A Microfluidic Chip for Liquid Metal Droplet Generation and Sorting
Abstract
:1. Introduction
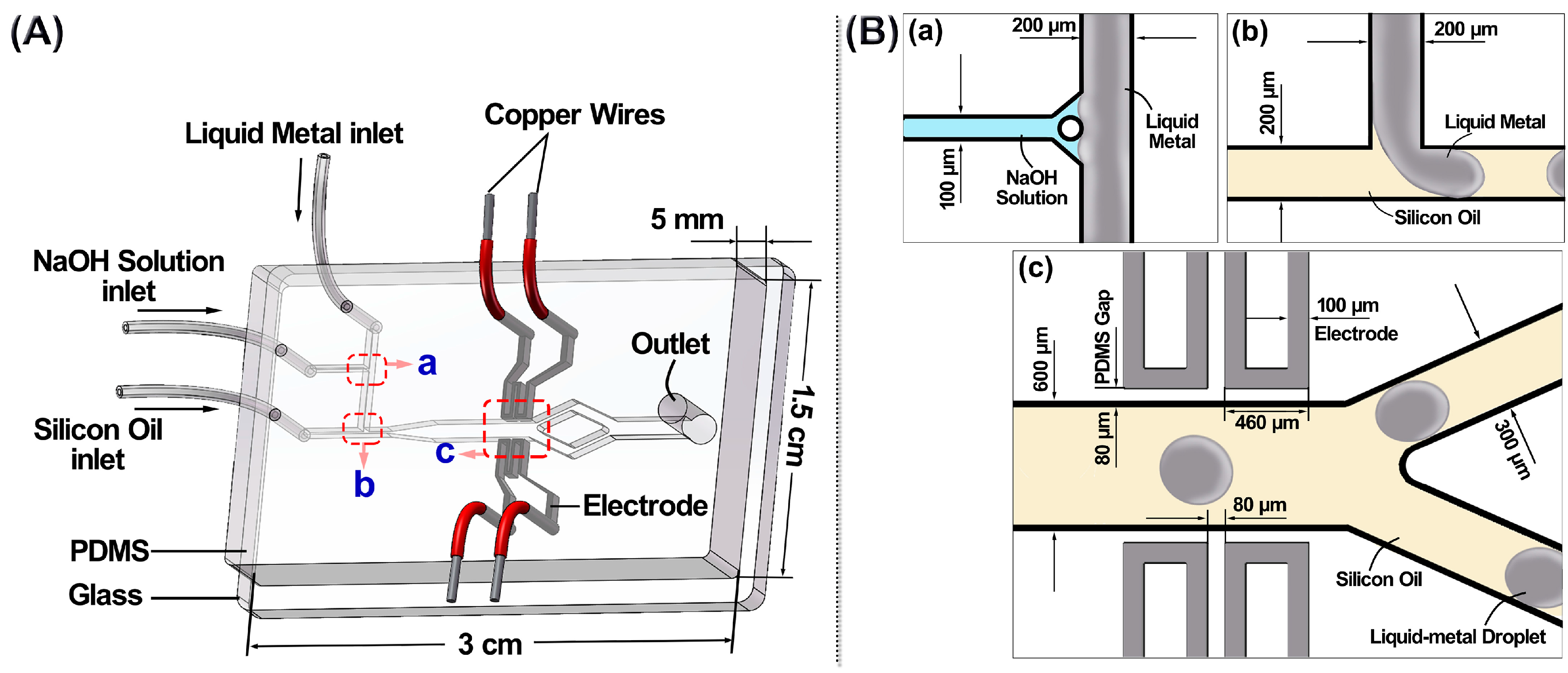
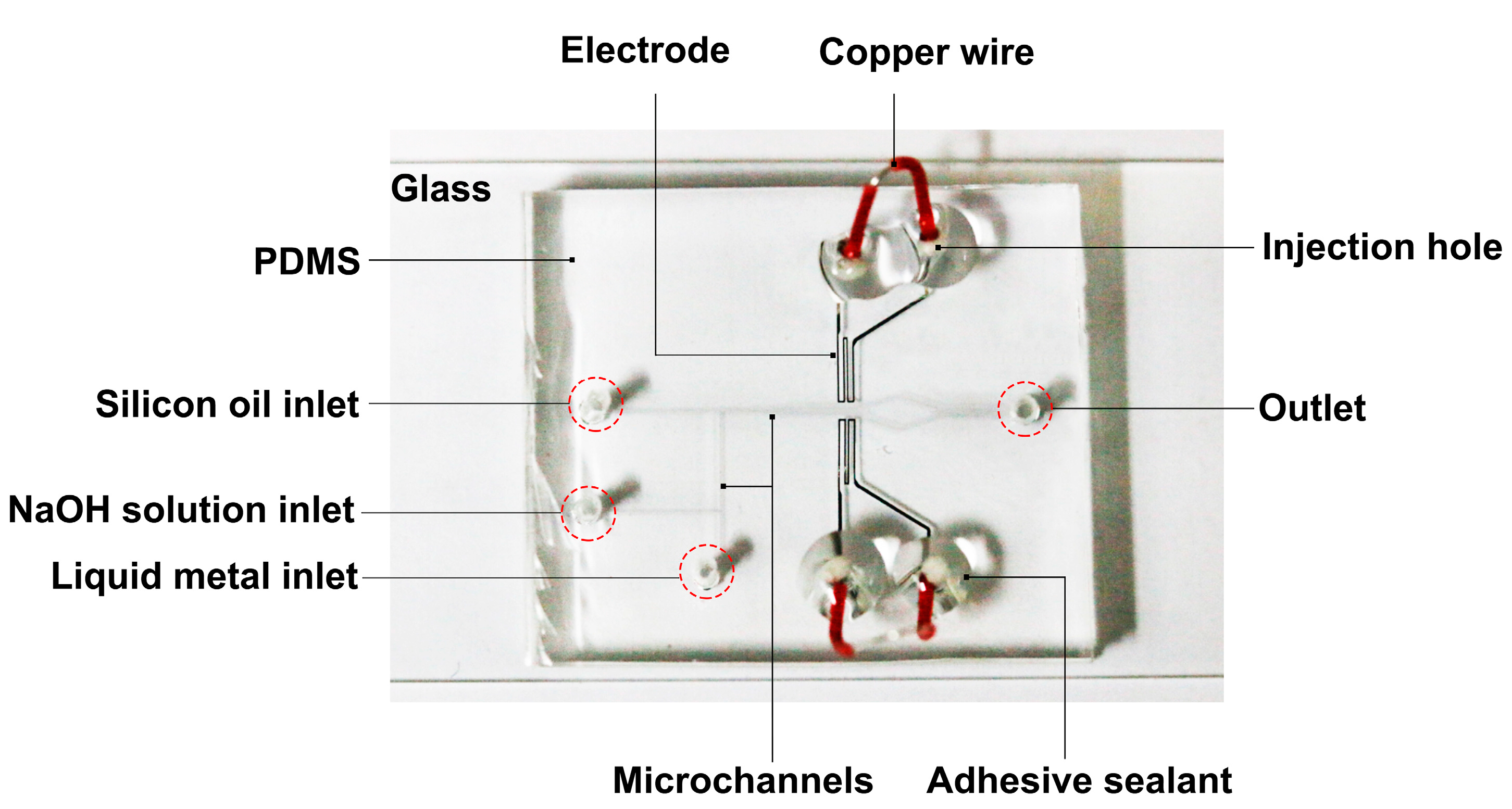
2. Liquid Metal Based Microfluidic Chip
3. Experimental Details
4. Results and Discussion
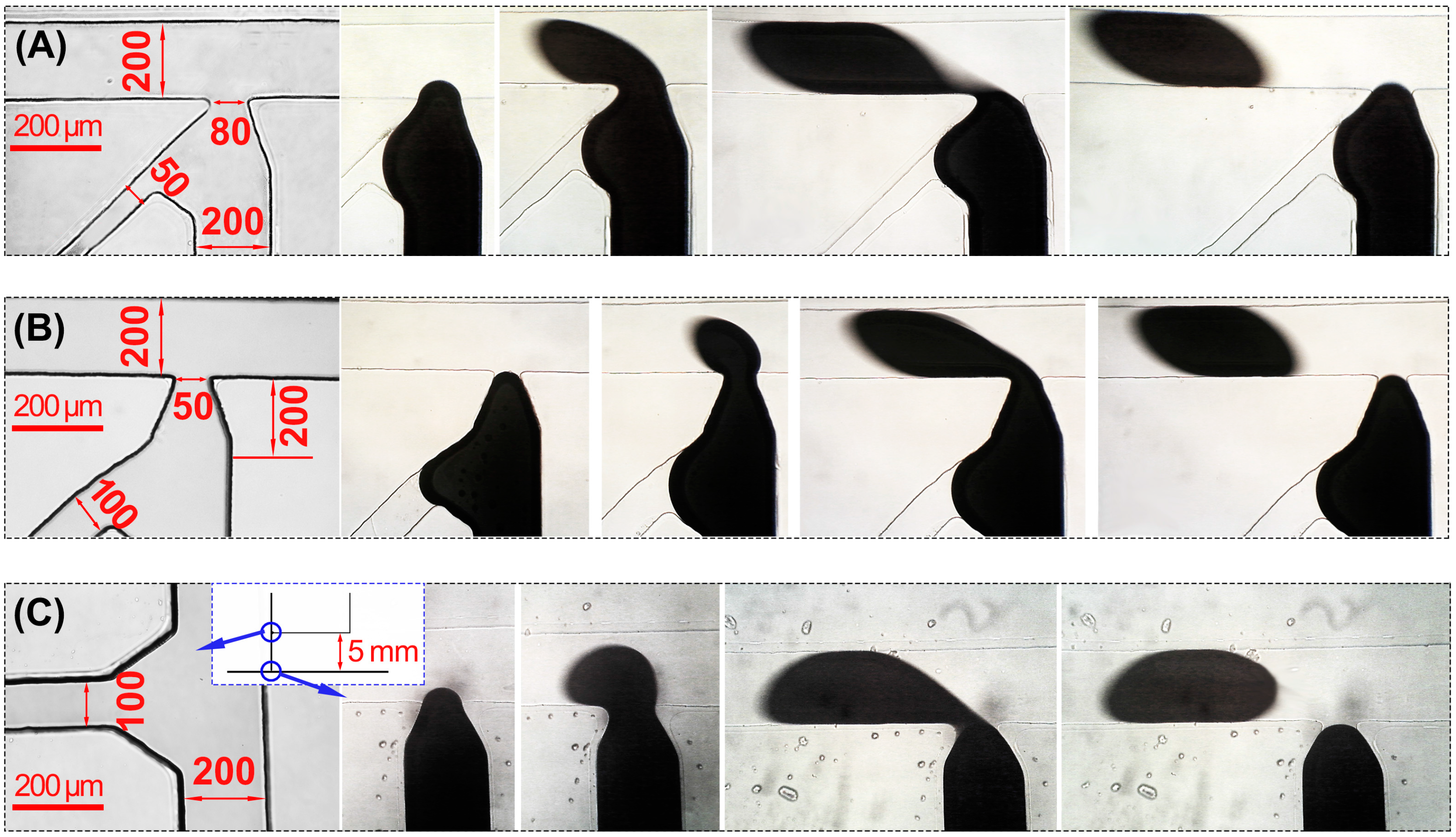
4.1. Microdroplet Generation on an F-Junction Chip
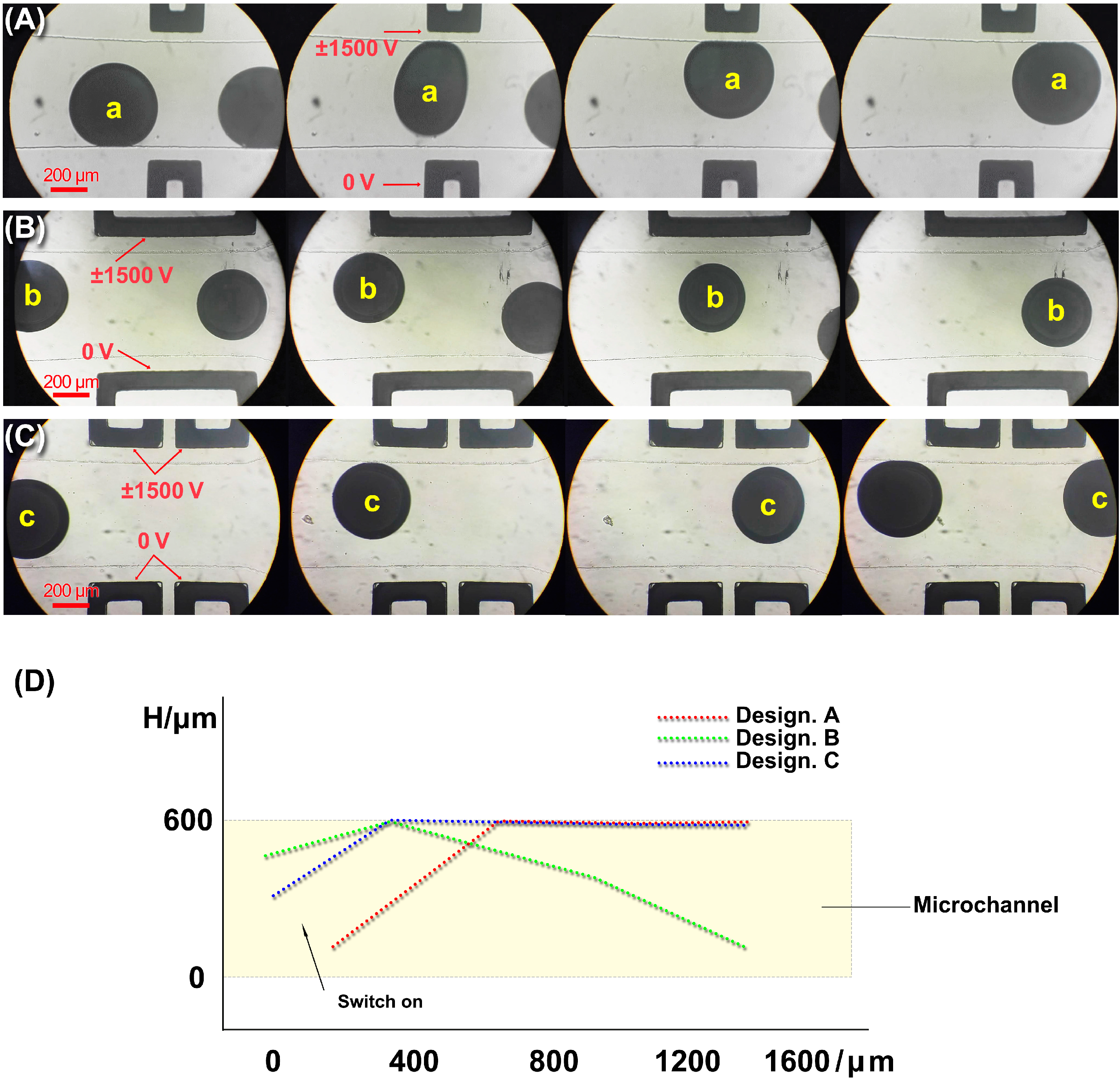
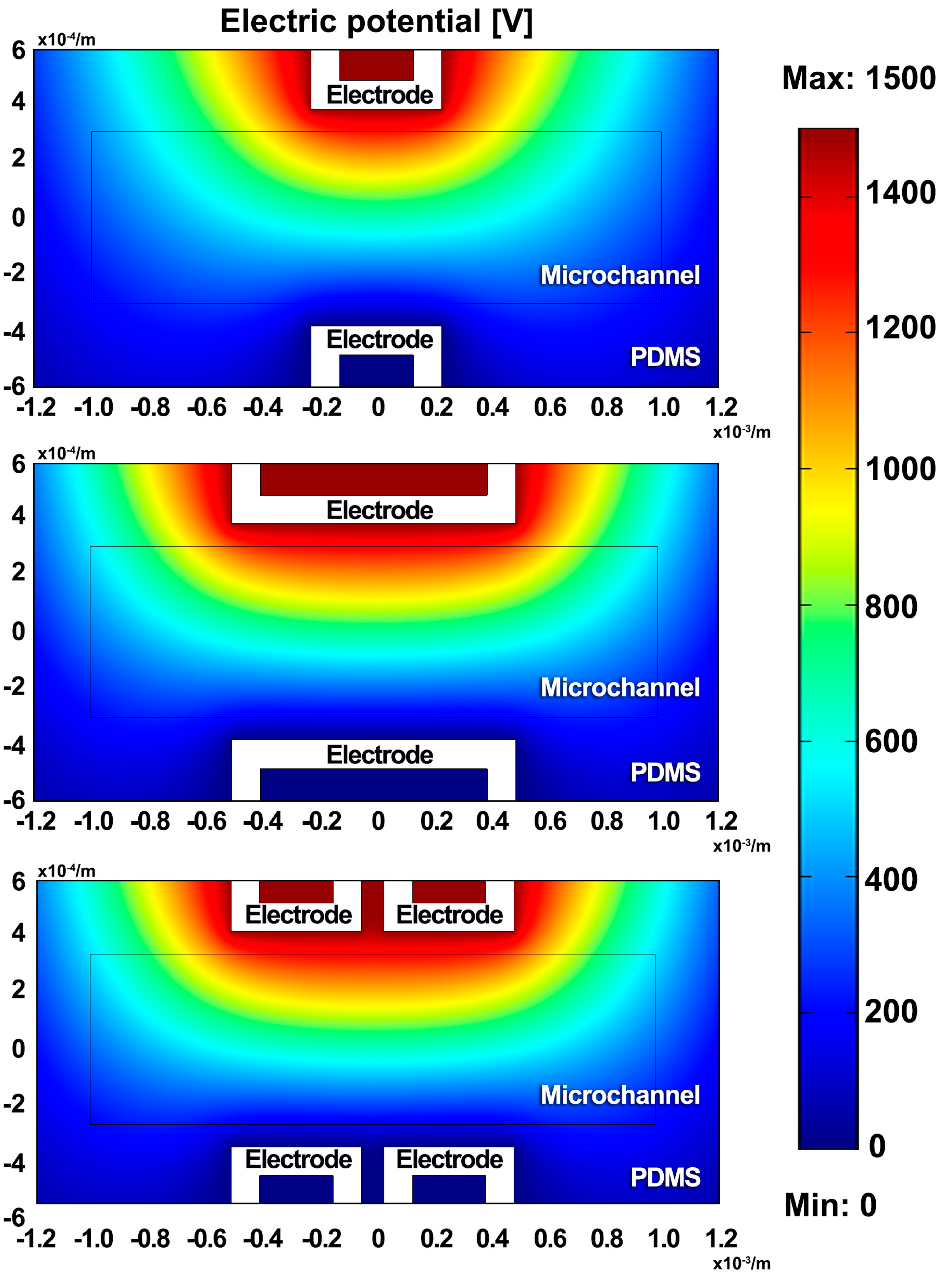
4.2. Liquid Metal Microdroplets Sorting
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Afrasiabi, R.; Soderberg, L.M.; Joensson, H.N.; Björk, P.; Andersson Svahn, H.; Linnros, J. Integration of a Droplet-Based Microfluidic System and Silicon Nanoribbon FET Sensor. Micromachines 2016, 7, 134. [Google Scholar] [CrossRef]
- Simon, M.G.; Li, Y.; Arulmoli, J.; McDonnell, L.P.; Akil, A.; Nourse, J.L.; Flanagan, L.A. Increasing label-free stem cell sorting capacity to reach transplantation-scale throughput. Biomicrofluidics 2014, 8, 064106. [Google Scholar] [CrossRef] [PubMed]
