Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications
Abstract
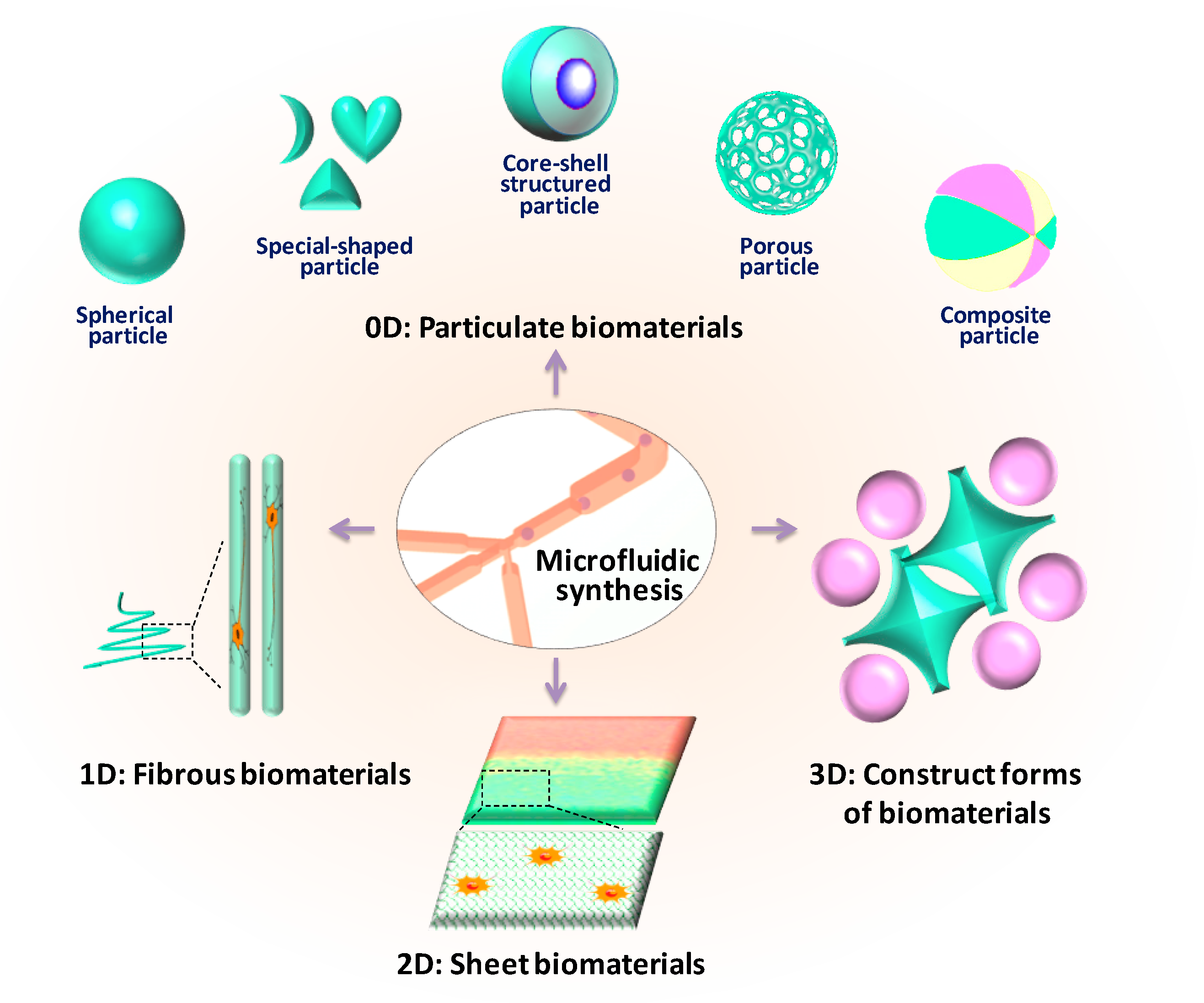
:1. Introduction
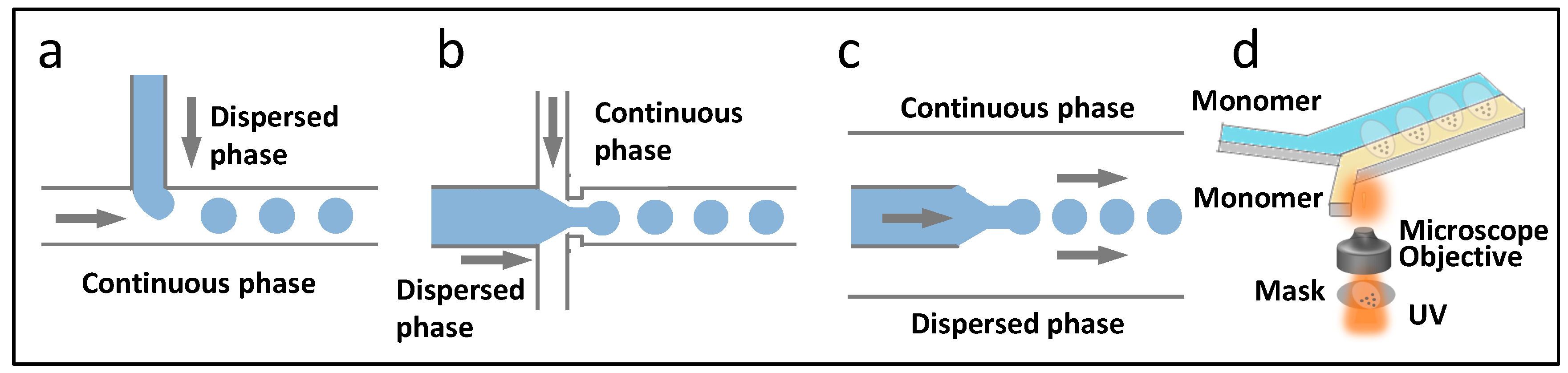
2. Particulate Biomaterials Synthesis and Applications
2.1. Particulate Biomaterials at Micro-Scale
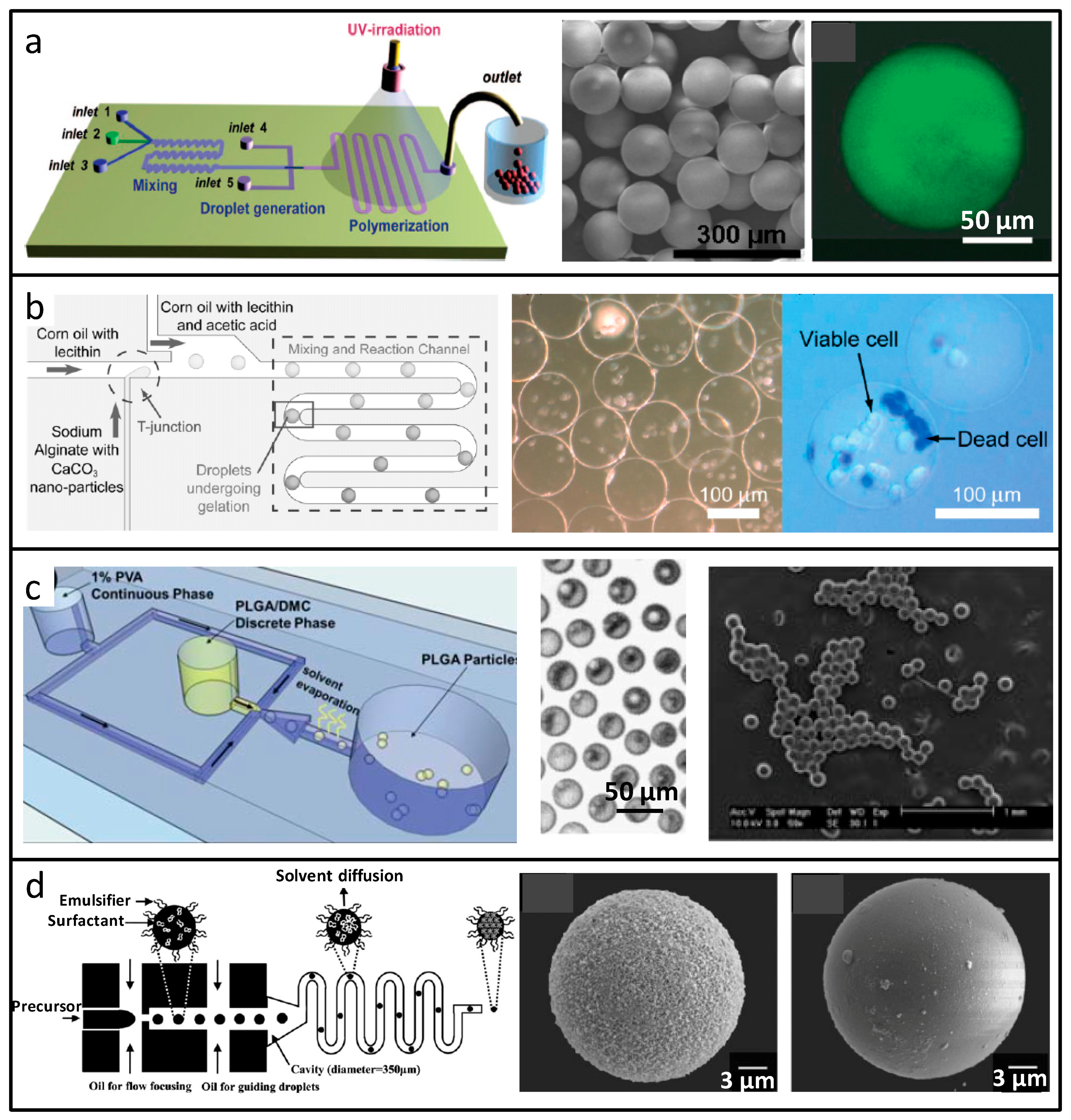
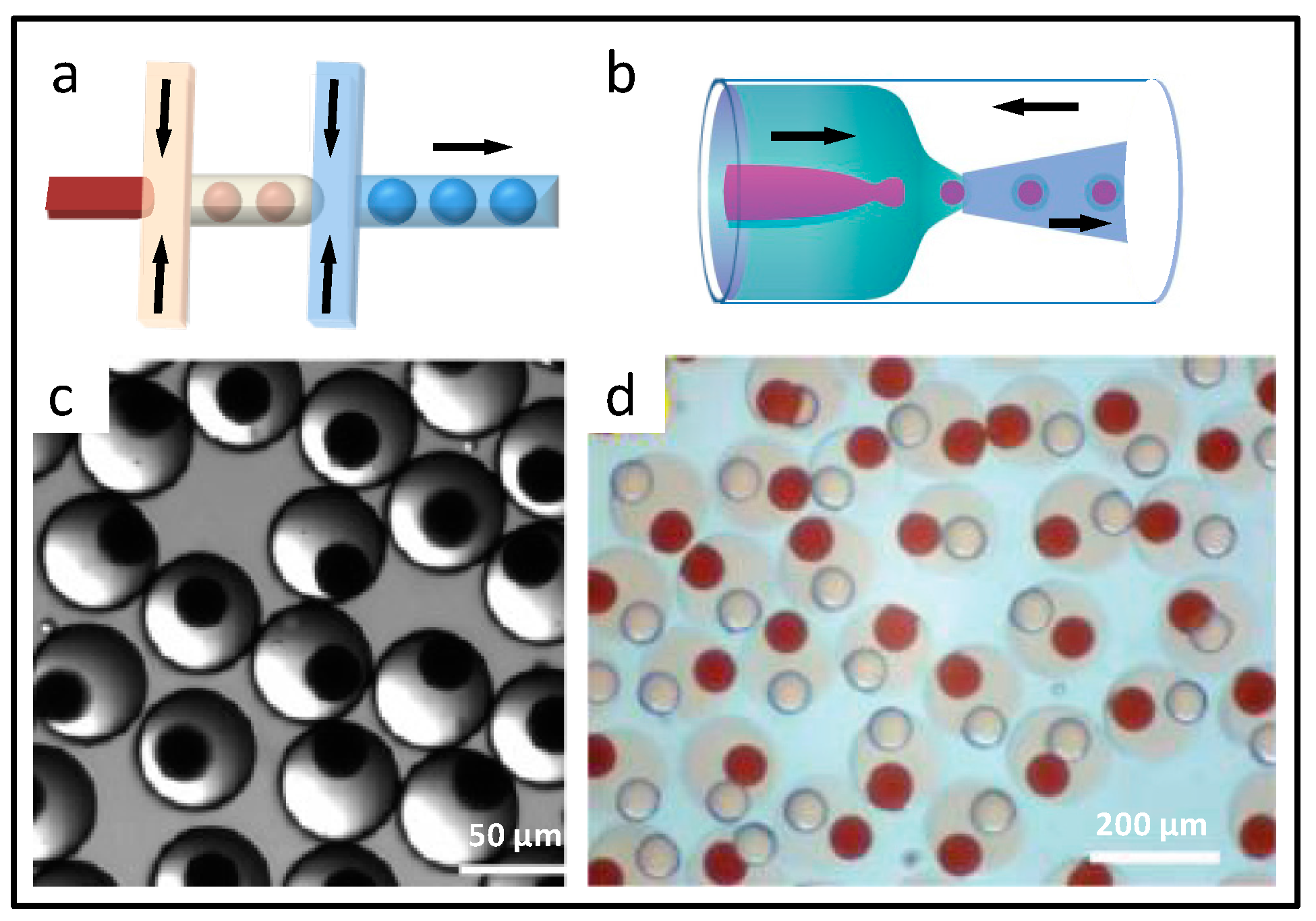
2.1.1. Spherical Microparticles
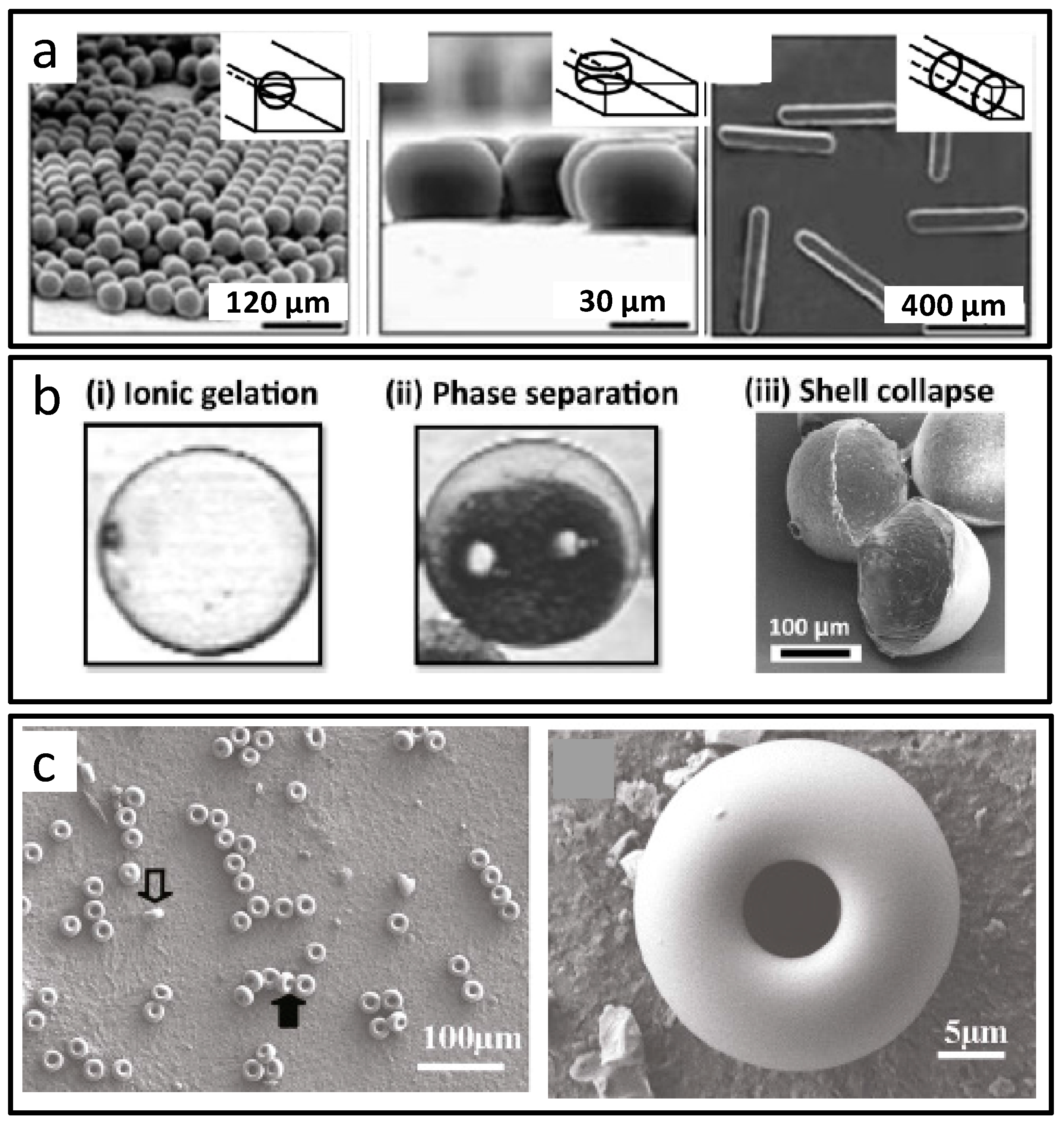
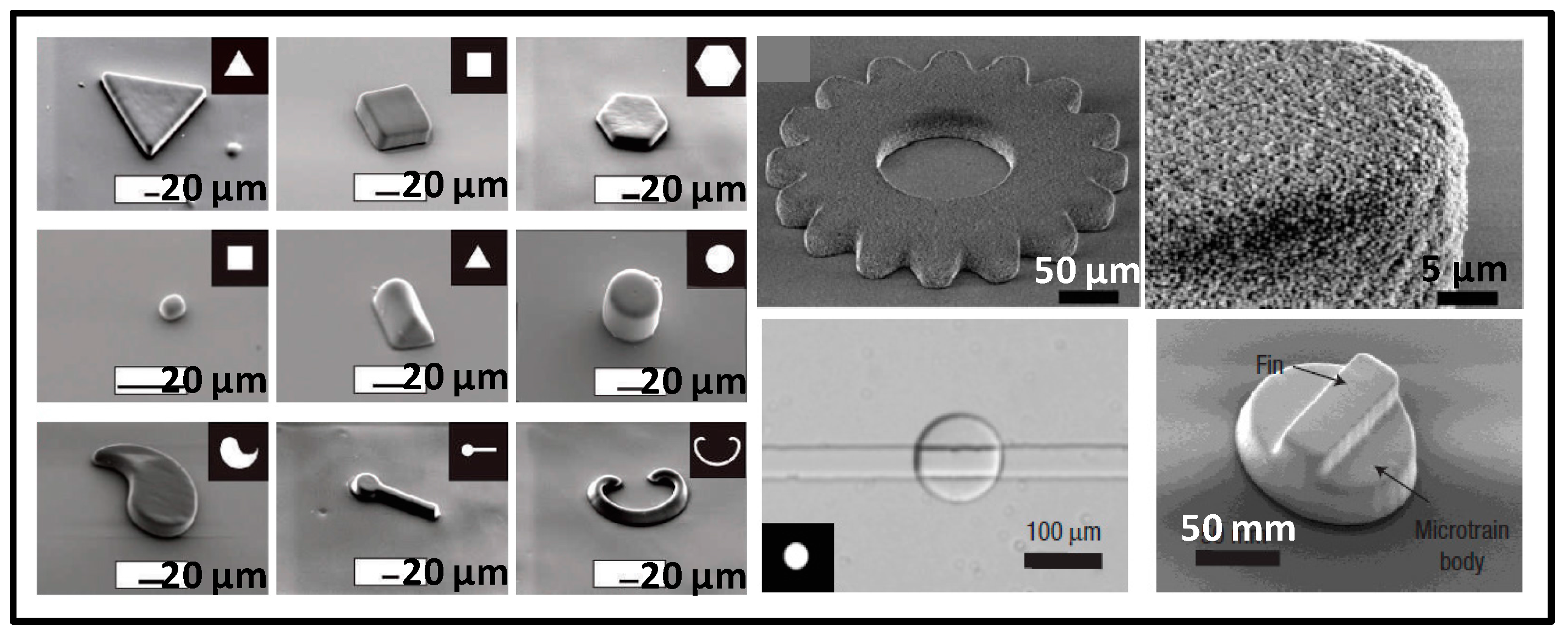
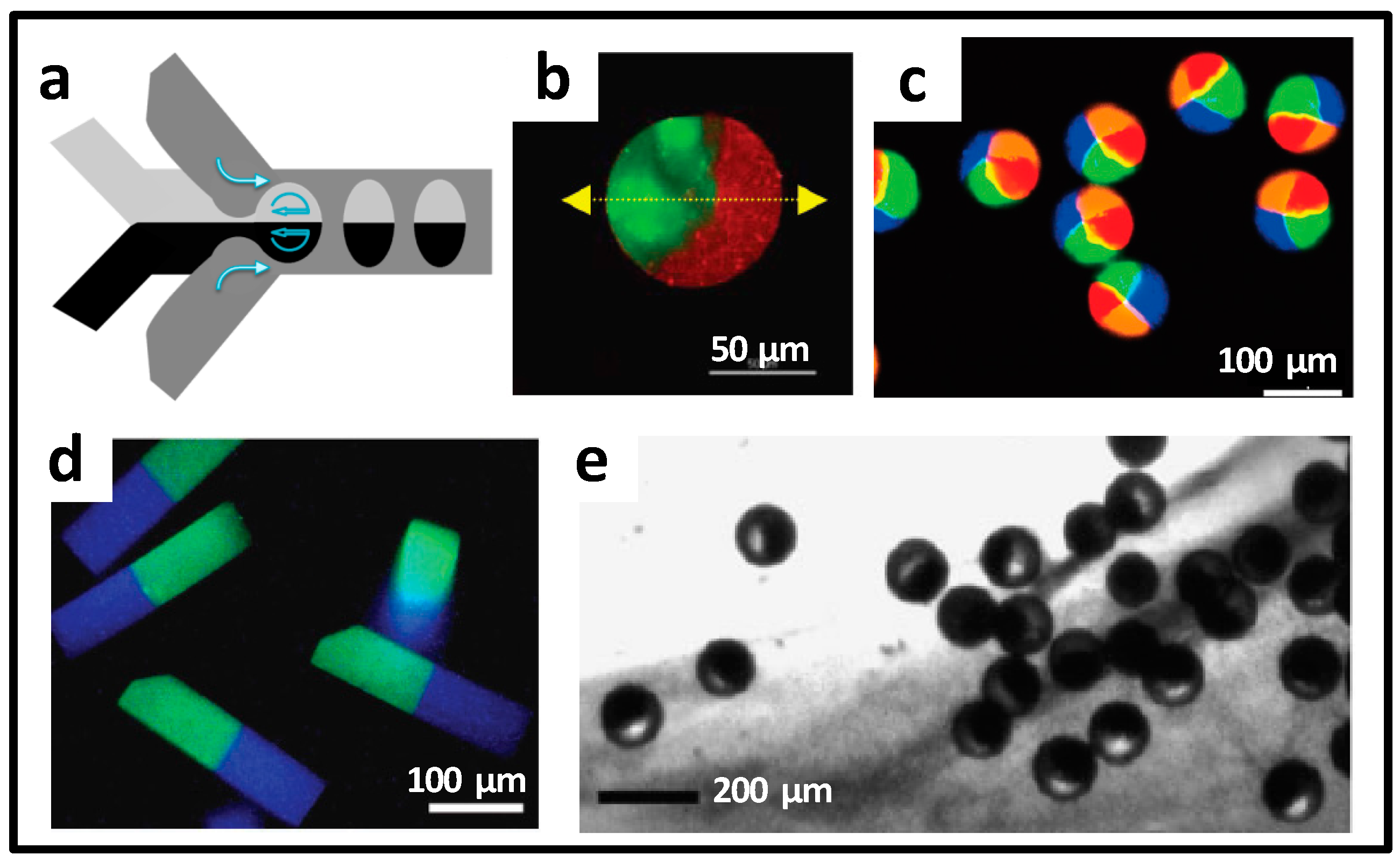
2.1.2. Special-Shaped Microparticles
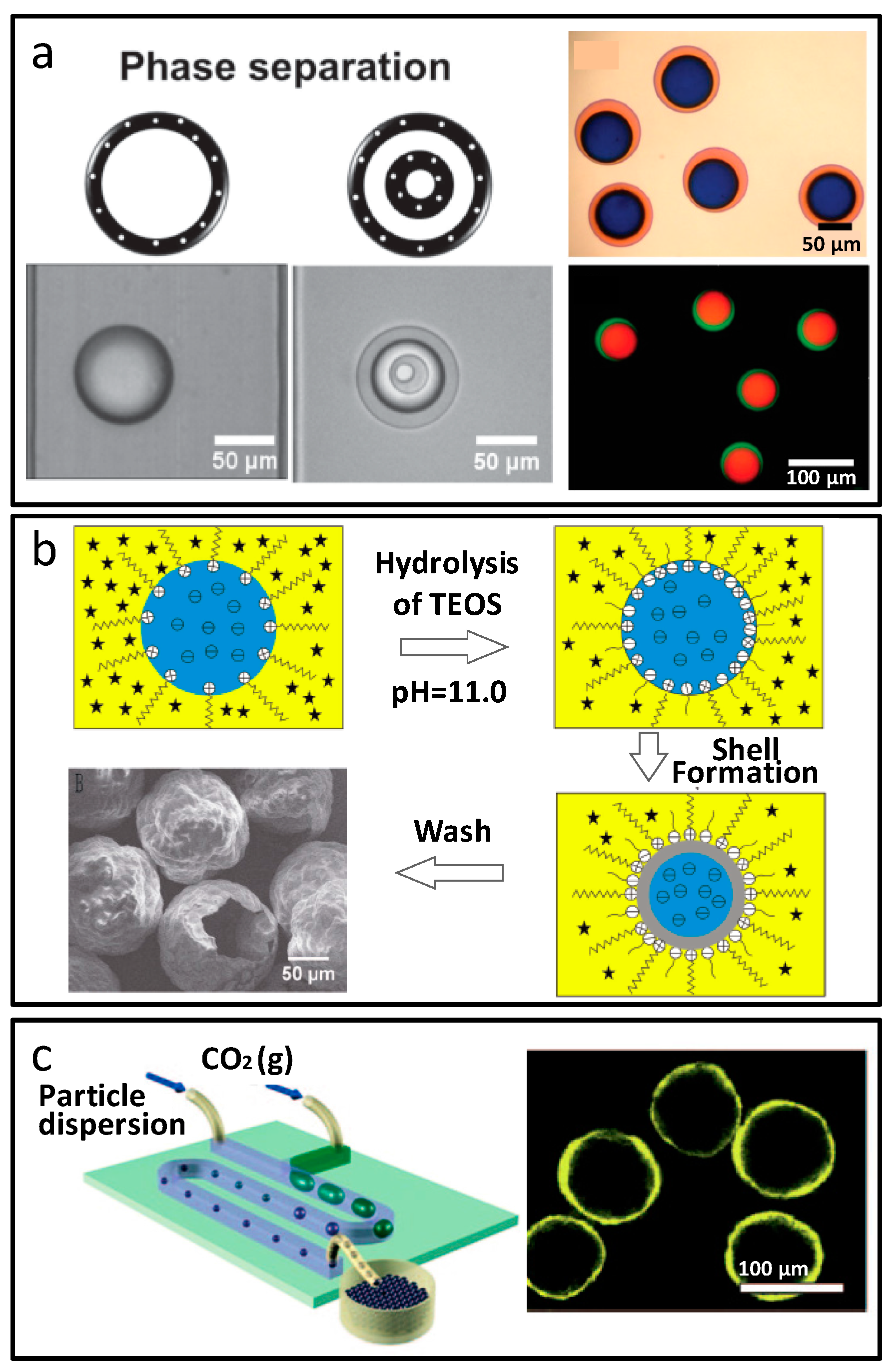
2.1.3. Core-Shell Structural Microparticles
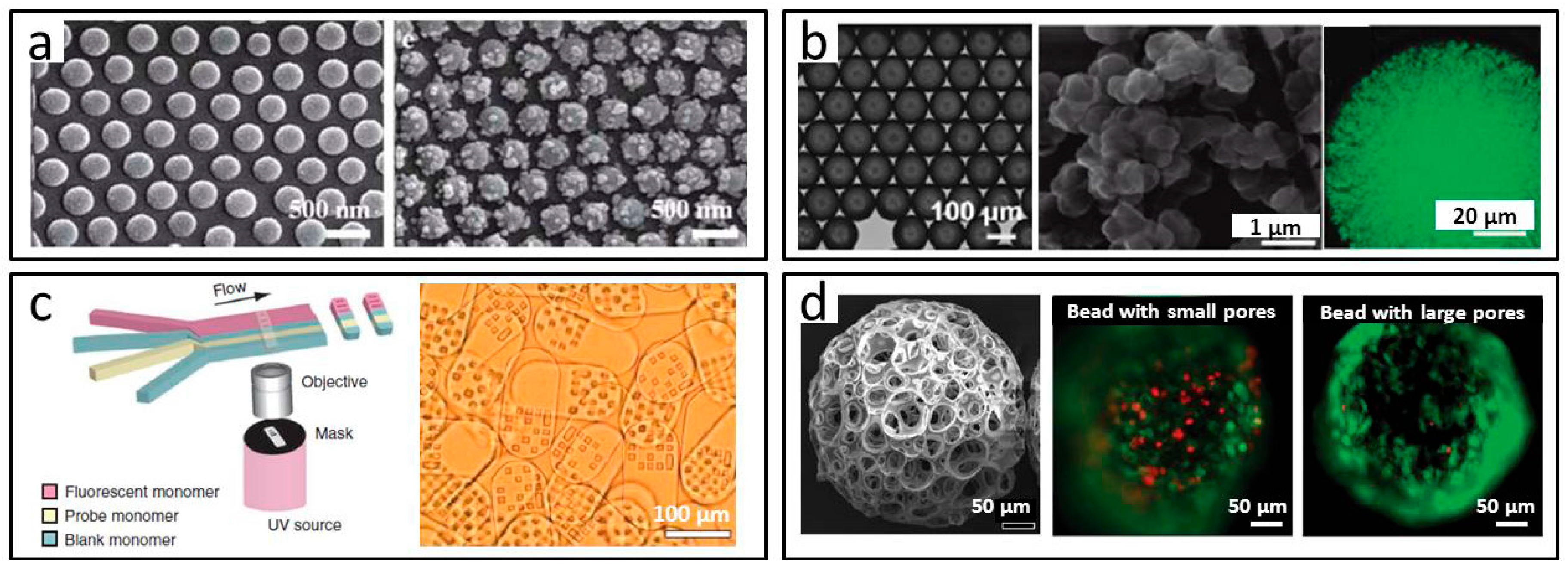
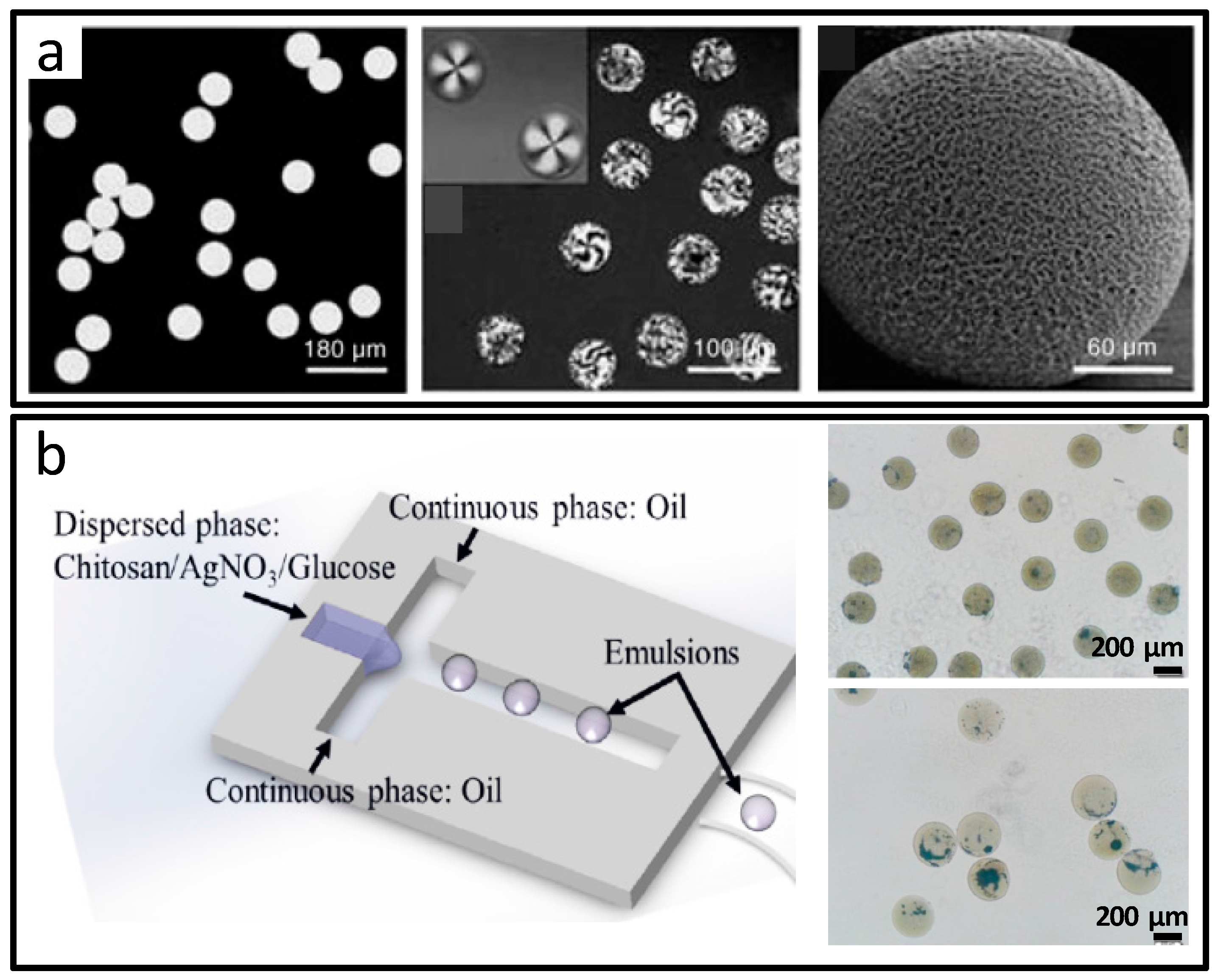
2.1.4. Porous Microparticles
2.1.5. Composite Microparticles
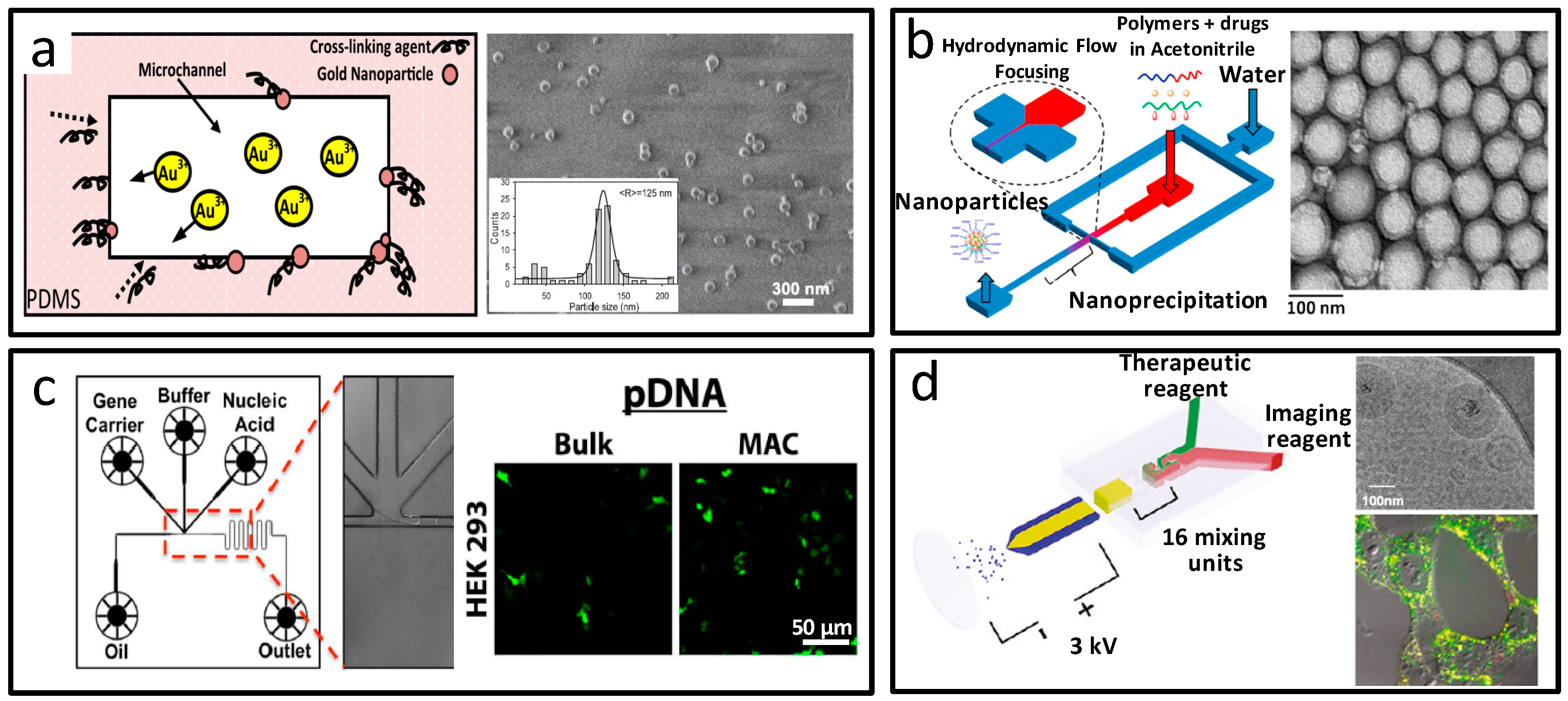
2.2. Particulate Biomaterials at the Nanoscale
3. Fibrous Biomaterials Synthesis and Applications
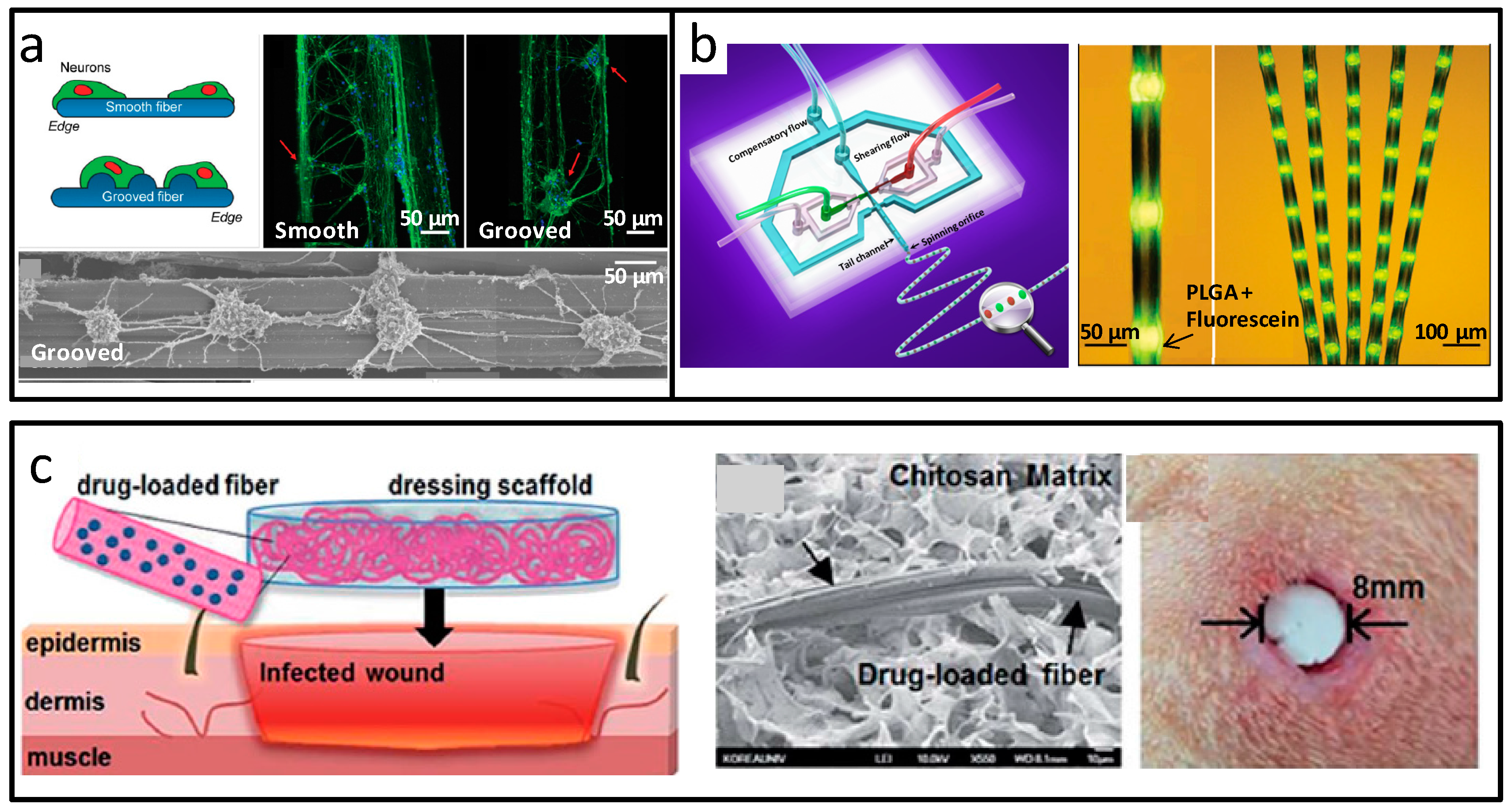
3.1. Fibrous Biomaterials at Micro-Scale
3.2. Fibrous Biomaterials at the Nanoscale
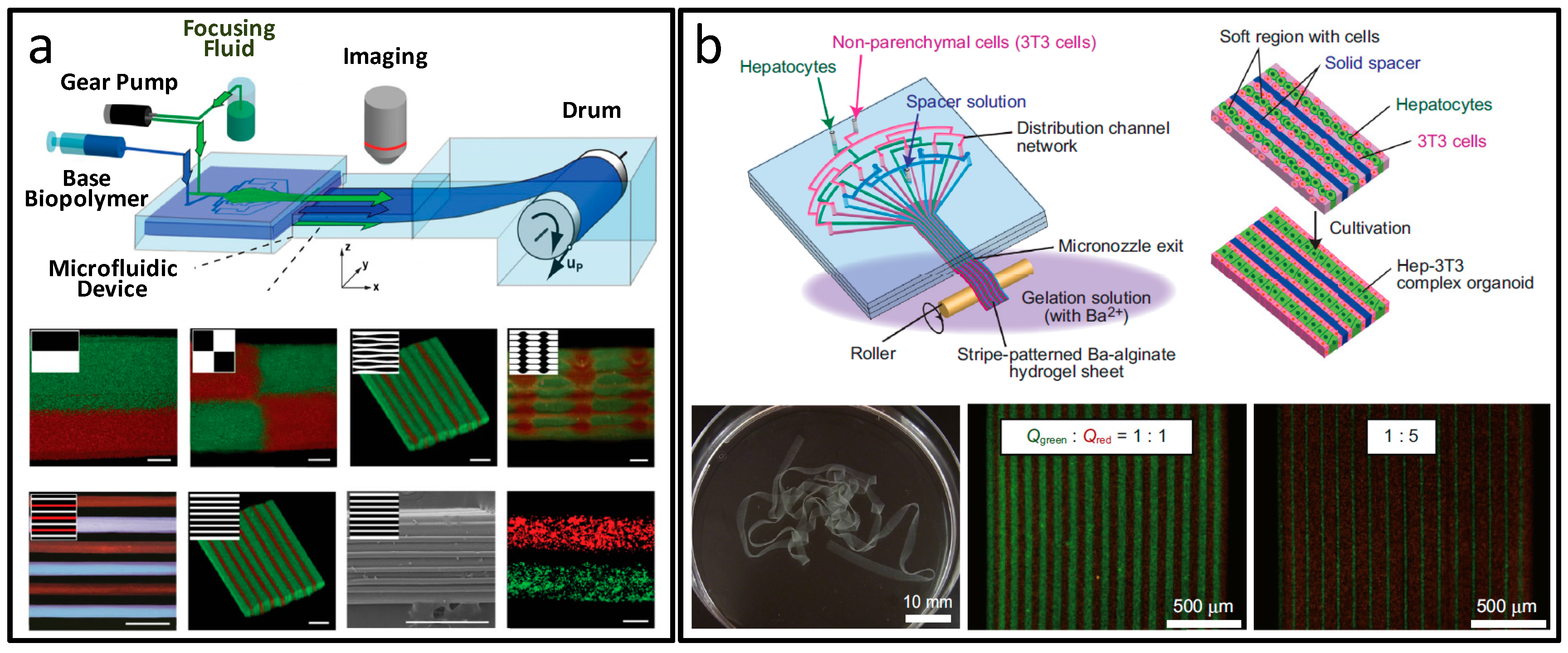
4. Sheet Biomaterials Synthesis and Applications
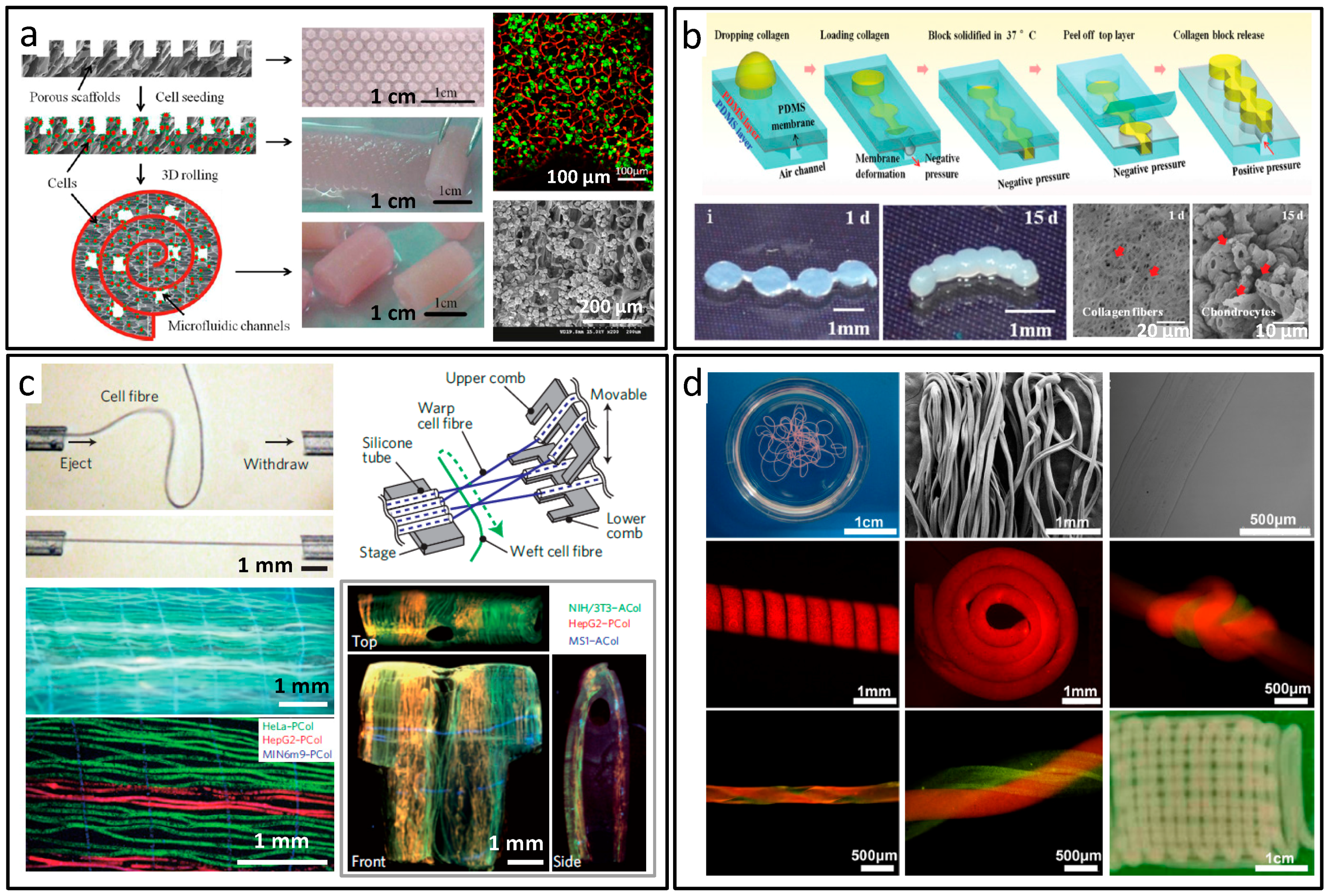
5. Construct Forms of Biomaterials Synthesis and Applications
6. Summaries and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ríos, Á.; Zougagh, M.; Avila, M. Miniaturization through lab-on-a-chip: Utopia or reality for routine laboratories? A review. Anal. Chim. Acta 2012, 740, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kricka, L.J. Miniaturization of analytical systems. Clin. Chem. 1998, 44, 2008–2014. [Google Scholar] [PubMed]
- Sauer, S.; Lange, B.M.H.; Gobom, J.; Nyarsik, L.; Seitz, H.; Lehrach, H. Miniaturization in functional genomics and proteomics. Nat. Rev. Genet. 2005, 6, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering Flows in Small Devices. Ann. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Psaltis, D.; Quake, S.R.; Yang, C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.I.; Tachikawa, K.; Manz, A. Microfluidics: Applications for analytical purposes in chemistry and biochemistry. Electrophoresis 2008, 29, 4443–4453. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where We Have Been and Where We Are Going. Ann. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Biomaterials in tissue engineering. Nat. Biotechnol. 1995, 13, 565–576. [Google Scholar] [CrossRef]
- Hench, L.L. Biomaterials: A forecast for the future. Biomaterials 1998, 19, 1419–1423. [Google Scholar] [CrossRef]
- Peppas, N.A.; Langer, R. New challenges in biomaterials. Science 1994, 263, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Saltzman, W.M. Biomaterials with hierarchically defined micro- and nanoscale structure. Biomaterials 2004, 25, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Choi, S.-Y.; Kim, Y.-K.; Yi, G.-R. Bulk synthesis of ordered macroporous silica particles for superhydrophobic coatings. J. Colloid Interface Sci. 2012, 386, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Y.; Zhang, H.; Tang, Y.; Wei, J.; Sun, W. Bulk Synthesis and Characterization of Ti3Al Nanoparticles by Flow-Levitation Method. J. Nanomater. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Rolland, J.P.; Maynor, B.W.; Euliss, L.E.; Exner, A.E.; Denison, G.M.; DeSimone, J.M. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005, 127, 10096–10100. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; Ropp, P.A.; DeSimone, J.M. Reductively labile PRINT particles for the delivery of doxorubicin to HeLa cells. J. Am. Chem. Soc. 2008, 130, 5008–5009. [Google Scholar] [CrossRef] [PubMed]
- Charcosset, C.; Limayem, I.; Fessi, H. The membrane emulsification process—A review. J. Chem. Technol. Biotechnol. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Stroock, A.D.; Cabodi, M. Microfluidic biomaterials. MRS Bull. 2006, 31, 114–119. [Google Scholar] [CrossRef]
- Kobel, S.; Lutolf, M.P. Biomaterials meet microfluidics: Building the next generation of artificial niches. Curr. Opin. Biotechnol. 2011, 22, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.K.; Tsai, S.S.H.; Wan, J.; Stone, H.A. Dripping and jetting in microfluidic multiphase flows applied to particle and fibre synthesis. J. Phys. D Appl. Phys. 2013, 46, 114002. [Google Scholar] [CrossRef] [PubMed]
