Preparation of an Acridinium Ester-Labeled Antibody and Its Application in GoldMag Nanoparticle-Based, Ultrasensitive Chemiluminescence Immunoassay for the Detection of Human Epididymis Protein 4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AE-Labeled Antibodies
2.3. Evaluation of the Labeling Effect
2.4. GMP-CLIA for HE4
2.5. Detection Limit and Functional Sensitivity Measurement
2.6. Clinical Application and Analysis
3. Results
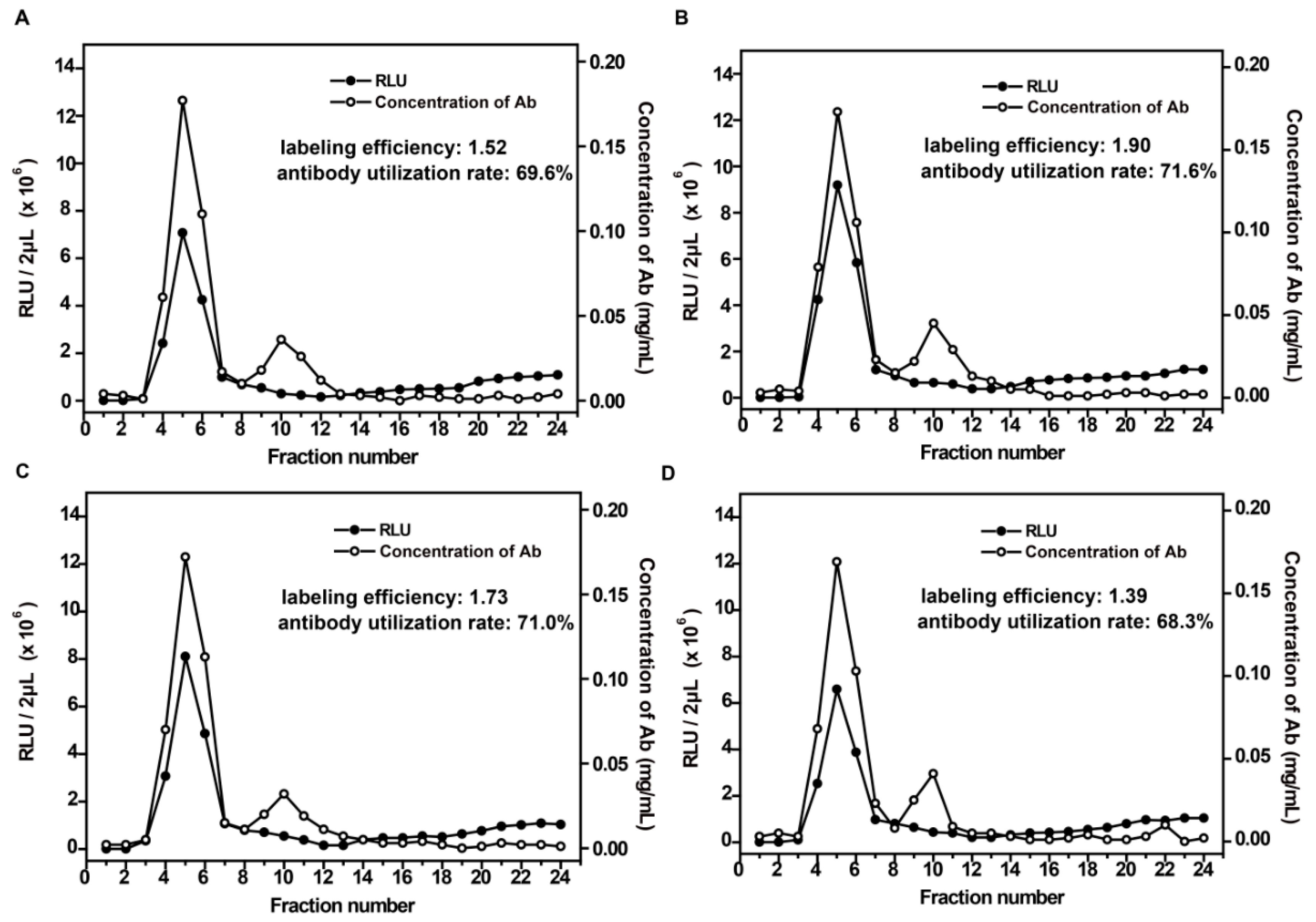
3.1. Characteristics of AE-Labeled Antibodies
3.2. Optimization of the Labeling Conditions
3.3. Evaluation of the Labeling System
3.4. Establishment of GMP-CLIA for Detection of HE4
3.5. Application in Human Serum Samples
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christodoulides, N.; Tran, M.; Floriano, P.N.; Rodriguez, M.; Goodey, A.; Ali, M.; Neikirk, D.; McDevitt, J.T. A microchip-based multianalyte assay system for the assessment of cardiac risk. Anal. Chem. 2002, 74, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Skogstrand, K.; Thorsen, P.; Norgaardpedersen, B.; Schendel, D.E.; Sørensen, L.C.; Hougaard, D.M. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin. Chem. 2005, 51, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Orozco, A.R.; Ruiz-Reyes, H.; Medina-Serriteno, N. Recent applications of chemiluminescence assays in clinical immunology. Mini Rev. Med. Chem. 2010, 10, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Kricka, L.J. Application of bioluminescence and chemiluminescence in biomedical sciences. Methods Enzymol. 2000, 305, 333–345. [Google Scholar] [PubMed]
- Falzarano, R.; Viggiani, V.; Michienzi, S.; Longo, F.; Tudini, S.; Frati, L.; Anastasi, E. Evaluation of a CLEIA automated assay system for the detection of a panel of tumor markers. Tumour Biol. 2013, 34, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, X.; Lin, Z.; Li, Z.; Ying, X.; Chen, G.; Lin, J.M. Development of a high-throughput, indirect antibody immobilization format chemiluminescence enzyme immunoassay (CLEIA) for the determination of progesterone in human serum. Luminescence 2008, 23, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Adam, A.; Loo, J.C.; Ong, H. A chemiluminescence enzyme immunoassay (CLEIA) for the determination of medroxyprogesterone acetate in human serum. J. Pharm. Biomed. Anal. 1995, 13, 885–891. [Google Scholar] [PubMed]
- Shi, G.; Tang, B.-J.; Wang, X.; Zhao, L.; Lin, J.-M. Microplate chemiluminescent enzyme immunoassay for the quantitative analysis of free prostate-specific antigen in human serum. Chin. J. Anal. Chem. 2007, 35, 1541–1547. [Google Scholar]
