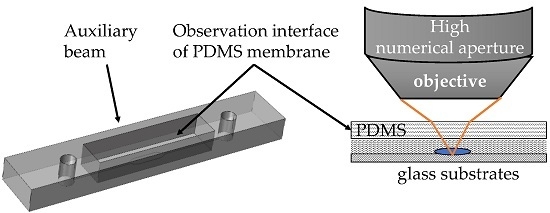
Observation Interface of PDMS Membrane in a Microfluidic Chip Based on One-Step Molding
Abstract
:1. Introduction
2. Materials and Methods
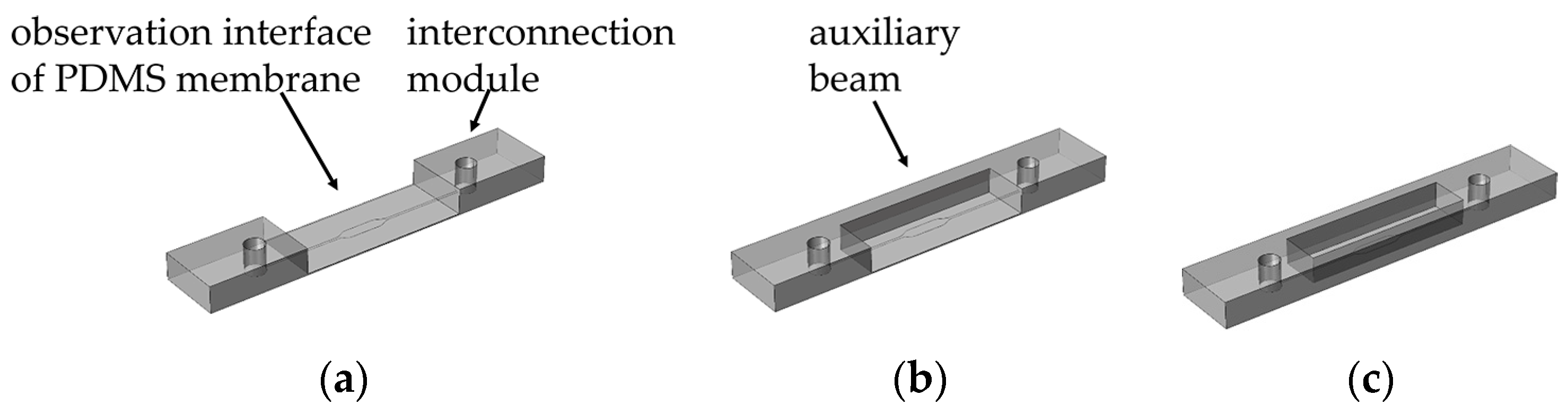
2.1. Design of Microfluidic Chips
2.2. Fabrication Process of One-Step Molding
2.3. Leakage Test
2.4. Experimental Procedure
3. Results and Discussion
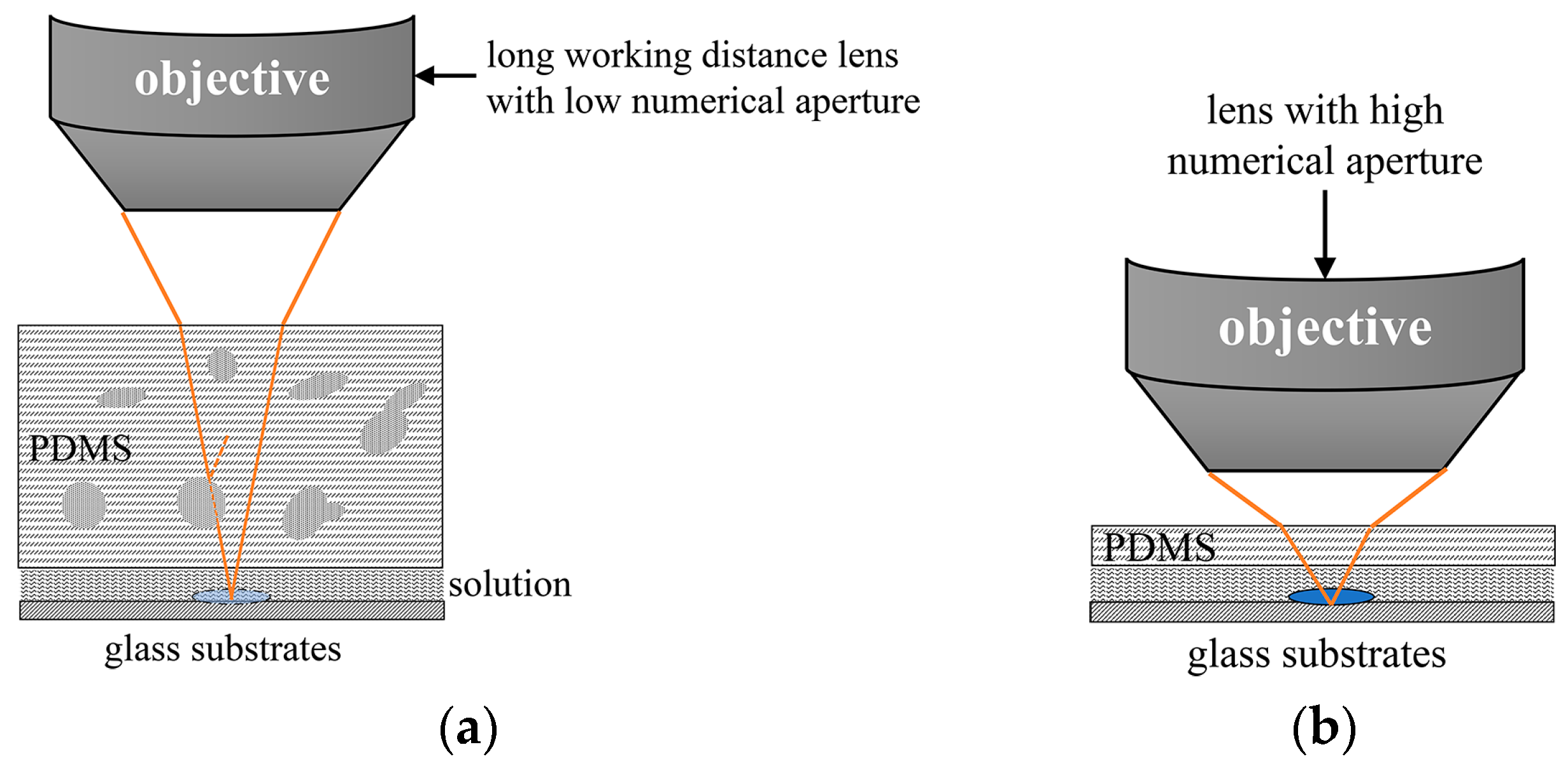
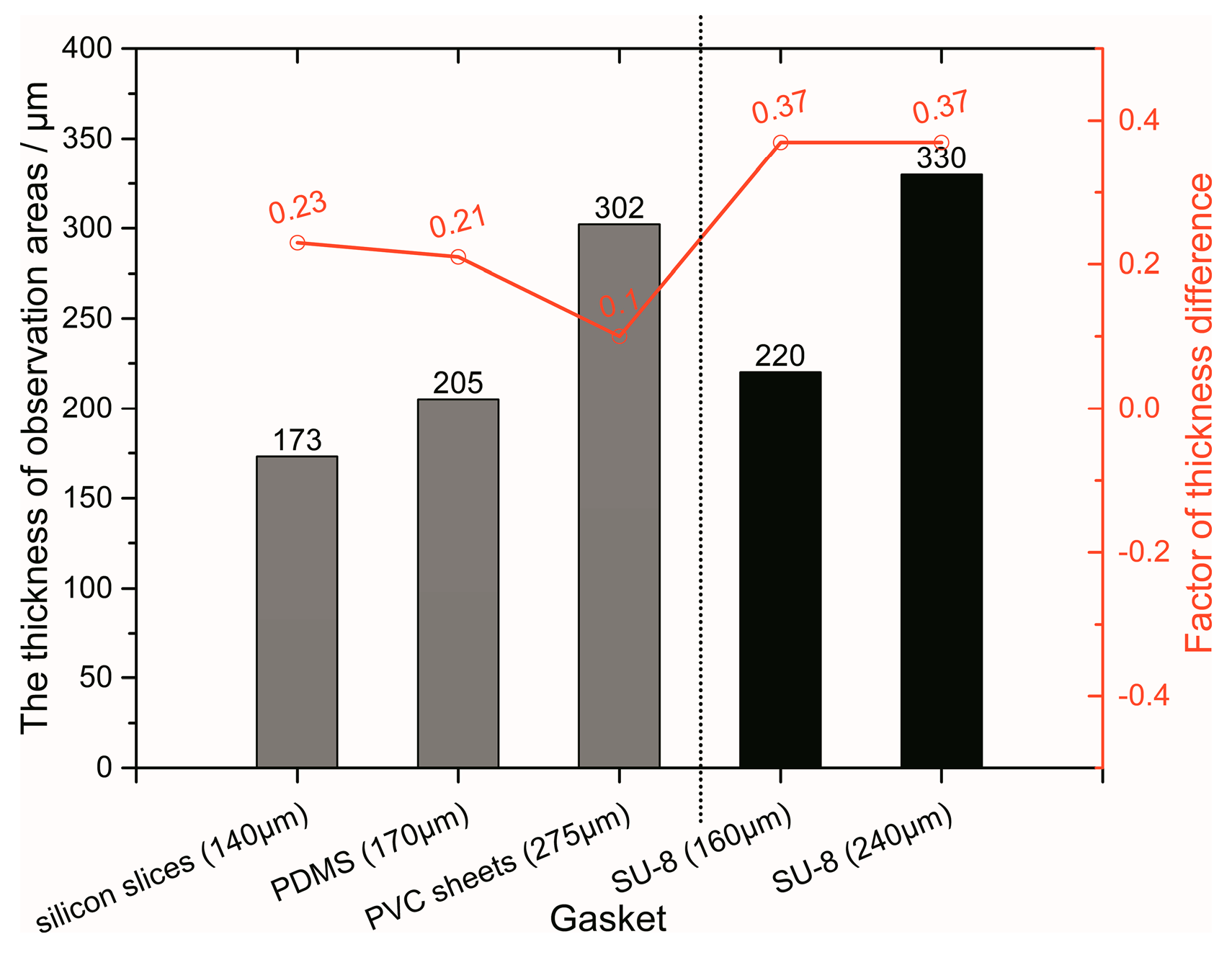
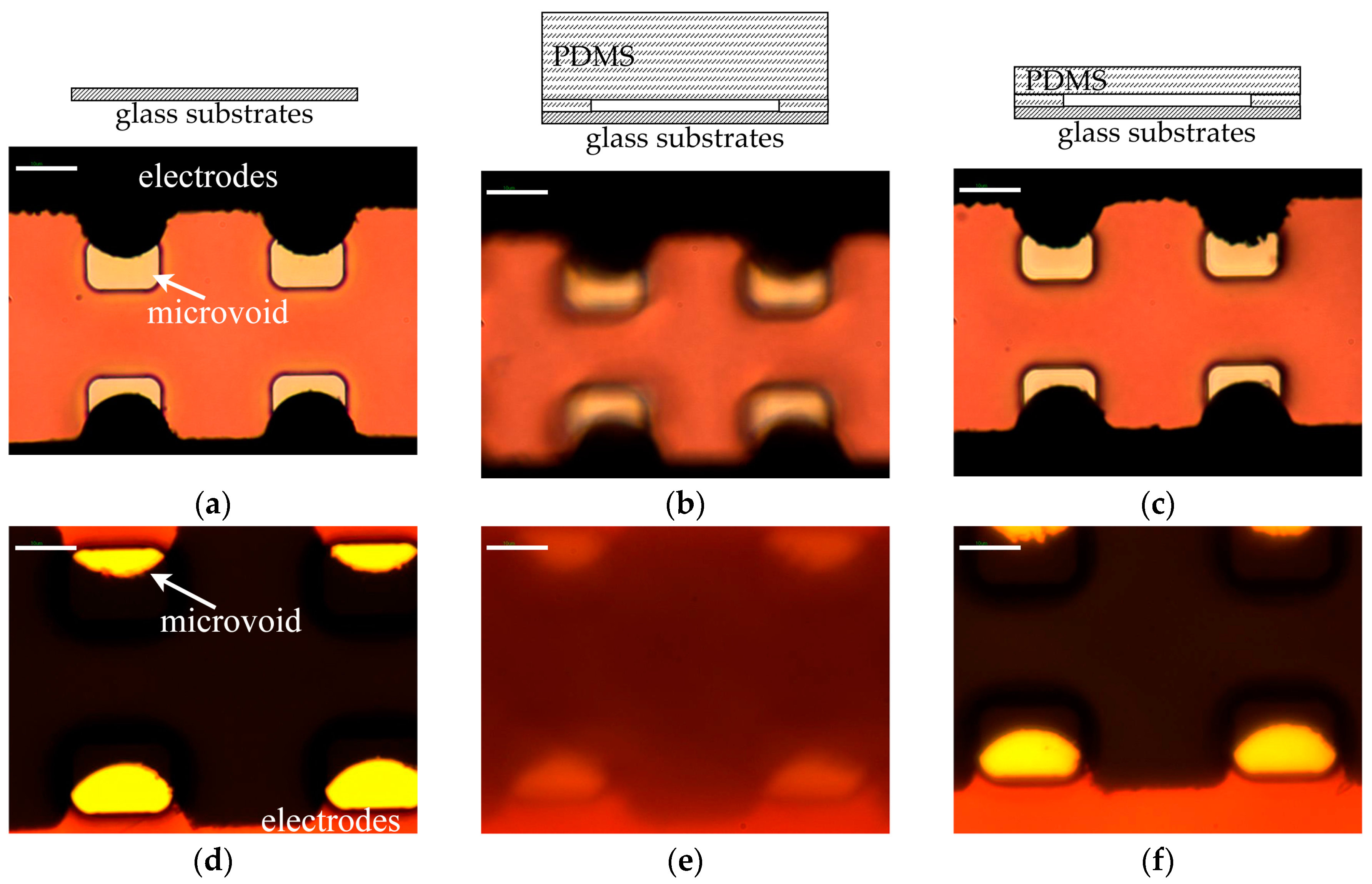
3.1. Optimization of Fabrication Process
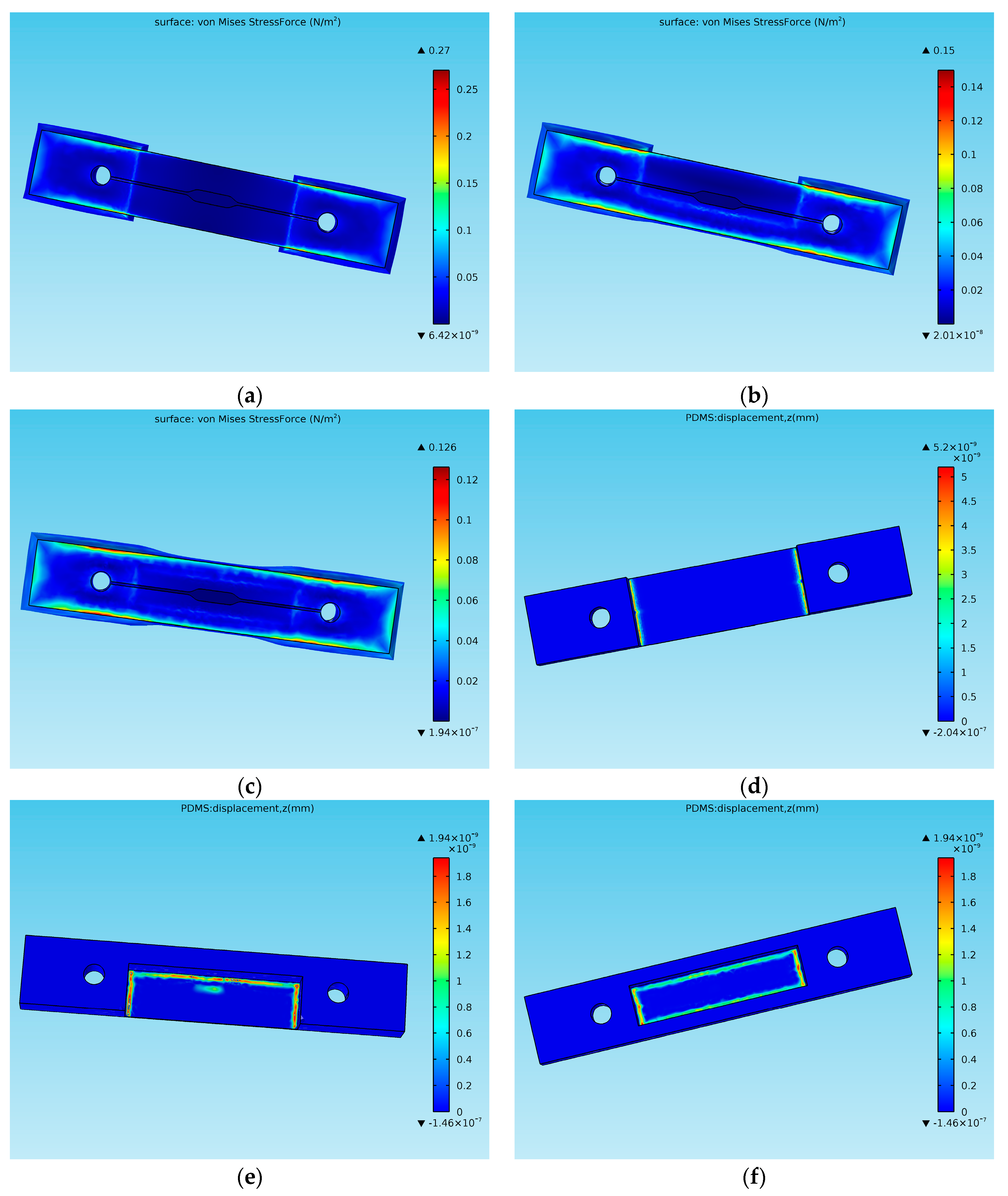
3.2. Selection of Auxiliary Beams
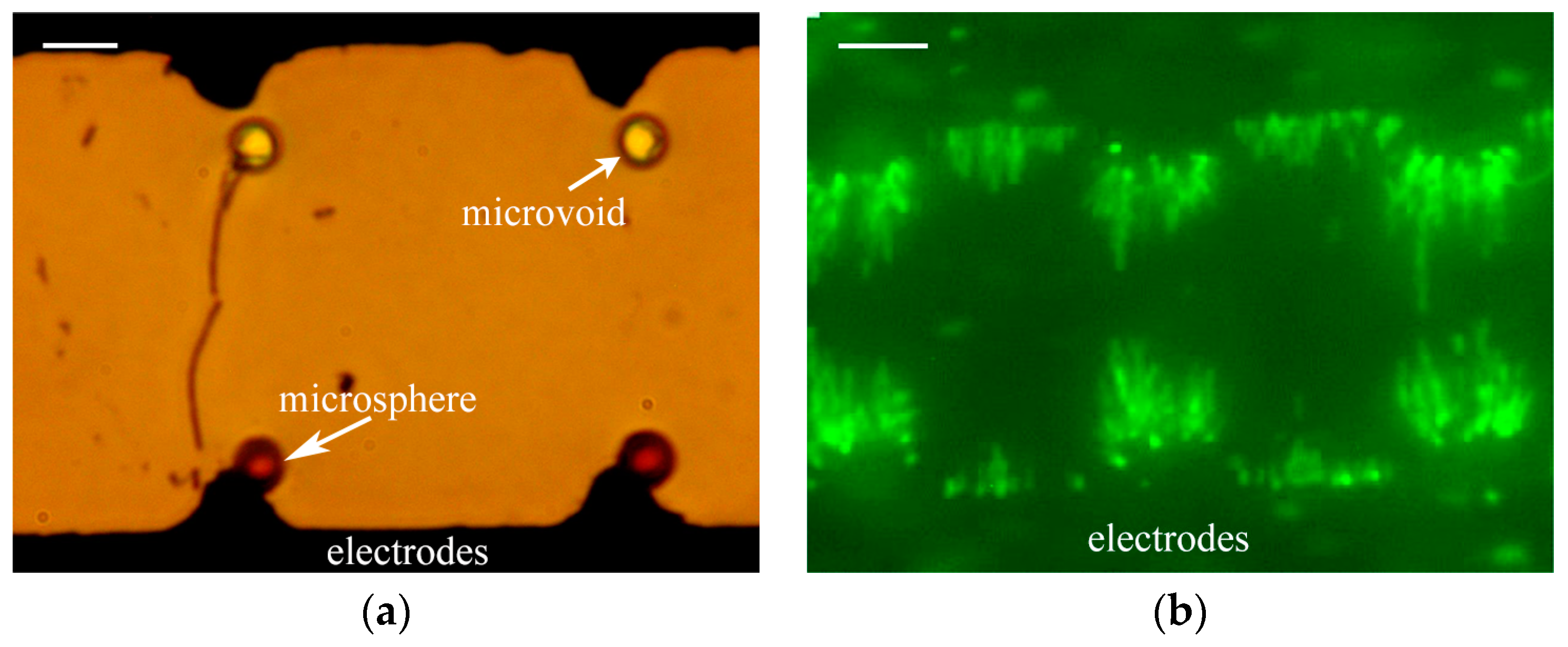
3.3. Observation Results and Applications