- Shim, T.S.; Kim, S.-H.; Yang, S.-M. Elaborate Design Strategies Toward Novel Microcarriers for Controlled Encapsulation and Release. Part. Part. Syst. Charact. 2013, 30, 9–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Gañán-Calvo, A.M.; Montanero, J.M.; Martín-Banderas, L.; Flores-Mosquera, M. Building functional materials for health care and pharmacy from microfluidic principles and Flow Focusing. Adv. Drug Deliv. Rev. 2013, 65, 1447–1469. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, X.; Wang, J.; Tian, H.; Zhao, P.; Tian, Y.; Gu, Y.; Wang, L.; Wang, C. Droplet Microfluidics for the Production of Microparticles and Nanoparticles. Micromachines 2017, 8, 22. [Google Scholar] [CrossRef]
- Yang, S.; Guo, F.; Kiraly, B.; Mao, X.; Lu, M.; Leong, K.W.; Huang, T.J. Microfluidic synthesis of multifunctional Janus particles for biomedical applications. Lab Chip 2012, 12, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, M.J.; Chu, L.Y. Functional Polymeric Microparticles Engineered from Controllable Microfluidic Emulsions. Acc. Chem. Res. 2014, 47, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hashimoto, M.; Dang, T.T.; Hoare, T.; Kohane, D.S.; Whitesides, G.M.; Langer, R.; Anderson, D.G. Preparation of Monodisperse Biodegradable Polymer Microparticles Using a Microfluidic Flow-Focusing Device for Controlled Drug Delivery. Small 2009, 5, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ding, H.J.; Liu, J.; Chen, Y.; Zhao, X.Z. Shape-controlled production of biodegradable calcium alginate gel microparticles using a novel microfluidic device. Langmuir ACS J. Surf. Colloids 2006, 22, 9453–9457. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; deMello, A.J. Microdroplets: A sea of applications? Lab Chip 2008, 8, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef] [PubMed]
- Seemann, R.; Brinkmann, M.; Pfohl, T.; Herminghaus, S. Droplet based microfluidics. Rep. Prog. Phys. 2012, 75, 016601. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Saffari, A.; Kumar, S.; Günther, A.; Kumacheva, E. Microfluidic Synthesis of Polymer and Inorganic Particulate Materials. Ann. Rev. Mater. Res. 2010, 40, 415–443. [Google Scholar] [CrossRef]
- Tan, W.S.; Lewis, C.L.; Horelik, N.E.; Pregibon, D.C.; Doyle, P.S.; Yi, H. Hierarchical assembly of viral nanotemplates with encoded microparticles via nucleic acid hybridization. Langmuir ACS J. Surf. Colloids 2008, 24, 12483–12488. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Nakajima, M.; Tong, J.; Nabetani, H.; Seki, M. Preparation of Monodispersed Solid Lipid Microspheres Using a Microchannel Emulsification Technique. J. Colloid Interface Sci. 2000, 227, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pham, H.H.; Nie, Z.; MacDonald, B.; Guenther, A.; Kumacheva, E. Multi-step microfluidic polymerization reactions conducted in droplets: The internal trigger approach. J. Am. Chem. Soc. 2008, 130, 9935–9941. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Heo, Y.J.; Okitsu, T.; Matsunaga, Y.; Kawanishi, T.; Takeuchi, S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proc. Natl. Acad. Sci. USA 2010, 107, 17894–17898. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.H.; Takeuchi, S. Monodisperse Alginate Hydrogel Microbeads for Cell Encapsulation. Adv. Mater. 2007, 19, 2696–2701. [Google Scholar] [CrossRef]
- Hung, L.-H.; Teh, S.-Y.; Jester, J.; Lee, A.P. PLGA micro/nanosphere synthesis by droplet microfluidic solvent evaporation and extraction approaches. Lab Chip 2010, 10, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Vladisavljević, G.T.; Shahmohamadi, H.; Das, D.B.; Ekanem, E.E.; Tauanov, Z.; Sharma, L. Glass capillary microfluidics for production of monodispersed poly (dl-lactic acid) and polycaprolactone microparticles: Experiments and numerical simulations. J. Colloid Interface Sci. 2014, 418, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Ono, T.; Kimura, Y. Continuous fabrication of monodisperse polylactide microspheres by droplet-to-particle technology using microfluidic emulsification and emulsion–solvent diffusion. Soft Matter 2011, 7, 9894–9897. [Google Scholar] [CrossRef]
- Lee, I.; Yoo, Y.; Cheng, Z.; Jeong, H.-K. Generation of Monodisperse Mesoporous Silica Microspheres with Controllable Size and Surface Morphology in a Microfluidic Device. Adv. Funct. Mater. 2008, 18, 4014–4021. [Google Scholar] [CrossRef]