- Yesiloz, G.; Boybay, M.S.; Ren, C.L. Label-free high-throughput detection and content sensing of individual droplets in microfluidic systems. Lab Chip 2015, 15, 4008–4019. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Maw, M.M.; Song, Y.; Pan, X.; Sun, Y.; Li, D. Detection of size spectrum of microalgae cells in an integrated underwater microfluidic device. J. Exp. Mar. Biol. Ecol. 2015, 473, 129–137. [Google Scholar] [CrossRef]
- Isgor, P.K.; Marcali, M.; Keser, M.; Elbuken, C. Microfluidic droplet content detection using integrated capacitive sensors. Sens. Actuators B Chem. 2015, 10, 669–675. [Google Scholar] [CrossRef]
- Wong, R.D.P.; Posner, J.D.; Santos, V.J. Flexible microfluidic normal force sensor skin for tactile feedback. Sens. Actuators A Phys. 2012, 179, 62–69. [Google Scholar] [CrossRef]
- Gao, M.; Gui, L. Development of a Multi-Stage Electroosmotic Flow Pump Using Liquid Metal Electrodes. Micromachines 2016, 7, 165. [Google Scholar] [CrossRef]
- Harman, G.G. Hard Gallium Alloys for Use as Low Contact Resistance Electrodes and for Bonding Thermocouples into Samples. Rev. Sci. Instrum. 1960, 31, 717–720. [Google Scholar] [CrossRef]
