3D Printing of Artificial Blood Vessel: Study on Multi-Parameter Optimization Design for Vascular Molding Effect in Alginate and Gelatin
Abstract
:1. Introduction
2. System and Process
3. Theory
4. Experiment Procedure
4.1. Materials
4.2. Experimental Procedure
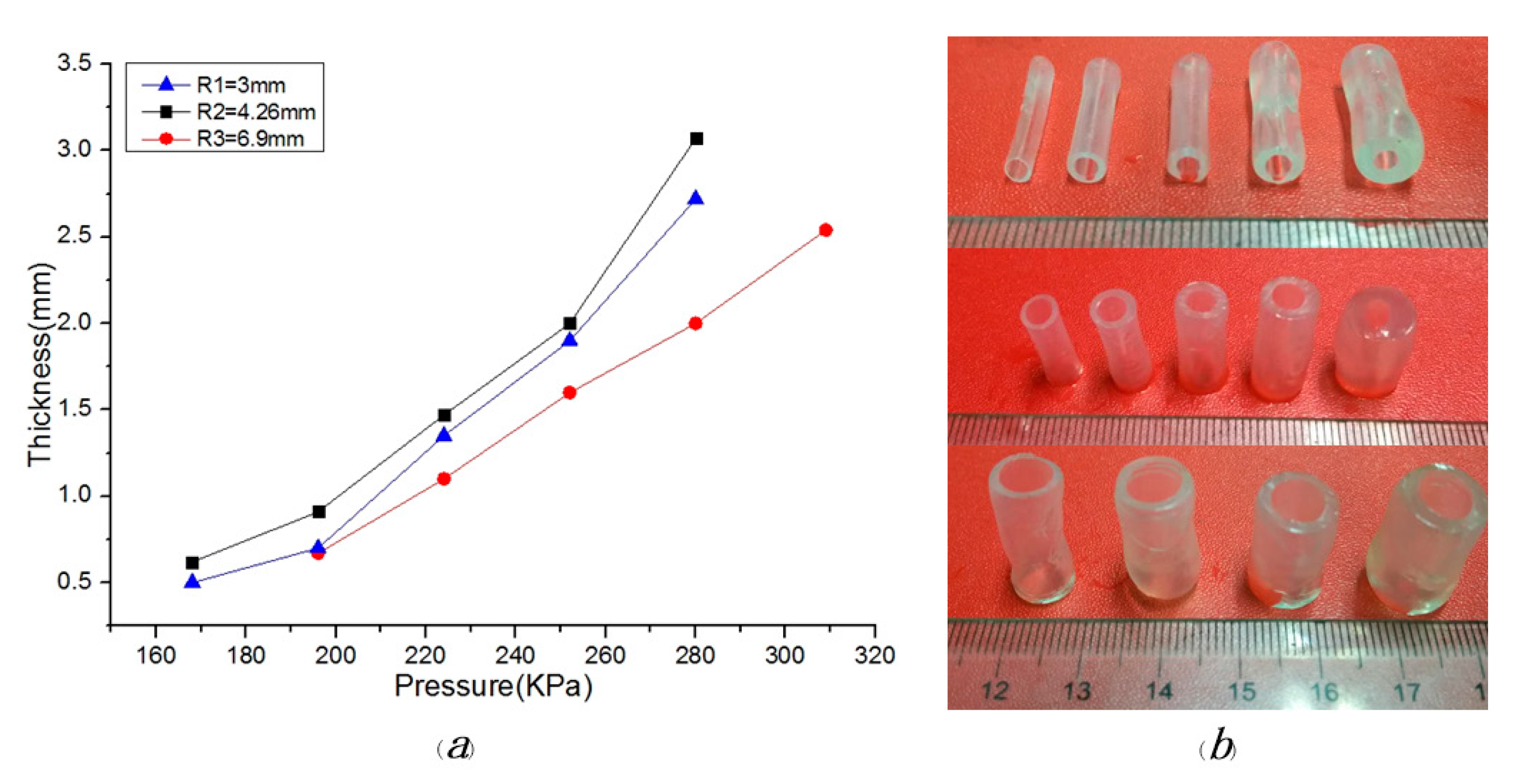
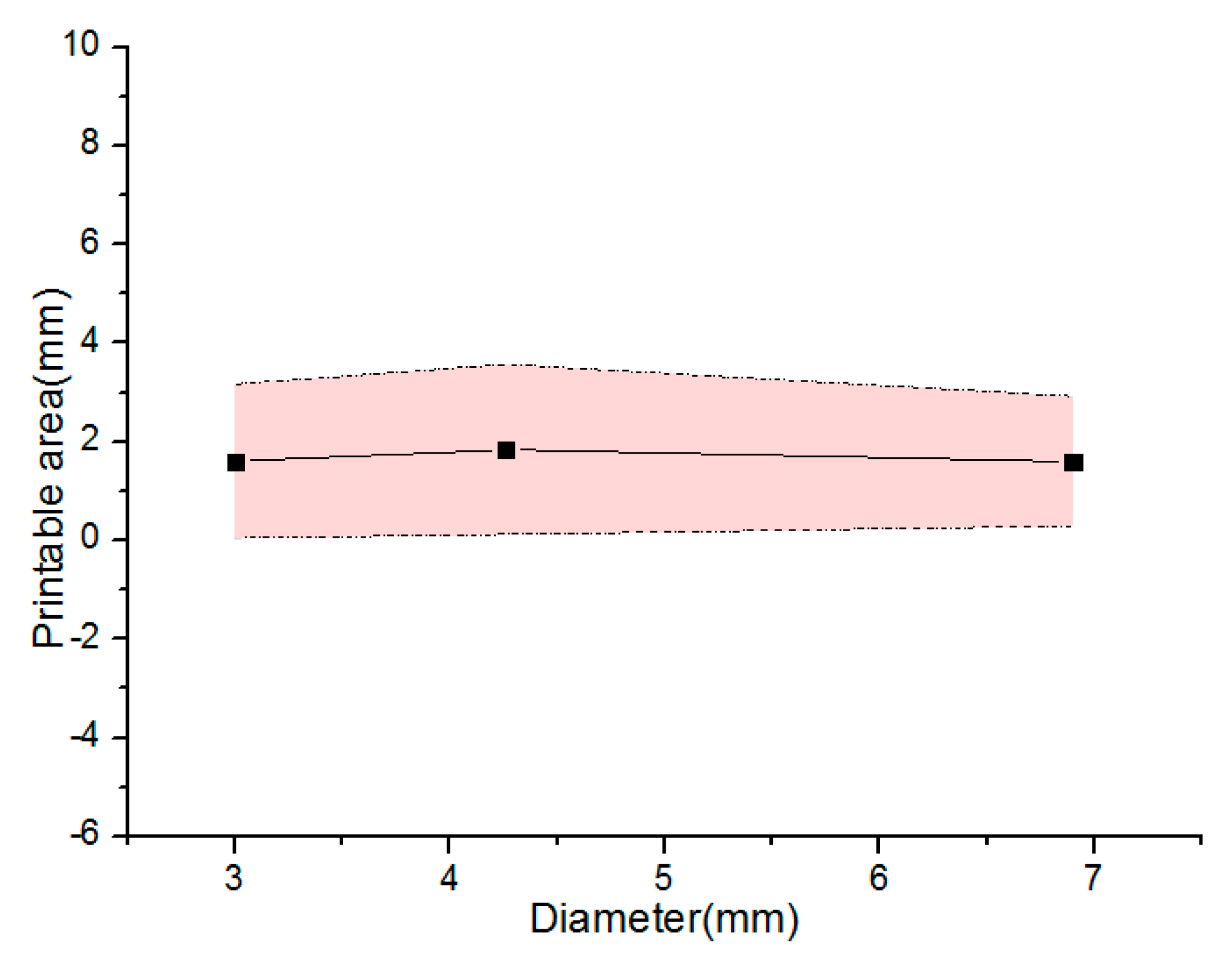
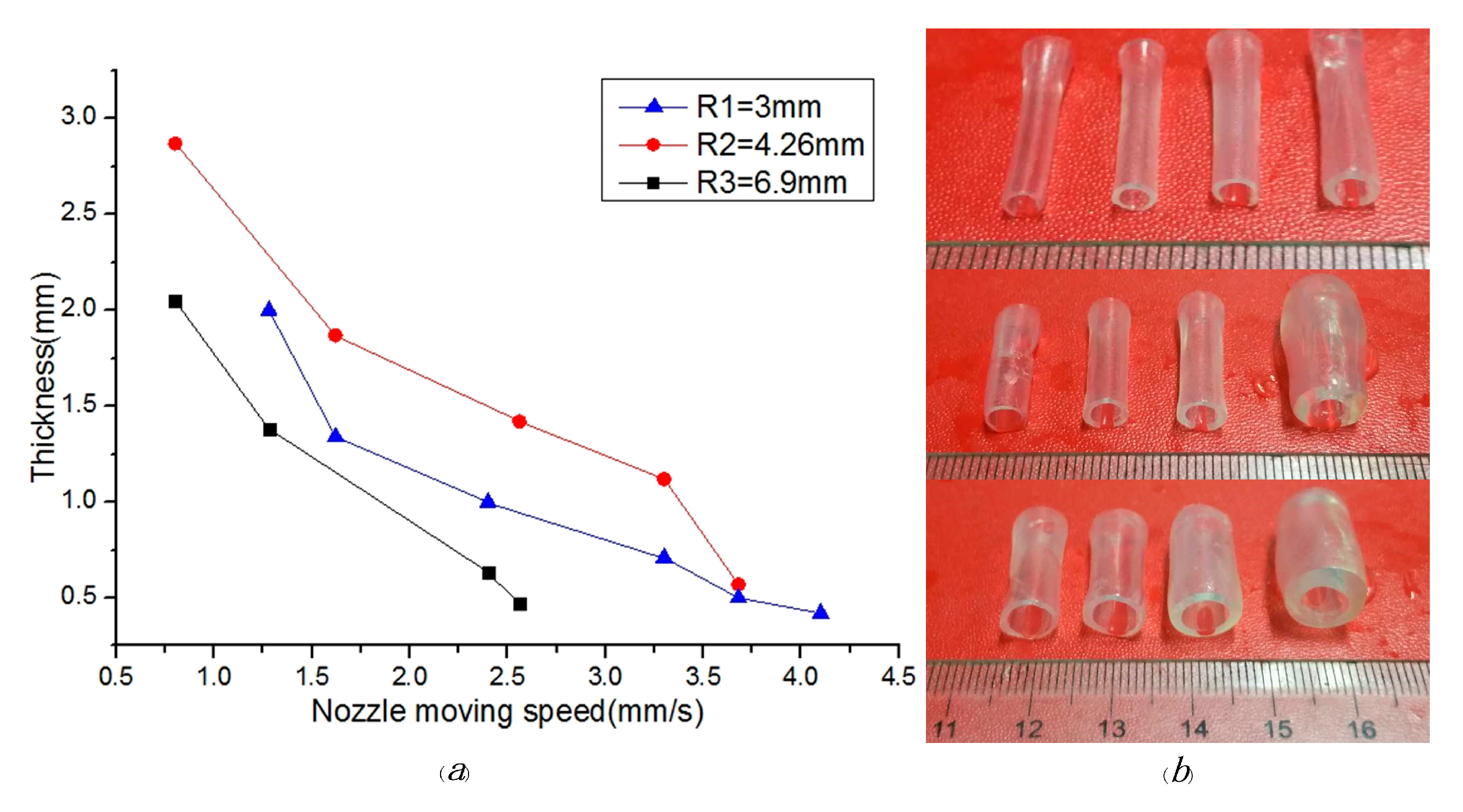
5. Results and Discussion
5.1. Material Viscosity
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits. Biomater. Sci. 2015, 3, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Can, K.; Ozler, S.B.; Inci, I.; Karakas, E.; Irmak, S.; Gozuacik, D.; Taralp, A.; Koc, B. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnol. Bioeng. 