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of Monodisperse Particles by Using Microfluidics: Control over Size, Shape, and Composition. Angew. Chem. Int. Ed. 2005, 44, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, B.; Qin, J. Synthesis of shape-controlled particles based on synergistic effect of geometry confinement, double emulsion template, and polymerization quenching. Microfluid. Nanofluid. 2011, 12, 33–39. [Google Scholar] [CrossRef]
- Ekanem, E.E.; Zhang, Z.; Vladisavljević, G.T. Facile microfluidic production of composite polymer core-shell microcapsules and crescent-shaped microparticles. J. Colloid Interface Sci. 2017, 498, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Gaillard, C.; Douliez, J.-P. Template-Free Formation of Monodisperse Doughnut-Shaped Silica Microparticles by Droplet-Based Microfluidics. Chem. Mater. 2011, 23, 4660–4662. [Google Scholar] [CrossRef]
- Dendukuri, D.; Pregibon, D.C.; Collins, J.; Hatton, T.A.; Doyle, P.S. Continuous-flow lithography for high-throughput microparticle synthesis. Nat. Mater. 2006, 5, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.F.; Panda, P.; Bao, Z.; Sandhage, K.H.; Hatton, T.A.; Lewis, J.A.; Doyle, P.S. Stop-Flow Lithography of Colloidal, Glass, and Silicon Microcomponents. Adv. Mater. 2008, 20, 4734–4739. [Google Scholar] [CrossRef]
- Chung, S.E.; Park, W.; Shin, S.; Lee, S.A.; Kwon, S. Guided and fluidic self-assembly of microstructures using railed microfluidic channels. Nat. Mater. 2008, 7, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeon, T.Y.; Choi, T.M.; Shim, T.S.; Kim, S.-H.; Yang, S.-M. Droplet Microfluidics for Producing Functional Microparticles. Langmuir 2014, 30, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Abate, A.R.; Lee, D.; Terentjev, E.M.; Weitz, D.A. Microfluidic Assembly of Magnetic Hydrogel Particles with Uniformly Anisotropic Structure. Adv. Mater. 2009, 21, 3201–3204. [Google Scholar] [CrossRef]
- Zhao, Y.; Shum, H.C.; Chen, H.; Adams, L.L.A.; Gu, Z.; Weitz, D.A. Microfluidic Generation of Multifunctional Quantum Dot Barcode Particles. J. Am. Chem. Soc. 2011, 133, 8790–8793. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, W.; Abbaspourrad, A.; Ahn, J.; Bader, A.; Bose, S.; Vegas, A.; Lin, J.; Tao, J.; Hang, T.; et al. Microfluidic Fabrication of Colloidal Nanomaterials-Encapsulated Microcapsules for Biomolecular Sensing. Nano Lett. 2017, 17, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H.; Weitz, D.A.; Lee, C.-S. One Step Formation of Controllable Complex Emulsions: From Functional Particles to Simultaneous Encapsulation of Hydrophilic and Hydrophobic Agents into Desired Position. Adv. Mater. 2013, 25, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, Y.; Zeng, C.; Wang, C.; Serra, C.A.; Zhang, L. Microfluidic preparation of yolk/shell ZIF-8/alginate hybrid microcapsules from Pickering emulsion. Chem. Eng. J. 2017, 307, 408–417. [Google Scholar] [CrossRef]
- Li, D.; Guan, Z.; Zhang, W.; Zhou, X.; Zhang, W.Y.; Zhuang, Z.; Wang, X.; Yang, C.J. Synthesis of Uniform-Size Hollow Silica Microspheres through Interfacial Polymerization in Monodisperse Water-in-Oil Droplets. ACS Appl. Mater. Interfaces 2010, 2, 2711–2714. [Google Scholar] [CrossRef]
- Park, J.I.; Nie, Z.; Kumachev, A.; Abdelrahman, A.I.; Binks, B.P.; Stone, H.A.; Kumacheva, E. A Microfluidic Approach to Chemically Driven Assembly of Colloidal Particles at Gas-Liquid Interfaces. Angew. Chem. Int. Ed. 2009, 48, 5300–5304. [Google Scholar] [CrossRef] [PubMed]
- Hentze, H.P.; Antonietti, M. Porous polymers and resins for biotechnological and biomedical applications. J. Biotechnol. 2002, 90, 27–53. [Google Scholar] [CrossRef]
- Lumelsky, Y.; Zoldan, J.; Levenberg, S.; Silverstein, M. Porous Polycaprolactone-Polystyrene Semi-interpenetrating Polymer Networks Synthesized within High Internal Phase Emulsions. Macromolecules 2008, 41, 1469–1474. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, S.-H.; Yang, S.-M. Microfluidic fabrication of SERS-active microspheres for molecular detection. Lab Chip 2011, 11, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, S.; Zhang, H.; Nie, Z.; Gourevich, I.; Voicu, D.; Deetz, M.; Kumacheva, E. Microfluidic Synthesis of Macroporous Copolymer Particles. Macromolecules 2008, 41, 3555–3561. [Google Scholar] [CrossRef]