- Gao, M.; Gui, L. A handy liquid metal based electroosmotic flow pump. Lab Chip 2014, 14, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Gui, L. Development of a fast thermal response microfluidic system using liquid metal. J. Micromech. Microeng. 2016, 26, 075005. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Lee, D.W. A galinstan-based inkjet printing system for highly stretchable electronics with self-healing capability. Lab Chip 2016, 16, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Glawdel, T.; Elbuken, C.; Ren, C.L. Droplet formation in microfluidic T-junction generators operating in the transitional regime. I. Experimental observations. Phys. Rev. E 2012, 85, 016322. [Google Scholar] [CrossRef] [PubMed]
- Glawdel, T.; Elbuken, C.; Ren, C.L. Droplet formation in microfluidic T-junction generators operating in the transitional regime. II. Modeling. Phys. Rev. E 2012, 85, 016323. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.; LeBlanc, B.E.; Kelley, M.; Fitzgerald, H.E.; Huff, G.H.; Han, A. Manipulating liquid metal droplets in microfluidic channels with minimized skin residues toward tunable RF applications. J. Microelectromech. Syst. 2015, 24, 1069–1076. [Google Scholar] [CrossRef]
- Dickey, M.D.; Chiechi, R.C.; Larsen, R.J.; Weiss, E.A.; Weitz, D.A.; Whitesides, G.M. Eutectic gallium-indium (EGaIn): A liquid metal alloy for the formation of stable structures in microchannels at room temperature. Adv. Funct. Mater. 2008, 18, 1097–1104. [Google Scholar] [CrossRef]
- Regan, M.J.; Kawamoto, E.H.; Lee, S.; Pershan, P.S.; Maskil, N.; Deutsch, M.; Magnussen, O.M.; Ocko, B.M.; Berman, L.E. Surface layering in liquid gallium: An X-ray reflectivity study. Phys. Rev. Lett. 1995, 75, 2498. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Parmar, M.; Lee, D.W. An oxidized liquid metal-based microfluidic platform for tunable electronic device applications. Lab Chip 2015, 15, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Hutter, T.; Bauer, W.A.C.; Elliott, S.R.; Huck, W.T. Formation of Spherical and Non-Spherical Eutectic Gallium-Indium Liquid-Metal Microdroplets in Microfluidic Channels at Room Temperature. Adv. Funct. Mater. 2012, 22, 2624–2631. [Google Scholar] [CrossRef]