2015, 112, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Tae-Yun, K.; Jung Ho, L.; Bum Jin, K.; Jo, A.K.; Jung Min, H.; Byoung Soo, K.; Hyung Joon, C.; Jong-Won, R.; Dong-Woo, C. In vivo endothelization of tubular vascular grafts through in situ recruitment of endothelial and endothelial progenitor cells by RGD-fused mussel adhesive proteins. Biofabrication 2015, 7, 015007. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Gremmels, H.; van Deventer, K.; Fledderus, J.O.; Oner, F.C.; Verhaar, M.C.; Dhert, W.J.; Alblas, J. Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture. J. Control. Release 2014, 184, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of Cell Viability and Functionality in Vessel-like Bioprintable Cell-Laden Tubular Channels. J. Biomech. Eng. 2013, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, S.; Rauf, S.; Hauser, C. 3D bioprinting technology for regenerative medicine applications. Int. J. Bioprint. 2016, 2. [Google Scholar] [CrossRef]
- Falguni, P.; Gantelius, J.; Svahn, H.A. 3D Bioprinting of Tissue/Organ Models. Angew. Chem. Int. Ed. 2016, 55, 4650–4665. [Google Scholar] [CrossRef]
- Paulsen, S.J.; Miller, J.S. Tissue vascularization through 3D printing: Will technology bring us flow? Dev. Dyn. 2015, 244, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, H.; Lan, H.; Liu, T. Organ regeneration: Integration application of cell encapsulation and 3D bioprinting. Virtual Phys. Prototyp. 2017, 1–11. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Nakamura, M.; Henmi, C.; Yamaguchi, K.; Mochizuki, S.; Nakagawa, H.; Takiura, K. Development of a three-dimensional bioprinter: Construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J. Biomech. Eng. 2009, 131, 035001. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, T.H.J.; Perelaer, J.; de Laat, A.W.M.; Schubert, U.S. Inkjet Printing of Narrow Conductive Tracks on Untreated Polymeric Substrates. Adv. Mater. 2008, 20, 343–345. [Google Scholar] [CrossRef]
- Saunders, R.E.; Brian, D. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Mao, S.; Sun, W.; Yao, R. The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication 2015, 7, 045002. [Google Scholar] [CrossRef] [PubMed]
- Lothar, K.; Brandt, O.; Deiwick, A.; Chichkov, B. Laser assisted bioprinting at different wavelengths and pulse durations with a metal dynamic release layer: A parametric study. Int. J. Bioprint. 2017, 3. [Google Scholar] [CrossRef]
- He, P.J.; Katis, I.N.; Eason, R.W.; Sones, C.L. Laser direct-write for fabrication of three-dimensional paper-based devices. Lab Chip 2016, 16, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Bourget, J.M.; Kerouredan, O.; Medina, M.; Remy, M.; Thebaud, N.B.; Bareille, R.; Chassande, O.; Amedee, J.; Catros, S.; Devillard, R. Patterning of Endothelial Cells and Mesenchymal Stem Cells by Laser-Assisted Bioprinting to Study Cell Migration. BioMed Res. Int. 2016, 3569843. [Google Scholar] [CrossRef] [PubMed]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Tovar, G.E.; Borchers, K. Bioprinting of artificial blood vessels: Current approaches towards a demanding goal. Eur. J. Cardio-Thorac. Surg. 2014, 46, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Gaebel, R.; Ma, N.; Liu, J.; Guan, J.; Koch, L.; Klopsch, C.; Gruene, M.; Toelk, A.; Wang, W.; Mark, P.; et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 2011, 32, 9218–9230. [Google Scholar] [CrossRef] [PubMed]