- Pregibon, D.C.; Toner, M.; Doyle, P.S. Multifunctional encoded particles for high-throughput biomolecule analysis. Science 2007, 315, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, D.C.; Chapin, S.C.; Srinivas, R.L.; Doyle, P.S. Bar-coded hydrogel microparticles for protein detection: Synthesis, assay and scanning. Nat. Protoc. 2011, 6, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hui, Y.S.; Zhang, M.; Yu, Y.; Wen, W.; Qin, J. Facile Synthesis of Biomimetic Honeycomb Material with Biological Functionality. Small 2013, 9, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Yeh, Y.-C.; Zhang, Y.; Sung, H.-W.; Xia, Y. Uniform Beads with Controllable Pore Sizes for Biomedical Applications. Small 2010, 6, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Wang, L.-S.; Chen, S.-Y.; Huang, M.-C.; Li, Y.-H.; Lin, Y.-C.; Chen, P.-F.; Shaw, J.-F.; Huang, K.-S. Microfluidic assisted synthesis of silver nanoparticle–chitosan composite microparticles for antibacterial applications. Int. J. Pharm. 2016, 510, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Marquis, M.; Renard, D.; Cathala, B. Microfluidic Generation and Selective Degradation of Biopolymer-Based Janus Microbeads. Biomacromolecules 2012, 13, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Onoe, H.; Takinoue, M.; Takeuchi, S. Controlled Synthesis of 3D Multi-Compartmental Particles with Centrifuge-Based Microdroplet Formation from a Multi-Barrelled Capillary. Adv. Mater. 2012, 24, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Choi, D.G.; Roh, Y.H.; Shim, M.S.; Bong, K.W. Microfluidic Synthesis of pH-Sensitive Multicompartmental Microparticles for Multimodulated Drug Release. Small 2016, 12, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Kim, J.-W.; Weitz, D.A. Janus Supraparticles by Induced Phase Separation of Nanoparticles in Droplets. Adv. Mater. 2009, 21, 1949–1953. [Google Scholar] [CrossRef]
- Ma, J.; Lee, S.M.; Yi, C.; Li, C.W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y. Microfluidic Synthesis of Nanohybrids. Small 2017, 13, 1604084. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Du, L.; Wang, Y.; Lu, Y.; Xu, J. Controllable preparation of particles with microfluidics. Particuology 2011, 9, 545–558. [Google Scholar] [CrossRef]
- Krishna, K.S.; Li, Y.; Li, S.; Kumar, C.S.S.R. Lab-on-a-chip synthesis of inorganic nanomaterials and quantum dots for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 1470–1495. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hassan, A.; Sandre, O.; Neveu, S.; Cabuil, V. Synthesis of Goethite by Separation of the Nucleation and Growth Processes of Ferrihydrite Nanoparticles Using Microfluidics. Angew. Chem. Int. Ed. 2009, 48, 2342–2345. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hassan, A.; Bazzi, R.; Cabuil, V. Multistep Continuous-Flow Microsynthesis of Magnetic and Fluorescent γ-Fe2O3@SiO2Core/Shell Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 7180–7183. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, H.; Chen, K.-J.; Guo, F.; Lin, W.-Y.; Chen, Y.-C.; Phung, D.L.; Tseng, H.-R.; Shen, C.K.F. A digital microfluidic droplet generator produces self-assembled supramolecular nanoparticles for targeted cell imaging. Nanotechnology 2010, 21, 445603. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Z.; Terepka, A.D.; Yang, H. Synthesis of Silver Nanoparticles in a Continuous Flow Tubular Microreactor. Nano Lett. 2004, 4, 2227–2232. [Google Scholar] [CrossRef]
- Wagner, J.; Kirner, T.; Mayer, G.; Albert, J.; Köhler, J.M. Generation of metal nanoparticles in a microchannel reactor. Chem. Eng. J. 2004, 101, 251–260. [Google Scholar] [CrossRef]
- SadAbadi, H.; Badilescu, S.; Packirisamy, M.; Wüthrich, R. Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens. Bioelectron. 2013, 44, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Knauer, A.; Thete, A.; Li, S.; Romanus, H.; Csáki, A.; Fritzsche, W.; Köhler, J.M. Au/Ag/Au double shell nanoparticles with narrow size distribution obtained by continuous micro segmented flow synthesis. Chem. Eng. J. 2011, 166, 1164–1169. [Google Scholar] [CrossRef]