- Li, G.; Parmar, M.; Kim, D.; Lee, J.B.J.; Lee, D.W. PDMS based coplanar microfluidic channels for the surface reduction of oxidized Galinstan. Lab Chip 2014, 14, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.C.; Fisher, J.S.; Lee, A.I.; Cristini, V.; Lee, A.P. Design of microfluidic channel geometries for the control of droplet volume, chemical concentration, and sorting. Lab Chip 2004, 4, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Takinoue, M.; Atsumi, Y.; Yoshikawa, K. Rotary motion driven by a direct current electric field. Appl. Phys. Lett. 2010, 96, 104105. [Google Scholar] [CrossRef] [Green Version]
- Guzman, A.R.; Kim, H.S.; de Figueiredo, P.; Han, A. A three-dimensional electrode for highly efficient electrocoalescence-based droplet merging. Biomed. Microdevices 2015, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yao, S. Electrostatic charging and control of droplets in microfluidic devices. Lab Chip 2013, 13, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chuang, H.S.; Wereley, S.T. Dynamic manipulation by light and electric fields: Micrometer particles to microliter droplets. Langmuir 2010, 26, 7656–7660. [Google Scholar] [CrossRef] [PubMed]
- Renaudot, R.; Agache, V.; Daunay, B.; Lambert, P.; Kumemura, M.; Fouillet, Y.; Fujita, H. Optimization of liquid dielectrophoresis (LDEP) digital microfluidic transduction for biomedical applications. Micromachines 2011, 2, 258–273. [Google Scholar] [CrossRef]
- Dash, S.; Mohanty, S.; Pradhan, S.; Mishra, B.K. CFD design of a microfluidic device for continuous dielectrophoretic separation of charged gold nanoparticles. J. Taiwan Inst. Chem. Eng. 2016, 58, 39–48. [Google Scholar] [CrossRef]
- Fan, S.K.; Huang, P.W.; Wang, T.T.; Peng, Y.H. Cross-scale electric manipulations of cells and droplets by frequency-modulated dielectrophoresis and electrowetting. Lab Chip 2008, 8, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Aubry, N. Transport and deformation of droplets in a microdevice using dielectrophoresis. Electrophoresis 2007, 28, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.A. Dielectrophoresis: The Behavior of Neutral Matter in Nonuniform Electric Fields; Cambridge University Press: Cambridge, UK, 1978; Volume 80. [Google Scholar]
