(1), 8. https://doi.org/10.3390/mi8010008