- Kolishetti, N.; Dhar, S.; Valencia, P.M.; Lin, L.Q.; Karnik, R.; Lippard, S.J.; Langer, R.; Farokhzad, O.C. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 17939–17944. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Pridgen, E.M.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano 2013, 7, 10671–10680. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhang, L.; Liu, C.; Li, X.; Hu, G.; Sun, J.; Jiang, X. Microfluidic based high throughput synthesis of lipid-polymer hybrid nanoparticles with tunable diameters. Biomicrofluidics 2015, 9, 052604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, Q.; Wang, J.; Zhang, S.; Ding, B.; Wei, Y.; Dong, M.; Ryu, J.-Y.; Yoon, T.-Y.; Shi, X.; et al. Microfluidic Synthesis of Hybrid Nanoparticles with Controlled Lipid Layers: Understanding Flexibility-Regulated Cell–Nanoparticle Interaction. ACS Nano 2015, 9, 9912–9921. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; He, J.; Hourwitz, M.J.; Yang, Y.; Fourkas, J.T.; Han, X.; Nie, Z. Continuous Microfluidic Self-Assembly of Hybrid Janus-Like Vesicular Motors: Autonomous Propulsion and Controlled Release. Small 2015, 11, 3762–3767. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, C.L.; Ho, Y.-P.; Lin, C.; Engbersen, J.F.J.; Leong, K.W. Microfluidic Preparation of Polymer-Nucleic Acid Nanocomplexes Improves Nonviral Gene Transfer. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Belliveau, N.M.; Huft, J.; Lin, P.J.C.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, H.; Shi, X.; Wu, H.; Hanagata, N. Microfluidic generation of chitosan/CpG oligodeoxynucleotide nanoparticles with enhanced cellular uptake and immunostimulatory properties. Lab Chip 2014, 14, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, Q.; Wang, J.; Sun, J.; Shi, X.; Jiang, X. Microfluidic Synthesis of Rigid Nanovesicles for Hydrophilic Reagents Delivery. Angew. Chem. Int. Ed. 2015, 54, 3952–3956. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, J.; Choi, J.S.; Chen, K.J.; Hou, S.; Yan, M.; Lin, W.Y.; Chen, K.S.; Ro, T.; Lipshutz, G.S.; et al. A High-Throughput Platform for Formulating and Screening Multifunctional Nanoparticles Capable of Simultaneous Delivery of Genes and Transcription Factors. Angew. Chem. 2016, 55, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L.; Mao, Y.; Lee, L.J. Static micromixer-coaxial electrospray synthesis of theranostic lipoplexes. ACS Nano 2012, 6, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Ozcelik, A.; Grigsby, C.L.; Zhao, Y.; Guo, F.; Leong, K.W.; Huang, T.J. Microfluidic hydrodynamic focusing for synthesis of nanomaterials. Nano Today 2016, 11, 778–792. [Google Scholar] [CrossRef]
- Lu, M.Q.; Yang, S.K.; Ho, Y.P.; Grigsby, C.L.; Leong, K.W.; Huang, T.J. Shape-Controlled Synthesis of Hybrid Nanomaterials via Three-Dimensional Hydrodynamic Focusing. Acs Nano 2014, 8, 10026–10034. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Q.; Ho, Y.P.; Grigsby, C.L.; Nawaz, A.A.; Leong, K.W.; Huang, T.J. Three-Dimensional Hydrodynamic Focusing Method for Polyplex Synthesis. Acs Nano 2014, 8, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jun, Y.; Qin, J.; Lee, S.-H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Choi, Y.Y.; Chae, S.-K.; Moon, J.-H.; Chang, J.-Y.; Lee, S.-H. Microfluidic Spinning of Flat Alginate Fibers with Grooves for Cell-Aligning Scaffolds. Adv. Mater. 2012, 24, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.M.; Park, Y.; Park, J.Y.; Lee, K.; Sun, K.; Khademhosseini, A.; Lee, S.H. Controlled cellular orientation on PLGA microfibers with defined diameters. Biomed. Microdevices 2009, 11, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Mun, C.H.; Kang, E.; No, D.Y.; Ju, J.; Lee, S.H. One-stop microfiber spinning and fabrication of a fibrous cell-encapsulated scaffold on a single microfluidic platform. Biofabrication 2014, 6, 024108. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ostrovidov, S.; Zhao, Y.; Liang, X.; Kasuya, M.; Kurihara, K.; Nakajima, K.; Bae, H.; Wu, H.; Khademhosseini, A. Microfluidic Spinning of Cell-Responsive Grooved Microfibers. Adv. Funct. Mater. 2015, 25, 2250–2259. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, H.; Ma, J.; Lykkemark, S.; Xu, H.; Qin, J. Flexible Fabrication of Biomimetic Bamboo-Like Hybrid Microfibers. Adv. Mater. 2014, 26, 2494–2499. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jeong, G.S.; Choi, Y.Y.; Lee, K.H.; Khademhosseini, A.; Lee, S.H. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater. 2011, 10, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Mun, C.H.; Lee, S.H. Microfluidic spinning of fibrous alginate carrier having highly enhanced drug loading capability and delayed release profile. RSC Adv. 2015, 5, 15172–15181. [Google Scholar] [CrossRef]