- XIAMETER®. Available online: https://www.xiameter.com/en/Products/Pages/ProductDetail.aspx?pid=01013084&R=X68EN&C=US#characteristicsAnchor (accessed on 23 January 2017).
- Kuo, A.C. Poly(dimethylsiloxane). In Polymer Data Handbook; Oxford University Press: Oxford, UK, 1999; pp. 411–435. [Google Scholar]
- Gao, M.; Gui, L. Micro thermal lab in a droplet. In Proceedings of the International Mechanical Engineering Congress and Exposition, San Diego, CA, USA, 15–21 November 2013.


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Gao, M.; Gui, L. A Microfluidic Chip for Liquid Metal Droplet Generation and Sorting. Micromachines 2017, 8, 39. https://doi.org/10.3390/mi8020039
Tian L, Gao M, Gui L. A Microfluidic Chip for Liquid Metal Droplet Generation and Sorting. Micromachines. 2017; 8(2):39. https://doi.org/10.3390/mi8020039
Chicago/Turabian StyleTian, Lu, Meng Gao, and Lin Gui. 2017. "A Microfluidic Chip for Liquid Metal Droplet Generation and Sorting" Micromachines 8, no. 2: 39. https://doi.org/10.3390/mi8020039
APA StyleTian, L., Gao, M., & Gui, L. (2017). A Microfluidic Chip for Liquid Metal Droplet Generation and Sorting. Micromachines, 8(2), 39. https://doi.org/10.3390/mi8020039






