Haptic-Based Manipulation Scheme of Magnetic Nanoparticles in a Multi-Branch Blood Vessel for Targeted Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Magnetic Nano-Manipulation for Targeted Drug Delivery
2.2. Virtual Tele-Nano-Manipulation System
2.2.1. System Overview
2.2.2. System Architecture
2.3. Haptic Interaction for Manipulations of MNPs
2.3.1. Overview
2.3.2. Physics Engine for Particles Simulations inside Blood Vessels
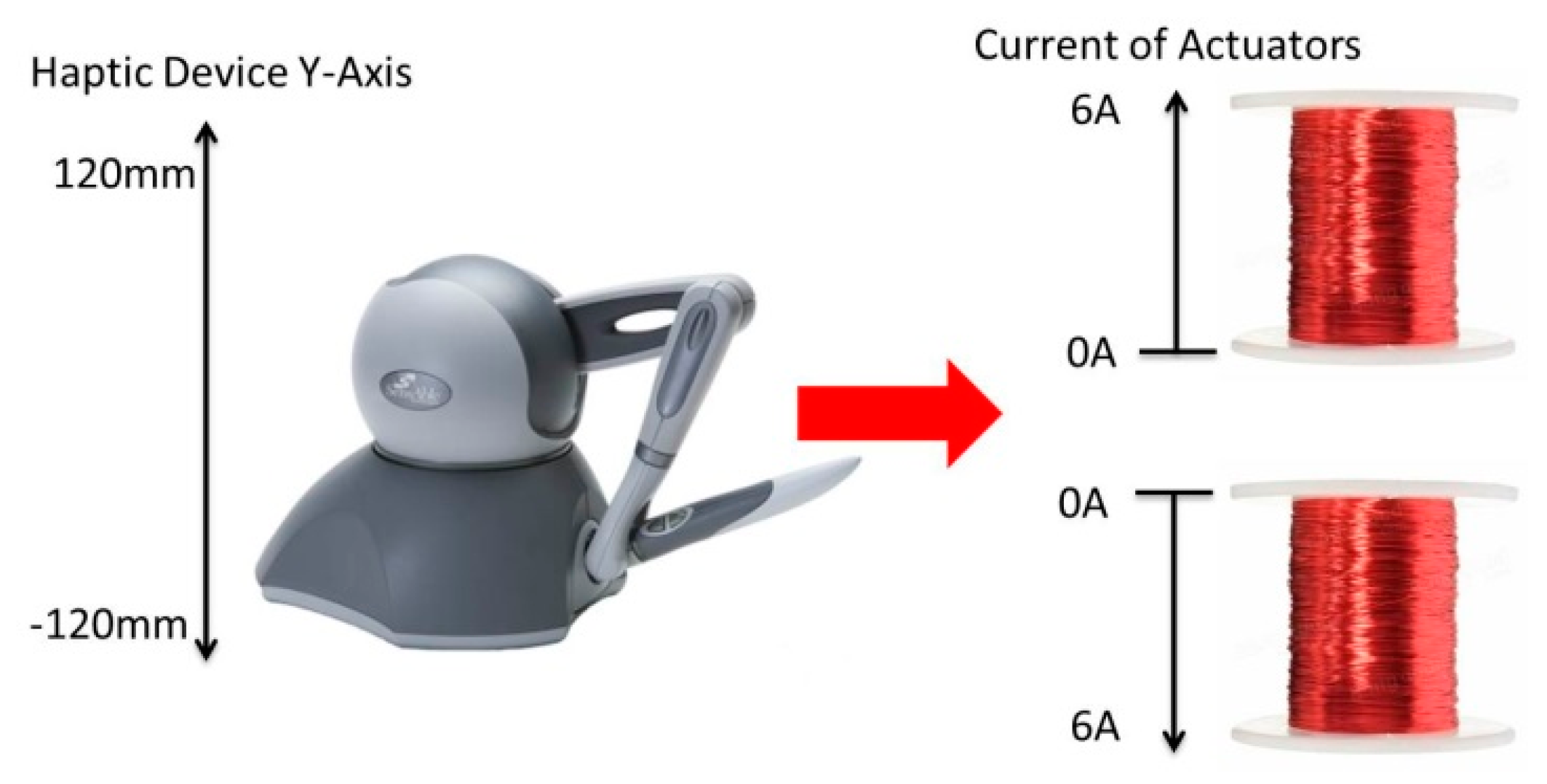
2.3.3. Mapping Framework
2.3.4. Forbidden Region Virtual Fixtures inside Blood Vessels
2.3.5. Haptic Rendering for Multi Particles in a Multi-Branch Blood Vessel
2.4. User Studies for the Virtual Tele-Nano-Manipulation
3. Results
4. Discussion
5. Conclusions and Future Works
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Bar, J.; Herbst, R.S.; Onn, A. Targeted drug delivery strategies to treat lung metastasis. Expert Opin. Drug Deliv. 2009, 6, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Ferreira, A. Guidelines for the design of magnetic nanorobots to cross the blood–brain barrier. IEEE Trans. Robot. 2014, 30, 81–92. [Google Scholar] [CrossRef]
- Nacev, A.; Beni, C.; Bruno, O.; Shapiro, B. The behaviors of ferromagnetic nano-particles in and around blood vessels under applied magnetic fields. J. Magn. Magn. Mater. 2011, 323, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.; Kulkarni, S.; Nacev, A.; Muro, S.; Stepanov, P.Y.; Weinberg, I.N. Open challenges in magnetic drug targeting. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.-B.; Martel, S. Magnetic microparticle steering within the constraints of an mri system: Proof of concept of a novel targeting approach. Biomed. Microdevices 2007, 9, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Martel, S.; Mathieu, J.-B.; Felfoul, O.; Chanu, A.; Aboussouan, E.; Tamaz, S.; Pouponneau, P.; Yahia, L.H.; Beaudoin, G.; Soulez, G. A computer-assisted protocol for endovascular target interventions using a clinical mri system for controlling untethered microdevices and future nanorobots. Comput. Aided Surg. 2008, 13, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.S.; Ferreira, P.; Eleutério, R.; de Korte, C.L.; Misra, S. Magnetic-based closed-loop control of paramagnetic microparticles using ultrasound feedback. In Proceedings of the 2014 IEEE International Conference on Robotics and Automation (ICRA), Hong Kong, China, 31 May–7 June 2014; pp. 3807–3812. [Google Scholar]
- Zhang, X.; Le, T.-A.; Yoon, J. Development of a magnetic nanoparticles guidance system for interleaved actuation and mpi-based monitoring. In Proceedings of the 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Daejeon, Korea, 9–14 October 2016; pp. 5279–5284. [Google Scholar]