- Barni, F.; Lewis, S.W.; Berti, A.; Miskelly, G.M.; Lago, G. Forensic application of the luminol reaction as a preumptivetest for latent blood detection. Talanta 2007, 72, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Lin, J.M.; Ying, X. Development of a highly sensitive and selective microplate chemiluminescence enzyme immunoassay for the determination of free thyroxine in human serum. Int. J. Biol. Sci. 2007, 3, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, X.; Li, Z.J.; Ren, S.Q.; Chen, G.N.; Ying, X.T.; Lin, J.M. Development of a sensitive, rapid, biotin-streptavidin based chemiluminescent enzyme immunoassay for human thyroid stimulating hormone. Talanta 2008, 75, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Y.; Song, C.; Dong, B.; Liu, Z.; Zhang, K.; Li, H.; Sun, Y.; Wei, Y.; Yang, A.; et al. Highly sensitive microplate chemiluminescence enzyme immunoassay for the determination of staphylococcal enterotoxin B based on a pair of specific monoclonal antibodies and its application to various matrices. Anal. Chem. 2010, 82, 7758–7765. [Google Scholar] [CrossRef] [PubMed]
- Rauch, P.; Poplstein, M.; Hochel, I.; Fukal, L.; Ferri, E.; Abagnato, C.A.; Girotti, S.; Roda, A. Enhanced chemiluminescent sandwich enzyme immunoassay for hen egg lysozyme. J. Biolumin. Chemilumin. 1995, 10, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ohnishi, N.; Takahashi, Y.; Kondo, A. Application of magnetic nanoparticles in full-automated chemiluminescent enzyme immunoassay. J. Magn. Magn. Mater. 2009, 321, 1686–1688. [Google Scholar] [CrossRef]
- Yang, C.C.; Yang, S.Y.; Ho, C.S.; Chang, J.F.; Liu, B.H.; Huang, K.W. Development of antibody functionalized magnetic nanoparticles for the immunoassay of carcinoembryonic antigen: A feasibility study for clinical use. J. Nanobiotechnol. 2014, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Li, H.; Lin, J.M. Development of a highly sensitive magnetic particle-based chemiluminescence enzyme immunoassay for thyroid stimulating hormone and comparison with two other immunoassays. Clin. Chim. Acta 2010, 411, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Goya, G.F.; Berquo, T.S.; Fonseca, F.C. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Cui, Y.; Hui, W.; Wang, H.; Wang, L.; Chen, C. Preparation and characterization of Fe3O4/Au composite particles. Sci. China Ser. B Chem. 2004, 47, 152–158. [Google Scholar] [CrossRef]