- He, X.H.; Wang, W.; Deng, K.; Xie, R.; Ju, X.; Liu, Z.; Chu, L. Microfluidic fabrication of chitosan microfibers with controllable internals from tubular to peapodlike structures. RSC Adv. 2015, 5, 928–936. [Google Scholar] [CrossRef]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.-H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.-K.; Kang, E.; Khademhosseini, A.; Lee, S.-H. Micro/Nanometer-Scale Fiber with Highly Ordered Structures by Mimicking the Spinning Process of Silkworm. Adv. Mater. 2013, 25, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, X.; Jiang, L.; Qin, J. Flexible Generation of Gradient Electrospinning Nanofibers Using a Microfluidic Assisted Approach. Langmuir 2012, 28, 10026–10032. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; McAllister, A.; Zhang, B.; Radisic, M.; Günther, A. Mosaic Hydrogels: One-Step Formation of Multiscale Soft Materials. Adv. Mater. 2012, 24, 3650–3658. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, X.; Chinnathambi, S.; Wu, H.; Hanagata, N. Generation of microgrooved silica nanotube membranes with sustained drug delivery and cell contact guidance ability by using a Teflon microfluidic chip. Sci. Technol. Adv. Mater. 2013, 14, 015005. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Yamakoshi, K.; Yajima, Y.; Utoh, R.; Yamada, M.; Seki, M. Preparation of stripe-patterned heterogeneous hydrogel sheets using microfluidic devices for high-density coculture of hepatocytes and fibroblasts. J. Biosci. Bioeng. 2013, 116, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Kothandaraman, A.; Edirisinghe, M.; Huang, J. Porous Polymeric Films from Microbubbles Generated Using a T-Junction Microfluidic Device. Langmuir 2016, 32, 13377–13385. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.G.; Lee, K.H.; Khademhosseini, A.; Lee, S.H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip 2012, 12, 45–59. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mao, M.; Liu, Y.; Zhu, L.; Li, D. Bottom-up fabrication of 3D cell-laden microfluidic constructs. Mater. Lett. 2013, 90, 93–96. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, Z.; Ma, J.; Shi, Y.; Xu, H.; Lykkemark, S.; Qin, J. Flexible Fabrication of Shape-Controlled Collagen Building Blocks for Self-Assembly of 3D Microtissues. Small 2015, 11, 3666–3675. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Iwase, M.; Yamada, M.; Yajima, Y.; Seki, M. Fabrication of multilayered vascular tissues using microfluidic agarose hydrogel platforms. Biotechnol. J. 2016, 11, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Huang, Q.; Shi, Q.; Wang, H.; Liu, X.; Seki, M.; Nakajima, M.; Fukuda, T. Magnetic assembly of microfluidic spun alginate microfibers for fabricating three-dimensional cell-laden hydrogel constructs. Microfluid. Nanofluid. 2015, 19, 1169–1180. [Google Scholar] [CrossRef]
- Lee, K.H.; Shin, S.J.; Kim, C.-B.; Kim, J.K.; Cho, Y.W.; Chung, B.G.; Lee, S.-H. Microfluidic synthesis of pure chitosan microfibers for bio-artificial liver chip. Lab Chip 2010, 10, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanian, S.; Qasaimeh, M.A.; Akbari, M.; Tamayol, A.; Juncker, D. Microfluidic direct writer with integrated declogging mechanism for fabricating cell-laden hydrogel constructs. Biomed. Microdevices 2014, 16, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sun, J.; Bolderson, J.; Zhong, M.; Dalby, M.J.; Cusack, M.; Yin, H.; Fan, H.; Zhang, X. Continuous Fabrication and Assembly of Spatial Cell-Laden Fibers for a Tissue-Like Construct via a Photolithographic-Based Microfluidic Chip. ACS Appl. Mater. Interfaces 2017, 9, 14606–14617. [Google Scholar] [CrossRef] [PubMed]
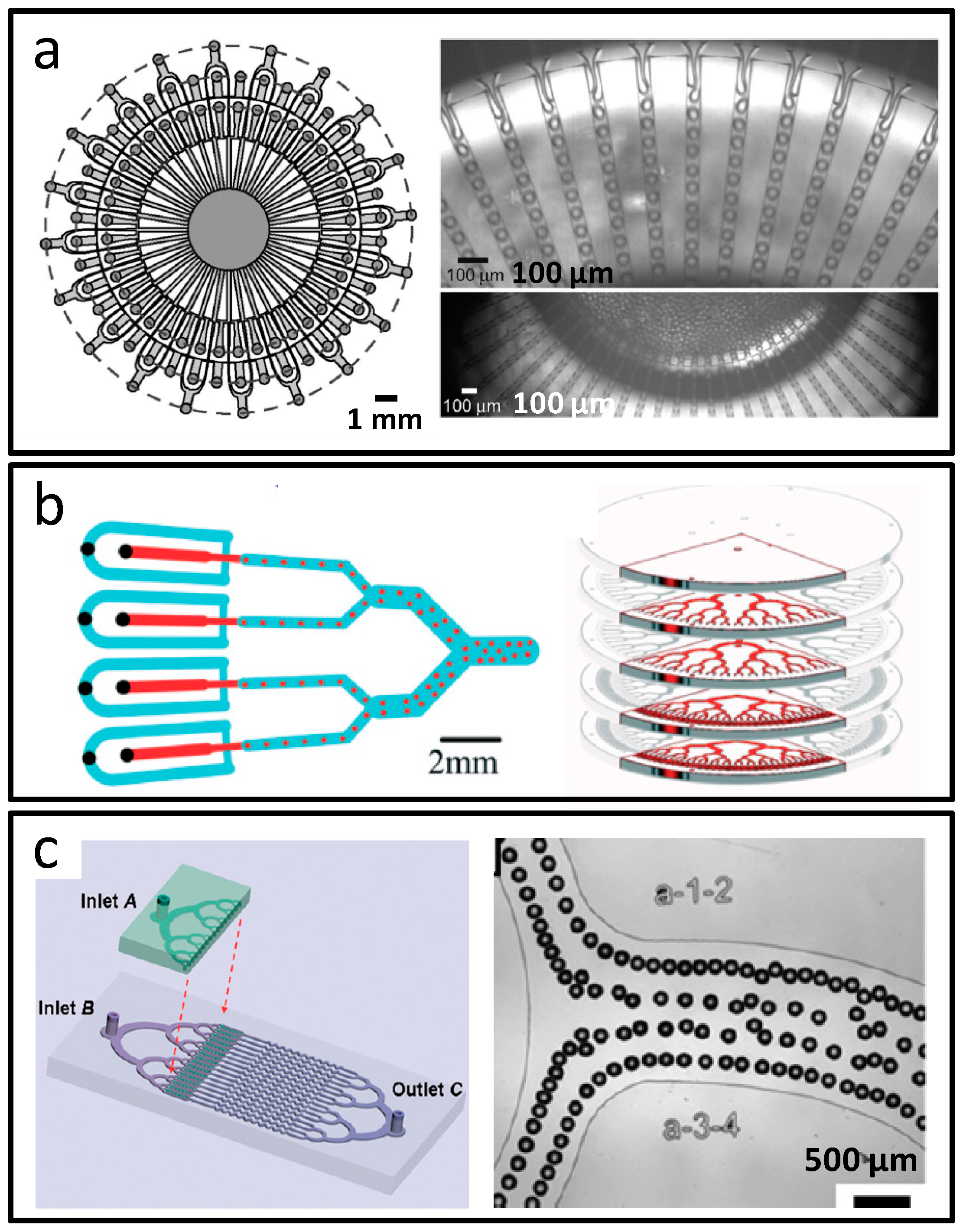