- Kucukgul, C.; Ozler, S.B.; Inci, I.; Karakas, E.; Irmak, S.; Gozuacik, D.; Taralp, A.; Koc, B. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnol. Bioeng. 2015, 112, 811–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Liu, Z.; Lin, Z.; Qiu, J.; Liu, Y.; Liu, A.; Wang, Y.; Xiang, M.; Chen, B.; Fu, J.; et al. 3D Bioprinting of Vessel-like Structures with Multilevel Fluidic Channels. ACS Biomater. Sci. Eng. 2017, 3, 399–408. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, H.; Chairinnas. The synchronization among nozzle extrusion, nozzle speed and rotating speed based on 3D vessel bioprinter. In Proceedings of the 2016 International Conference on Instrumentation, Control and Automation (ICA), Bandung, Indonesia, 29–31 August 2016. [Google Scholar]
- Liu, H.; Zhou, H.; Lan, H.; Liu, F.; Wang, X. Multinozzle Multichannel Temperature Deposition System for Construction of a Blood Vessel. SLAS Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
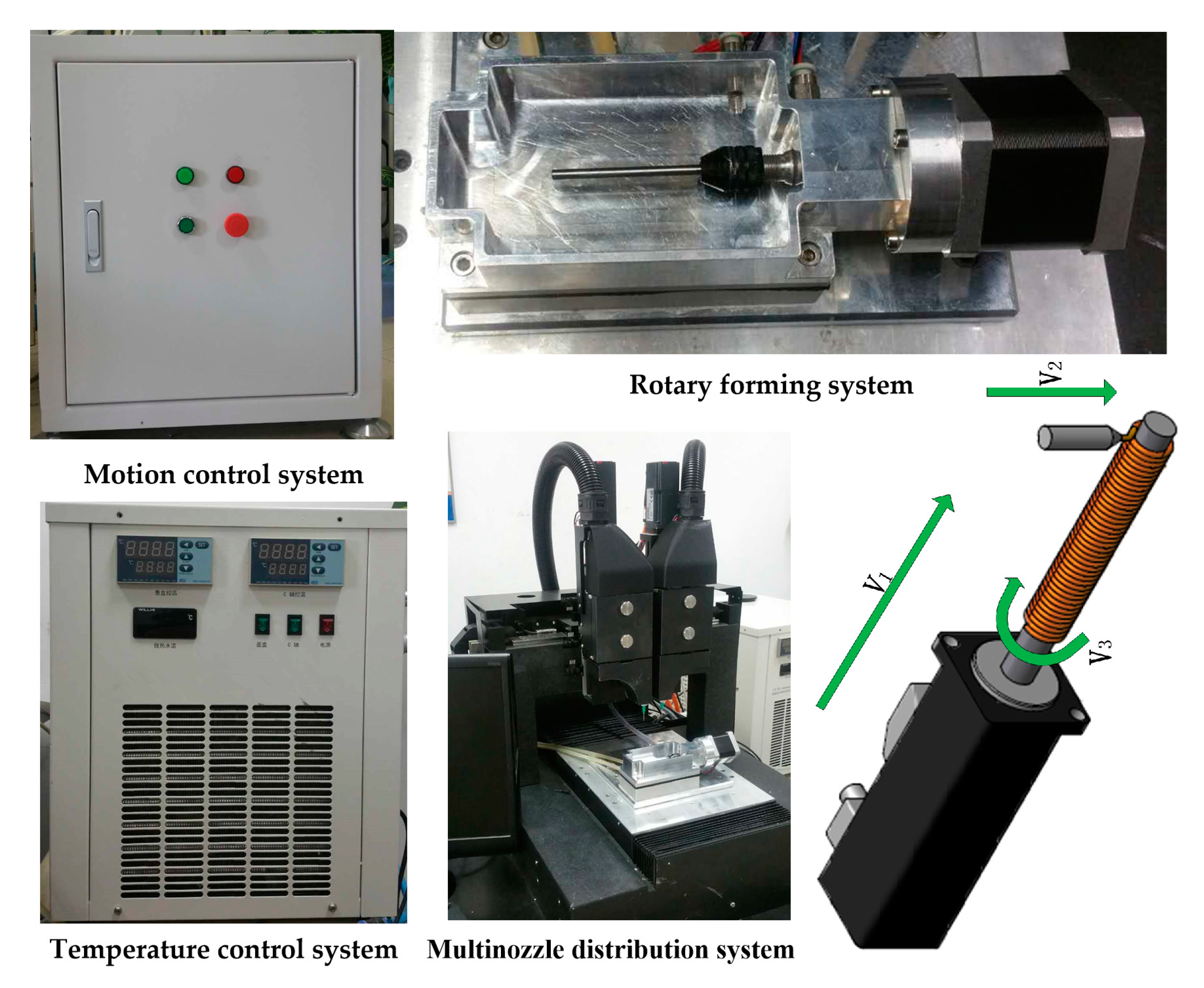
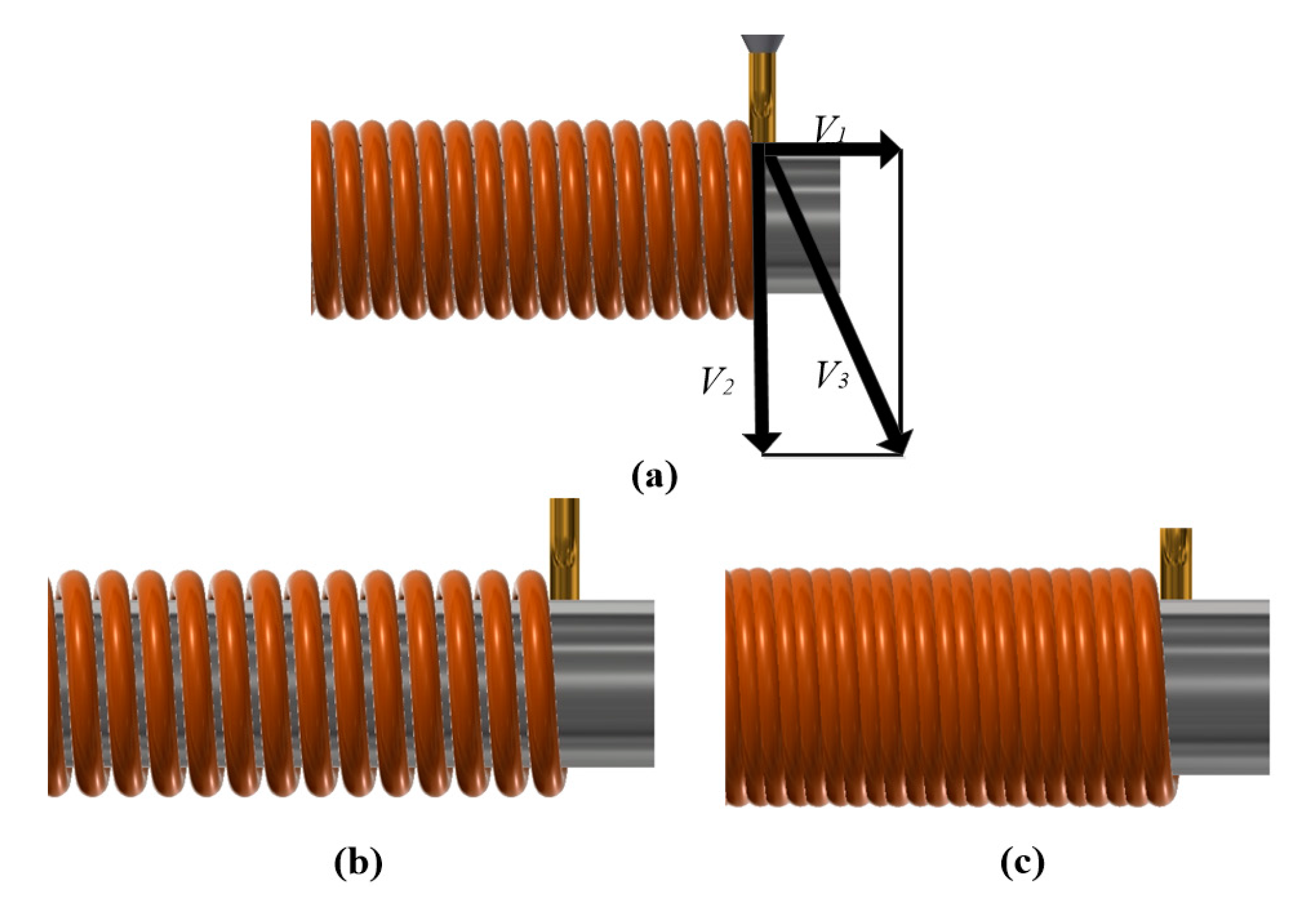
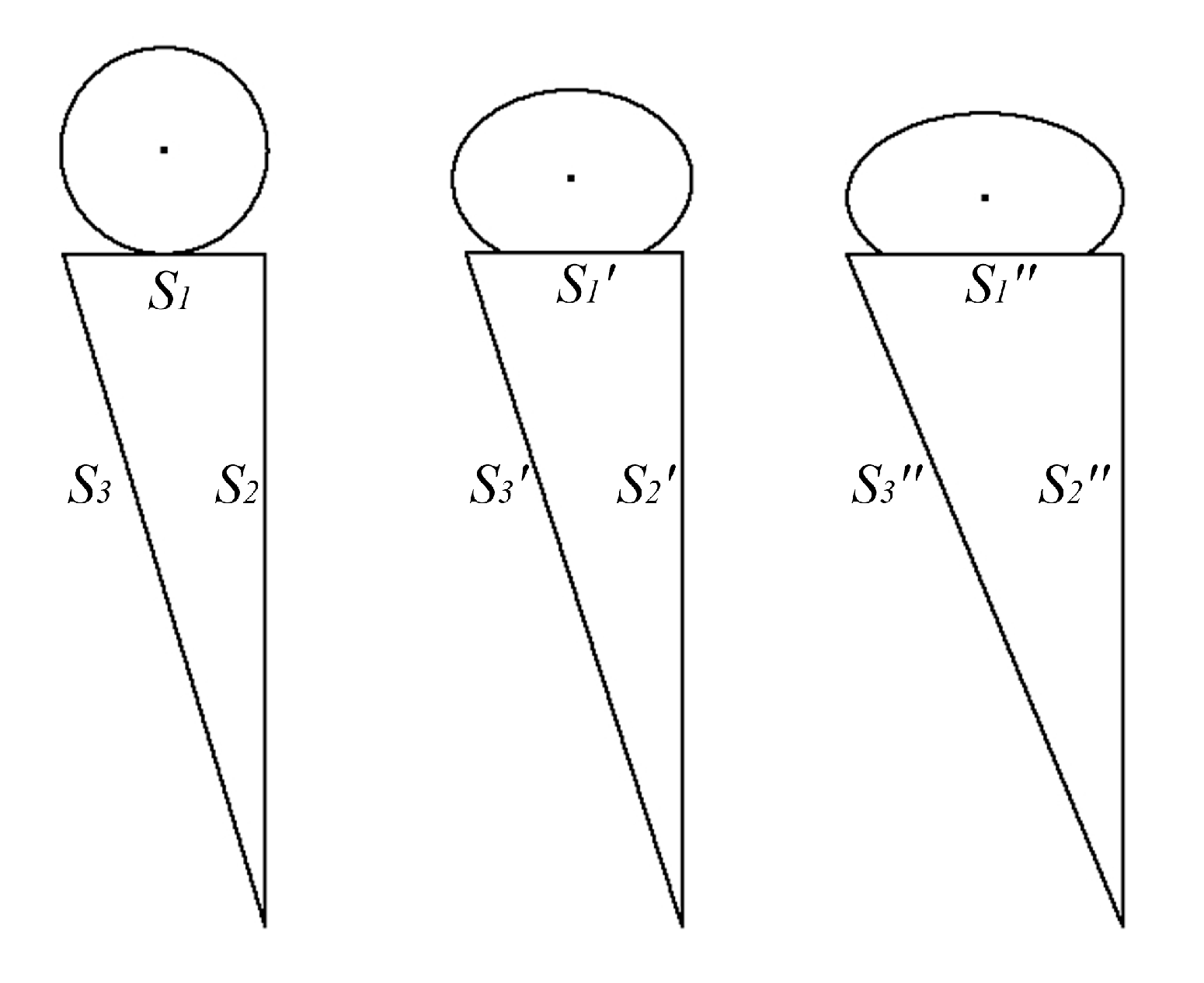
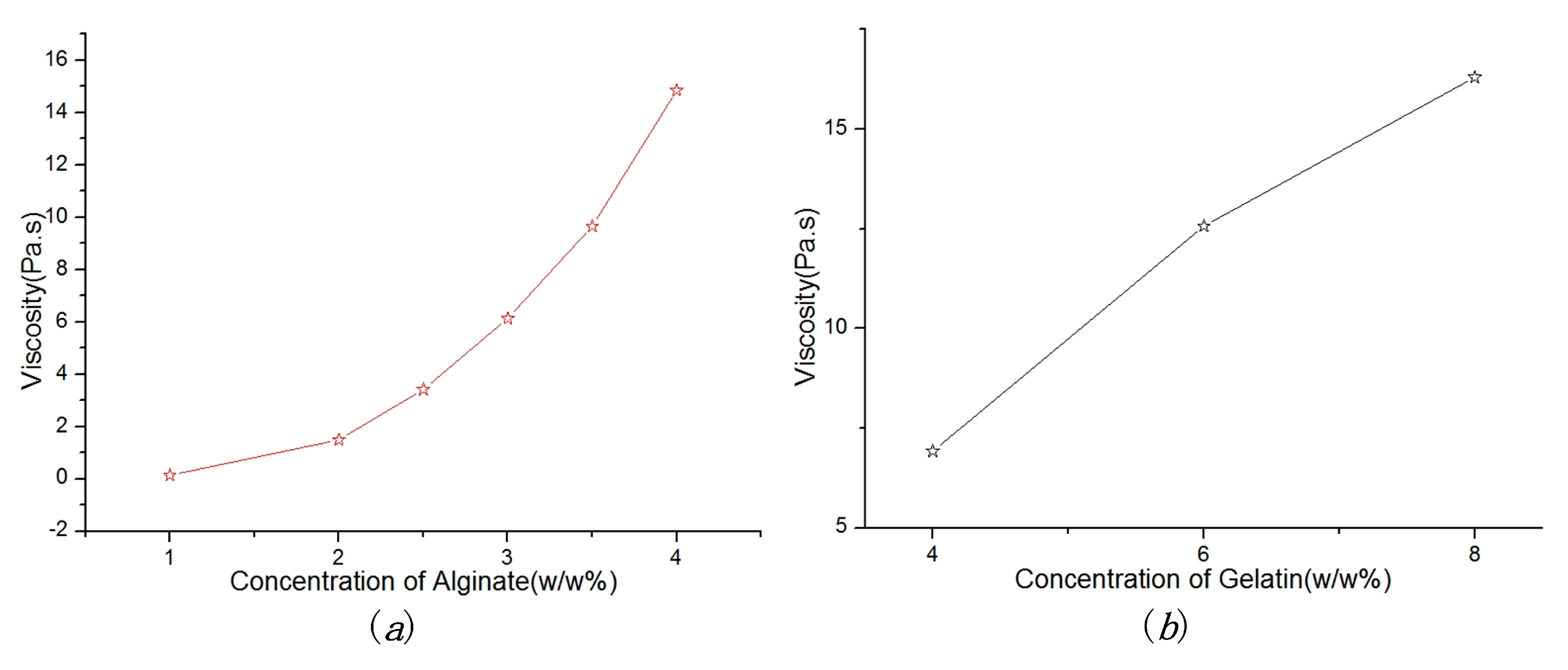


| Parameters | Value |
|---|---|
| Dimensions (X × Y × Z) | 150 × 150 × 150 mm3 |
| Position resolution | ±5 μm |
| Temperature range | 0–60 °C |
| Print speed | 0.1–50 mm/s |
| Pressure range | 0–1 MPa |
| Concentration of Alginate (% w/w) | Concentration of Gelatin (% w/w) | Diameter (mm) | Calcium Chloride (% w/w) |
|---|---|---|---|
| 3% | 4% | 3 | 5% |
| 3% | 6% | 4.26 | |
| 3% | 8% | 6.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhou, H.; Lan, H.; Liu, T.; Liu, X.; Yu, H. 3D Printing of Artificial Blood Vessel: Study on Multi-Parameter Optimization Design for Vascular Molding Effect in Alginate and Gelatin. Micromachines 2017, 8, 237. https://doi.org/10.3390/mi8080237
Liu H, Zhou H, Lan H, Liu T, Liu X, Yu H. 3D Printing of Artificial Blood Vessel: Study on Multi-Parameter Optimization Design for Vascular Molding Effect in Alginate and Gelatin. Micromachines. 2017; 8(8):237. https://doi.org/10.3390/mi8080237
Chicago/Turabian StyleLiu, Huanbao, Huixing Zhou, Haiming Lan, Tianyu Liu, Xiaolong Liu, and Hejie Yu. 2017. "3D Printing of Artificial Blood Vessel: Study on Multi-Parameter Optimization Design for Vascular Molding Effect in Alginate and Gelatin" Micromachines 8, no. 8: 237. https://doi.org/10.3390/mi8080237