- Zhang, X.; Le, T.-A.; Yoon, J. Development of a real time imaging-based guidance system of magnetic nanoparticles for targeted drug delivery. J. Magn. Magn. Mater. 2017, 427, 345–351. [Google Scholar] [CrossRef]
- Le, T.-A.; Zhang, X.; Hoshiar, A.K.; Yoon, J. Real-time two-dimensional magnetic particle imaging for electromagnetic navigation in targeted drug delivery. Sensors 2017, 17, 2050. [Google Scholar] [CrossRef] [PubMed]
- Yesin, K.B.; Vollmers, K.; Nelson, B.J. Modeling and control of untethered biomicrorobots in a fluidic environment using electromagnetic fields. Int. J. Robot. Res. 2006, 25, 527–536. [Google Scholar] [CrossRef]
- Kummer, M.P.; Abbott, J.J.; Kratochvil, B.E.; Borer, R.; Sengul, A.; Nelson, B.J. Octomag: An electromagnetic system for 5-dof wireless micromanipulation. IEEE Trans. Robot. 2010, 26, 1006–1017. [Google Scholar] [CrossRef]
- Komaee, A.; Shapiro, B. Steering a ferromagnetic particle by optimal magnetic feedback control. IEEE Trans. Control Syst. Technol. 2012, 20, 1011–1024. [Google Scholar] [CrossRef]
- Nacev, A.; Komaee, A.; Sarwar, A.; Probst, R.; Kim, S.H.; Emmert-Buck, M.; Shapiro, B. Towards control of magnetic fluids in patients: Directing therapeutic nanoparticles to disease locations. IEEE Control Syst. 2012, 32, 32–74. [Google Scholar] [CrossRef]
- Komaee, A. Feedback control for transportation of magnetic fluids with minimal dispersion: A first step toward targeted magnetic drug delivery. IEEE Trans. Control Syst. Technol. 2017, 25, 129–144. [Google Scholar] [CrossRef]
- Pacchierotti, C.; Magdanz, V.; Medina-Sánchez, M.; Schmidt, O.G.; Prattichizzo, D.; Misra, S. Intuitive control of self-propelled microjets with haptic feedback. J. Micro-Bio Robot. 2015, 10, 37–53. [Google Scholar] [CrossRef]
- Van West, E.; Yamamoto, A.; Higuchi, T. The concept of “haptic tweezer”, a non-contact object handling system using levitation techniques and haptics. Mechatronics 2007, 17, 345–356. [Google Scholar] [CrossRef]
- Pacoret, C.; Régnier, S. Invited article: A review of haptic optical tweezers for an interactive microworld exploration. Rev. Sci. Instrum. 2013, 84, 081301. [Google Scholar] [CrossRef] [PubMed]
- Basdogan, C.; Kiraz, A.; Bukusoglu, I.; Varol, A.; Doğanay, S. Haptic guidance for improved task performance in steering microparticles with optical tweezers. Opt. Express 2007, 15, 11616–11621. [Google Scholar] [CrossRef] [PubMed]
- Mehrtash, M.; Tsuda, N.; Khamesee, M.B. Bilateral macro–micro teleoperation using magnetic levitation. IEEE/ASME Trans. Mechatron. 2011, 16, 459–469. [Google Scholar] [CrossRef]