- Yang, D.; Ma, J.; Zhang, Q.; Li, N.; Yang, J.; Raju, P.A.; Peng, M.; Luo, Y.; Hui, W.; Chen, C.; et al. Polyelectrolyte-coated gold Magnetic nanoparticles for immunoassay development: Toward point of care diagnostics for syphilis screening. Anal. Chem. 2013, 85, 6688–6695. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Hou, P.; Chen, M.; Hui, W.; Vermorken, A.; Luo, Z.; Li, H.; Li, Q.; Cui, Y. Gold magnetic nanoparticle conjugate-based lateral flow assay for the detection of IgM class antibodies related to TORCH infections. Int. J. Mol. Med. 2015, 36, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Zhang, S.; Zhang, C.; Wan, Y.; Zhu, J.; Zhao, G.; Wu, S.; Xi, D.; Zhang, Q.; Li, N.; et al. A Novel Lateral Flow Assay Based on GoldMag Nanoparticles and Its Clinical Applications for Genotyping of MTHFR C677T Polymorphisms. Nanoscale 2016, 8, 3579–3587. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Li, H.; Luo, Z.; Kong, J.; Wan, Y.; Niu, H.; Vermorken, F.; Van de Ven, W.; Zhang, X.; Li, F.; et al. Dextran-coated superparamagnetic nanoparticles as potential cancer drug carrier in vivo. Nanoscale 2015, 7, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ma, L.; Zhang, M.; Yang, J.; Du, H.; Ma, T.; Cui, Y. GoldMag particle-based chemiluminescene immunoassay for human high sensitive C-reactive protein. Chinese J. Cell. Mol. Immunol. 2015, 31, 1468–1505. [Google Scholar]
- Dodeigne, C.; Thunus, L.; Lejeune, R. Chemiluminescence as diagnostic tool. Talanta 2000, 51, 415–439. [Google Scholar] [CrossRef]
- Simpson, J.S.; Campbell, A.K.; Ryall, M.E.; Woodhead, J.S. A stable chemiluminescent-labelled antibody for immunological assays. Nature 1979, 279, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Weeks, I.; Sturgess, M.; Brown, R.C.; Woodhead, J.S. Immunoassays using acridinium esters. Methods Enzymol. 1986, 133, 366–387. [Google Scholar] [PubMed]
- Speeckaert, M.M.; Speeckaert, R.; Delanghe, J.R. Human epididymis protein 4 in cancer diagnostics: A promising and reliable tumor marker. Adv. Clin. Chem. 2013, 59, 1–21. [Google Scholar] [PubMed]
- Hellstrom, I.; Heagerty, P.J.; Swisher, E.M.; Liu, P.; Jaffar, J.; Agnew, K.; Hellstrom, K.E. Detection of the HE4 protein in urine as a biomarker for ovarian neoplasms. Cancer Lett. 2010, 296, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.-G.; He, Y.-F.; Liu, Y.-B.; Shen, D.-C.; Han, S.-Q.; Luo, Z.-F. Study of chemiluminescence characters of DMAE·NHS. Label. Immunoass. Clin. Med. 2004, 11, 163–167. [Google Scholar]
