Design and Near-Infrared Actuation of a Gold Nanorod–Polymer Microelectromechanical Device for On-Demand Drug Delivery
Abstract
:1. Introduction
2. Material and Methods
2.1. Design
2.2. Fabrication
2.2.1. Poly Dimethyl Siloxane (PDMS) Reservoir
2.2.2. Au-PDMS Membrane
2.2.3. Thin Bilayer Membrane
2.2.4. Reservoir Loading with Drug
2.2.5. Laser Drilling
2.2.6. Reservoir Filling for Drug-Release Measurements
2.2.7. In Vitro Drug-Release Studies
2.2.8. Thermal Data Collection
2.2.9. Deflection Measurements
3. Results
3.1. Characterization
3.1.1. Overall Device Characteristics
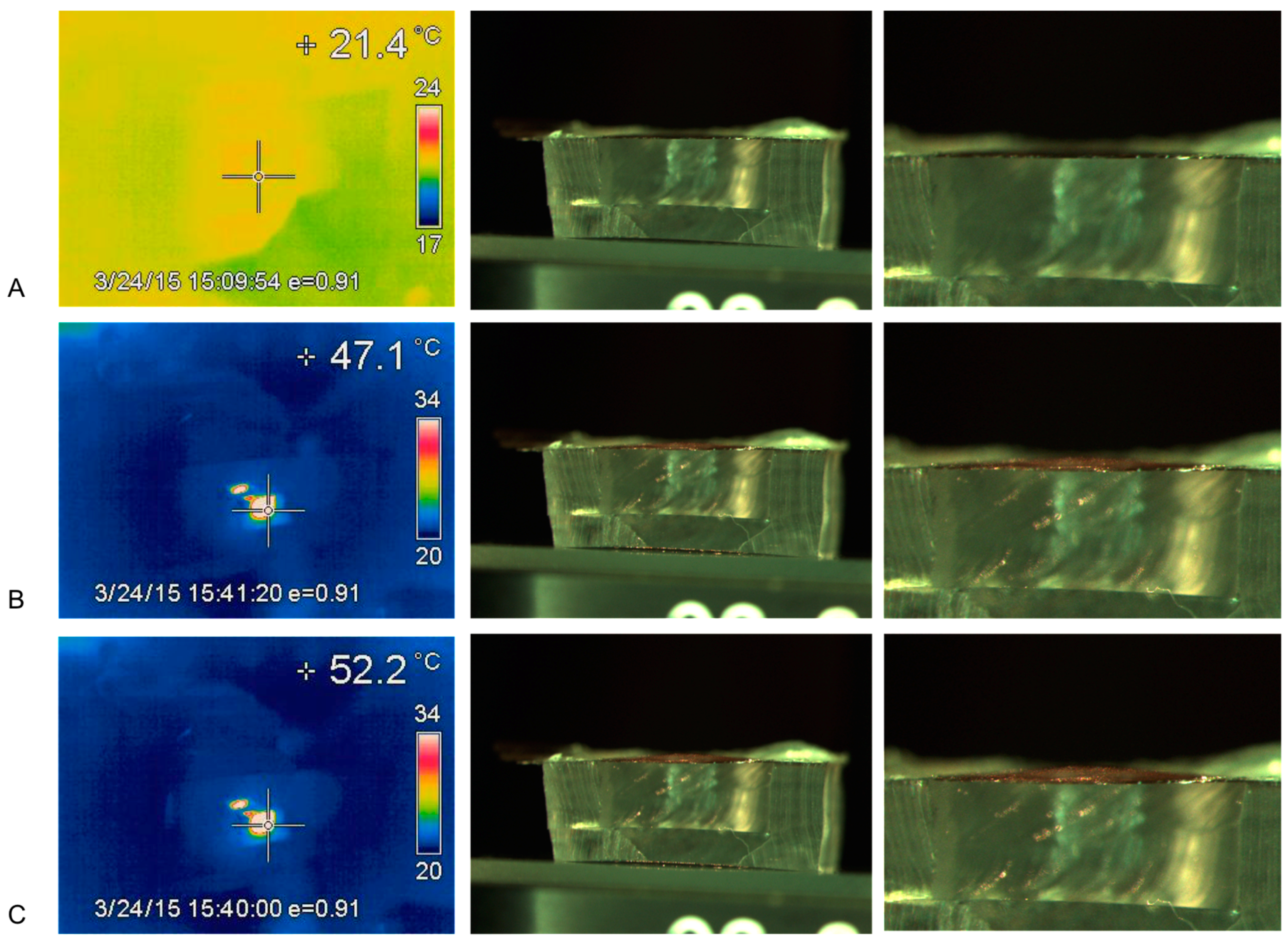
3.1.2. Thermal-Heating Measurements
3.1.3. Temperature vs. Membrane Deflection
3.2. Docetaxel (DTX) Drug Release
In Vitro Drug-Release Study
4. Discussion
Author Contributions
Conflicts of Interest
References
- Kiryukhin, M.V. Active drug release systems: Current status, applications and perspectives. Curr. Opin. Pharmacol. 2014, 18, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release 2014, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; Florence, A.T. Towards more effective advanced drug delivery systems. Int. J. Pharm. 2013, 454, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.K.; Gleave, M.E.; Yago, V.; Beraldi, E.; Hunter, W.L.; Burt, H.M. The suppression of human prostate tumor growth in mice by the intratumoral injection of a slow-release polymeric paste formulation of paclitaxel. Cancer Res. 2000, 60, 4146–4151. [Google Scholar] [PubMed]
- Owen, G.R.; Jackson, J.K.; Chehroudi, B.; Brunette, D.M.; Burt, H.M. An in vitro study of plasticized poly(lactic-co-glycolic acid) films as possible guided tissue regeneration membranes: Material properties and drug release kinetics. J. Biomed. Mater. Res. A 2010, 95, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Liggins, R.T.; Burt, H.M. Paclitaxel loaded poly(l-lactic acid) microspheres: Properties of microspheres made with low molecular weight polymers. Int. J. Pharm. 2001, 222, 19–33. [Google Scholar] [CrossRef]