- Nisisako, T.; Ando, T.; Hatsuzawa, T. High-volume production of single and compound emulsions in a microfluidic parallelization arrangement coupled with coaxial annular world-to-chip interfaces. Lab Chip 2012, 12, 3426–3435. [Google Scholar] [CrossRef] [PubMed]
- Conchouso, D.; Castro, D.; Khan, S.A.; Foulds, I.G. Three-dimensional parallelization of microfluidic droplet generators for a litre per hour volume production of single emulsions. Lab Chip 2014, 14, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Greener, J.; Voicu, D.; Kumacheva, E. Multiple modular microfluidic (M3) reactors for the synthesis of polymer particles. Lab Chip 2009, 9, 2715–2721. [Google Scholar] [CrossRef] [PubMed]


| Dimension of Product | Chip Design | Materials | Potential Biomedical Applications | Reference |
|---|---|---|---|---|
| 0D (spherical microparticles) | “Squeezing out” microchannel | Edible oil | Not mentioned in original work | [37] |
| 0D (spherical microparticles) | Flow-focusing | Poly tripropyleneglycol diacrylate (polyTPGDA) Polyurethane | Not mentioned in original work | [38] |
| 0D (spherical microparticles) | Coaxial | Polyacrylamide (PAM) | Glucose monitoring | [39] |
| 0D (spherical microparticles) | T-junction | Calcium alginate | Cell carrier | [40] |
| 0D (spherical microparticles) | Flow-focusing | Poly(lactide-co-glycolide) (PLGA) | Drug delivery | [41] |
| 0D (spherical microparticles) | Coaxial | Poly(dl-lactic acid) or polycaprolactone (PCL) | Drug delivery | [42] |
| 0D (spherical microparticles) | T-junction variant | Poly(lactic acid) (PLA) | Drug delivery | [43] |
| 0D (spherical microparticles) | Flow-focusing | Silica (SiO2) | Sensors Biomolecule delivery | [44] |
| 0D (special-shaped microparticles) | Flow-focusing | PolyTPGDA | Not mentioned in original work | [45] |
| 0D (special-shaped microparticles) | T-junction Flow-focusing | Poly(ethylene glycol) diacrylate (PEGDA) | Drug delivery Biological probes | [46] |
| 0D (special-shaped microparticles) | Coaxial | PLA | Cell trapping or immobilisation | [47] |
| 0D (special-shaped microparticles) | Flow-focusing | SiO2 | Controlled release Biosensing | [48] |
| 0D (special-shaped microparticles) | Lithography channel | PEGDA | Drug delivery Biosensors | [49] |
| 0D (special-shaped microparticles) | Lithography channel | Colloidal Glass SiO2 | Biosensor | [50] |
| 0D (core–shell microparticles) | Lithography channel | PEGDA | Cell assembly | [51] |
| 0D (core–shell microparticles) | Flow-focusing Double emulsions | Ferrofluid PAM | Magnetic imaging Micro-mixing | [53] |
| 0D (core–shell microparticles) | Coaxial Double emulsions | PEGDA Ethoxylated trimethylolpropane triacrylate (ETPTA) | Bioassays Cell culture | [54] |
| 0D (core–shell microparticles) | Coaxial Double emulsions | Polyethylene glycol (PEG) Colloidal nanosensors | Biomolecular sensing | [55] |
| 0D (core–shell microparticles) | Flow-focusing | PEGDA | Agent delivery | [56] |
| 0D (core–shell microparticles) | Coaxial | ZIF-8 Alginate | Drug carrier | [57] |
| 0D (core–shell microparticles) | Flow-focusing | SiO2 | Detoxification | [58] |
| 0D (core–shell microparticles) | T-junction | Poly(styrene-co-acrylic acid) | Ultrasonic MRI | [59] |
| 0D (porous microparticles) | Coaxial | SiO2 Silver ETPTA | Molecular detection | [62] |
| 0D (porous microparticles) | Flow-focusing | Poly(GMA-co-EGDMA) | Carriers of biologically active species | [63] |
| 0D (porous microparticles) | Lithography channel | PEG | Biomolecule analysis | [64] |
| 0D (porous microparticles) | Lithography channel | PEG | Protein detection | [65] |
| 0D (porous microparticles) | Flow-focusing | PLGA | Drug carrier Cell carrier | [66] |
| 0D (porous microparticles) | Coaxial | PLGA | Cell scaffold | [67] |
| 0D (composite microparticles) | Flow-focusing | Silver Chitosan | Antibacterial | [68] |
| 0D (composite microparticles) | Flow-focusing | Pectin Alginate Biopolymer | Controlled release | [69] |
| 0D (composite microparticles) | Coaxial Multi-Barrelled Capillary | Calcium alginate | Cell carrier | [70] |
| 0D (composite microparticles) | Lithography channel | PEGDA Ketal-containing diacrylamide | Drug release | [71] |
| 0D (composite microparticles) | Coaxial | PAM Poly(N-isopropylacrylamide) Iron oxide particles | Magnetically manipulation | [72] |