- Pacchierotti, C.; Scheggi, S.; Prattichizzo, D.; Misra, S. Haptic feedback for microrobotics applications: A review. Front. Robot. AI 2016, 3, 53. [Google Scholar] [CrossRef]
- Bowyer, S.A.; Davies, B.L.; y Baena, F.R. Active constraints/virtual fixtures: A survey. IEEE Trans. Robot. 2014, 30, 138–157. [Google Scholar] [CrossRef]
- Abbott, J.J.; Okamura, A.M. Stable forbidden-region virtual fixtures for bilateral telemanipulation. J. Dyn. Syst. Meas. Control 2006, 128, 53–64. [Google Scholar] [CrossRef]
- Okamura, A.M. Methods for haptic feedback in teleoperated robot-assisted surgery. Ind. Robot Int. J. 2004, 31, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Ammi, M.; Ferreira, A. Robotic assisted micromanipulation system using virtual fixtures and metaphors. In Proceedings of the 2007 IEEE International Conference on Robotics and Automation, Roma, Italy, 10–14 April 2007; pp. 454–460. [Google Scholar]
- Bolopion, A.; Xie, H.; Haliyo, D.S.; Régnier, S. Haptic teleoperation for 3-D microassembly of spherical objects. IEEE/ASME Trans. Mechatron. 2012, 17, 116–127. [Google Scholar] [CrossRef]
- Ghanbari, A.; Horan, B.; Nahavandi, S.; Chen, X.; Wang, W. Haptic microrobotic cell injection system. IEEE Syst. J. 2014, 8, 371–383. [Google Scholar] [CrossRef]
- Nothnagel, N.; Rahmer, J.; Gleich, B.; Halkola, A.; Buzug, T.M.; Borgert, J. Steering of magnetic devices with a magnetic particle imaging system. IEEE Trans. Biomed. Eng. 2016, 63, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zheng, J.-J.; Zhou, H.-J.; Zhang, B.-K. A new constraint-based virtual environment for haptic assembly training. Adv. Eng. Softw. 2016, 98, 58–68. [Google Scholar] [CrossRef]
- House, D.H.; Keyser, J.C. Foundations of Physically Based Modeling and Animation; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Do, T.D.; Noh, Y.; Kim, M.O.; Yoon, J. An optimized field function scheme for nanoparticle guidance in magnetic drug targeting systems. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–2 Octobet 2015; pp. 4388–4393. [Google Scholar]
- Tehrani, M.D.; Kim, M.O.; Yoon, J. A novel electromagnetic actuation system for magnetic nanoparticle guidance in blood vessels. IEEE Trans. Magn. 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Hoshiar, A.K.; Le, T.-A.; Amin, F.U.; Kim, M.O.; Yoon, J. Studies of aggregated nanoparticles steering during magnetic-guided drug delivery in the blood vessels. J. Magn. Magn. Mater. 2017, 427, 181–187. [Google Scholar] [CrossRef]