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiong, P.; Chen, X.; Xiong, Y.; Liu, G.; Tian, Y. Microstructure-enhanced liquid–liquid extraction in a real-time fluorescence detection microfluidic chip. Micromachines 2016, 7, 46. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, Y. Application of vertical electrodes in microfluidic channels for impedance analysis. Micromachines 2016, 7, 96. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Tanaka, J.; Kaneko, M.; Horade, M.; Ito, H.; Taniguchi, T.; Ohtani, T.; Sakata, Y. An on-chip RBC deformability checker significantly improves velocity-deformation correlation. Micromachines 2016, 7, 176. [Google Scholar] [CrossRef]
- Shim, J.S. Theoretic optimisation of microfluidic and magnetic self-assembly of carbon nanotubes. IET Micro Nano Lett. 2014, 9, 523–528. [Google Scholar] [CrossRef]
- Lee, C.-C.; Sui, G.; Elizarov, A.; Shu, C.J.; Shin, Y.-S.; Dooley, A.N.; Huang, J.; Daridon, A.; Wyatt, P.; Stout, D. Multistep synthesis of a radiolabeled imaging probe using integrated microfluidics. Science 2005, 310, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Huang, B.; Zhou, J.; Liang, Y.; Tian, J.; Ji, L.; Liang, X.; Ye, X. A microfluidic chip for cell patterning utilizing paired microwells and protein patterns. Micromachines 2017, 8, 1. [Google Scholar] [CrossRef]
- Abate, A.R.; Agresti, J.J.; Weitz, D.A. Microfluidic sorting with high-speed single-layer membrane valves. Appl. Phys. Lett. 2010, 96, 203509. [Google Scholar] [CrossRef]
- Lin, B.S.; Yang, Y.C.; Ho, C.Y.; Yang, H.Y.; Wang, H.Y. A PDMS-based cylindrical hybrid lens for enhanced fluorescence detection in microfluidic systems. Sensors 2014, 14, 2967–2980. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.J.; Zhang, Z.Y.; Lin, M.; Cao, X.C. Development of a simple and reliable pdms interconnect for high throughput microfluidic applications. Microsyst. Technol. 2013, 21, 147–154. [Google Scholar] [CrossRef]
- Song, M.; Lei, Y.; Sun, H. Comparison of spherical and non-spherical particles in microchannels under dielectrophoretic force. Microsyst. Technol. 2013, 21, 381–391. [Google Scholar] [CrossRef]
- Babahosseini, H.; Srinivasaraghavan, V.; Agah, M. Microfluidic chip bio-sensor for detection of cancer cells. In Proceedings of the 2012 IEEE Sensors, Taipei, Taiwan, 28–31 October 2012; pp. 1–4.
- Wu, C.-W.; Chang, S.-P.; Wu, C.-J.; Huang, S.-H. Cells adhered and cultured on microcantilevers. Microsyst. Technol. 2012, 19, 105–112. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 2010, 4, 026502. [Google Scholar] [CrossRef] [PubMed]
- Jo, B.H.; Van Lerberghe, L.M.; Motsegood, K.M.; Beebe, D.J. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J. Microelectromech. Syst. 2000, 9, 76–81. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Xiong, Y.; Xiong, P.; Hou, S.; Zhang, X.; Liu, G.; Tian, Y.; Zheng, J. A dielectrophoresis method to manipulate and monitor the shewanella oneidensis. J. Nanomater. Nanoeng. Nanosyst. 2016, 230, 85–90. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hou, S.; Chu, J.; Xiong, Y.; Xiong, P.; Liu, G.; Tian, Y. Observation Interface of PDMS Membrane in a Microfluidic Chip Based on One-Step Molding. Micromachines 2017, 8, 64. https://doi.org/10.3390/mi8030064
Chen X, Hou S, Chu J, Xiong Y, Xiong P, Liu G, Tian Y. Observation Interface of PDMS Membrane in a Microfluidic Chip Based on One-Step Molding. Micromachines. 2017; 8(3):64. https://doi.org/10.3390/mi8030064
Chicago/Turabian StyleChen, Xiangyu, Shuangyue Hou, Jian Chu, Ying Xiong, Penghui Xiong, Gang Liu, and Yangchao Tian. 2017. "Observation Interface of PDMS Membrane in a Microfluidic Chip Based on One-Step Molding" Micromachines 8, no. 3: 64. https://doi.org/10.3390/mi8030064
APA StyleChen, X., Hou, S., Chu, J., Xiong, Y., Xiong, P., Liu, G., & Tian, Y. (2017). Observation Interface of PDMS Membrane in a Microfluidic Chip Based on One-Step Molding. Micromachines, 8(3), 64. https://doi.org/10.3390/mi8030064