- Rak, J.; Skurski, P.; Blazejowski, J. Toward an understanding of the chemiluminescence accompanying the reaction of 9-carboxy-10-methylacridinium phenyl ester with hydrogen peroxide. J. Org. Chem. 1999, 64, 3002–3008. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, A.; Sharpe, D.; Wen, D. Effect of surfactants on the chemiluminescence of acridinium dimethylphenyl ester labels and their conjugates. Org. Biomol. Chem. 2011, 9, 5092–5103. [Google Scholar] [CrossRef] [PubMed]







| No | Trigger Solution | RLU1 a | RLU2 b |
|---|---|---|---|
| 1 | (A) 0.1% H2O2 | 17,239 ± 902 | 2266 ± 308 |
| (B) 0.25 M NaOH | |||
| 2 | (A) 0.1% H2O2 + 0.1 M HNO3 | 180,503 ± 6011 | 13,266 ± 927 |
| (B) 0.25 M NaOH | |||
| 3 | (A) 0.1% H2O2 + 0.1 M HNO3 | 484,743 ± 4813 | 55,142 ± 882 |
| (B) 0.25 M NaOH + 2% Tween-20 | |||
| 4 | (A) 0.1% H2O2 + 0.1 M HNO3 | 1,145,820 ± 18921 | 95,443 ± 730 |
| (B) 0.25 M NaOH + 2% Triton-100 |
| Item | AE-Labeled Antibody | Non-Labeled Antibody | Blank | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dilution factor | 103 | 104 | 105 | 106 | 107 | 108 | 103 | 104 | 105 | 106 | 107 | 108 | / |
| A450 | 1.692 | 1.425 | 0.764 | 0.394 | 0.201 | 0.101 | 1.797 | 1.569 | 0.887 | 0.476 | 0.245 | 0.138 | 0.078 |
| Detection Limit | Functional Sensitivity | |||
|---|---|---|---|---|
| Mean (n = 20) | 4231.70 | Conc. of HE4 (ng/mL) | Mean (n = 20) | CV (%) |
| SD | 784.48 | 0.20 | 9853 | 22 |
| Mean + 2SD | 5800.46 | 0.25 | 11,872 | 18 |
| DL (ng/mL) | 0.084 | 0.30 | 13,294 | 9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Zhang, M.; Wan, Y.; Cui, Y.; Ma, L. Preparation of an Acridinium Ester-Labeled Antibody and Its Application in GoldMag Nanoparticle-Based, Ultrasensitive Chemiluminescence Immunoassay for the Detection of Human Epididymis Protein 4. Micromachines 2017, 8, 149. https://doi.org/10.3390/mi8050149
Ma T, Zhang M, Wan Y, Cui Y, Ma L. Preparation of an Acridinium Ester-Labeled Antibody and Its Application in GoldMag Nanoparticle-Based, Ultrasensitive Chemiluminescence Immunoassay for the Detection of Human Epididymis Protein 4. Micromachines. 2017; 8(5):149. https://doi.org/10.3390/mi8050149
Chicago/Turabian StyleMa, Ting, Mengdan Zhang, Yinsheng Wan, Yali Cui, and Le Ma. 2017. "Preparation of an Acridinium Ester-Labeled Antibody and Its Application in GoldMag Nanoparticle-Based, Ultrasensitive Chemiluminescence Immunoassay for the Detection of Human Epididymis Protein 4" Micromachines 8, no. 5: 149. https://doi.org/10.3390/mi8050149