- Do, T.D.; Noh, Y.; Kim, M.O.; Yoon, J. In silico magnetic nanocontainers navigation in blood vessels: A feedback control approach. J. Nanosci. Nanotechnol. 2016, 16, 6368–6373. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Hoshiar, A.K.; Do, T.D.; Noh, Y.; Shah, S.A.; Khan, M.S.; Yoon, J.; Kim, M.O. Osmotin-loaded magnetic nanoparticles with electromagnetic guidance for the treatment of alzheimer’s disease. Nanoscale 2017, 9, 10619–10632. [Google Scholar] [CrossRef] [PubMed]
- Pranami, G. Understanding Nanoparticle Aggregation. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2009. [Google Scholar]
- Lee, J.; Moon, S.; Lim, J.; Gwak, M.-J.; Kim, J.G.; Chung, E.; Lee, J.-H. Imaging of the finger vein and blood flow for anti-spoofing authentication using a laser and a mems scanner. Sensors 2017, 17, 925. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.-H.; Ahmad, A.C.; Nizam, M.; Abdullah, B. Magnetic resonance imaging: Health effects and safety. In Proceedings of the International Conference on Non-Ionizing Radiation at UNITEN (ICNIR2003) Electromagnetic Fields and Our Health, Selangor, Malaysia, 20–22 October 2003. [Google Scholar]
- Hartwig, V.; Giovannetti, G.; Vanello, N.; Lombardi, M.; Landini, L.; Simi, S. Biological effects and safety in magnetic resonance imaging: A review. Int. J. Environ. Res. Public Health 2009, 6, 1778–1798. [Google Scholar] [CrossRef] [PubMed]














| Parameter | Value | Unit |
|---|---|---|
| MNP Diameter | 400–1000 | nm |
| MNP Density | 7200 | kg/ |
| Blood Density | 1050 | kg/ |
| Blood Viscosity | 0.004 | Pa·s |
| 570.7 |
| Current (A) | B (mT) | ∇B (T/m) |
|---|---|---|
| 1 | 28.2 | 0.43 |
| 2 | 54.1 | 0.92 |
| 3 | 79.8 | 1.39 |
| 4 | 106.3 | 1.90 |
| 5 | 132.6 | 2.40 |
| 6 | 160.7 | 2.8 |
| Analysis Parameter | Factor | F | p-Value |
|---|---|---|---|
| Success Rate | Haptic Cues | (2, 22) = 59.529 | <0.001 |
| Blood Velocity | (2.120, 23.321) = 20.240 | <0.001 | |
| Interaction | (8, 88) = 2.887 | 0.007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdipoor, V.; Afzal, M.R.; Le, T.-A.; Yoon, J. Haptic-Based Manipulation Scheme of Magnetic Nanoparticles in a Multi-Branch Blood Vessel for Targeted Drug Delivery. Micromachines 2018, 9, 14. https://doi.org/10.3390/mi9010014
Hamdipoor V, Afzal MR, Le T-A, Yoon J. Haptic-Based Manipulation Scheme of Magnetic Nanoparticles in a Multi-Branch Blood Vessel for Targeted Drug Delivery. Micromachines. 2018; 9(1):14. https://doi.org/10.3390/mi9010014
Chicago/Turabian StyleHamdipoor, Vahid, Muhammad Raheel Afzal, Tuan-Anh Le, and Jungwon Yoon. 2018. "Haptic-Based Manipulation Scheme of Magnetic Nanoparticles in a Multi-Branch Blood Vessel for Targeted Drug Delivery" Micromachines 9, no. 1: 14. https://doi.org/10.3390/mi9010014