| 0D(nanoparticles) | 3D flow-focusing | Goethite | Magnetically manipulation | [77] |
| 0D(nanoparticles) | 3D flow-focusing | SiO2-coated magnetic nanoparticles | MRI | [78] |
| 0D(nanoparticles) | Digital droplet generator | Supramolecular nanoparticles | Molecular imaging | [79] |
| 0D(nanoparticles) | Single channel | Silver | Biosensing | [80] |
| 0D(nanoparticles) | Single channel | Gold | Biosensing | [81] |
| 0D(nanoparticles) | Single channel | Gold | Biosensor for protein and polypeptide detection | [82] |
| 0D(nanoparticles) | T-junction variant | Au/Ag/Au | Plasmonic application | [83] |
| 0D(nanoparticles) | 2D flow-focusing | PEG-PLGA | Drug delivery | [84] |
| 0D(nanoparticles) | 3D flow-focusing | PEG-PLGA | Drug delivery | [85] |
| 0D(nanoparticles) | 3D flow-focusing | Lipid-PLGA | Drug delivery | [86] |
| 0D(nanoparticles) | 3D flow-focusing | Lipid-PLGA | Drug delivery | [87] |
| 0D(nanoparticles) | 2D flow-focusing | PEO45-b-PS45 Platinum nanoparticles Gold nanorods | Drug delivery | [88] |
| 0D(nanoparticles) | T-junction | Polyplexes | Nucleic acid delivery | [89] |
| 0D(nanoparticles) | Y-junction Herringbone structures | Lipid | Nucleic acid delivery | [90] |
| 0D(nanoparticles) | 2D flow-focusing | Chitosan | Nucleic acid delivery | [91] |
| 0D(nanoparticles) | 3D flow-focusing | Lipid-PLGA | Nucleic acid delivery | [92] |
| 0D(nanoparticles) | Digital droplet generator | Supramolecular nanoparticles | Nucleic acid delivery | [93] |
| 0D(nanoparticles) | Coaxial | Lipoplexes | Cancer treatment potential | [94] |
| 0D(nanoparticles) | 3D flow-focusing | Tetrathiafulvalene Au | Not mentioned in original work | [96] |
| 0D(nanoparticles) | 3D flow-focusing | Polyplexes | Therapeutics | [97] |
| 1D(microfibers) | Flow-focusing | Calcium alginate | Tissue engineering | [99] |
| 1D(microfibers) | T-junction variant | PLGA | Tissue engineering | [100] |
| 1D(microfibers) | Flow-focusing | Calcium alginate | Tissue engineering | [101] |
| 1D(microfibers) | T-junction | Methacrylamide-modified gelatin or alginate | Tissue engineering | [102] |
| 1D(microfibers) | Flow-focusing | Calcium alginate | Cells or biomolecules carrier | [103] |
| 1D(microfibers) | Flow-focusing | Calcium alginate | Cells or drug delivery | [104] |
| 1D(microfibers) | Flow-focusing | Calcium alginate | Drug carriers | [105] |
| 1D(microfibers) | Coaxial | Chitosan | Drug carriers | [106] |
| 1D(nanofibers) | Flow-focusing | Calcium alginate | Biomimetic material | [108] |
| 1D(nanofibers) | Y-junction | PLGA | Tissue engineering | [109] |
| 2D(sheet) | Multiple parallel microchannels | Calcium alginate | Tissue engineering | [110] |
| 2D(sheet) | Specifical patterning | Silica | Drug delivery Cell guidance | [111] |
| 2D(sheet) | Multiple parallel microchannels | Calcium alginate | Tissue engineering | [112] |
| 2D(sheet) | T-junction | Alginate | Drug delivery Tissue engineering | [113] |
| 3D(constructs) | Specifical patterning | Silk fibroin Chitosan | Tissue engineering | [115] |
| 3D(constructs) | Specifical patterning | Collagen | Tissue engineering | [116] |
| 3D(constructs) | Specifical patterning | Calcium alginate | Tissue engineering | [117] |
| 3D(constructs) | Flow-focusing | Calcium alginate | Tissue engineering | [118] |
| 3D(constructs) | Flow-focusing | Chitosan | Tissue engineering | [119] |
| 3D(constructs) | Coaxial | Calcium alginate | Tissue engineering | [120] |
| 3D(constructs) | T-junction | Calcium alginate | Tissue engineering | [121] |
| 3D(constructs) | Flow-focusing | Calcium alginate | Tissue engineering | [122] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, Y.; Liu, J. Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications. Micromachines 2017, 8, 255. https://doi.org/10.3390/mi8080255
Ma J, Wang Y, Liu J. Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications. Micromachines. 2017; 8(8):255. https://doi.org/10.3390/mi8080255
Chicago/Turabian StyleMa, Jingyun, Yachen Wang, and Jing Liu. 2017. "Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications" Micromachines 8, no. 8: 255. https://doi.org/10.3390/mi8080255
APA StyleMa, J., Wang, Y., & Liu, J. (2017). Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications. Micromachines, 8(8), 255. https://doi.org/10.3390/mi8080255