- LaVan, D.A.; McGuire, T.; Langer, R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 2003, 21, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.L.; Desai, T.A. Microfabricated drug delivery systems: From particles to pores. Adv. Drug Deliv. Rev. 2003, 55, 315–328. [Google Scholar] [CrossRef]
- Staples, M.; Daniel, K.; Cima, M.J.; Langer, R. Application of micro- and nano-electromechanical devices to drug delivery. Pharm. Res. 2006, 23, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Shawgo, R.S.; Grayson, A.C.R.; Li, Y.; Cima, M.J. BioMEMS for drug delivery. Curr. Opin. Solid State Mater. Sci. 2002, 6, 329–334. [Google Scholar] [CrossRef]
- Nuxoll, E. BioMEMS in drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jackson, J.K.; Chiao, M. Microfabricated drug delivery devices: Design, fabrication and applications. Adv. Funct. Mater. 2017, 27, 1703606. [Google Scholar] [CrossRef]
- Pirmoradi, F.N.; Jackson, J.K.; Burt, H.M.; Chiao, M. A magnetically controlled MEMS device for drug delivery: Design, fabrication, and testing. Lap Chip 2011, 11, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Shademani, A.; Zhang, H.; Jackson, J.K.; Chiao, M. Active regulation of on-demand drug delivery by magnetically triggerable microspouters. Adv. Funct. Mater. 2017, 27, 1604558. [Google Scholar] [CrossRef]
- Zachkani, P.; Jackson, J.K.; Pirmoradi, F.N.; Chiao, M. A cylindrical magnetically-actuated drug delivery device proposed for minimally invasive treatment of prostate cancer. RSC Adv. 2015, 5, 98087–98096. [Google Scholar] [CrossRef]
- Pirmoradi, F.N.; Jackson, J.K.; Burt, H.M.; Chiao, M. On-demand controlled release of docetaxel from a battery-less MEMS drug delivery device. Lap Chip 2011, 11, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Ziaie, B.; Baldi, A.; Lei, M.; Gu, Y.; Siegel, R.A. Hard and soft micromachining for BioMEMS: Review of techniques and examples of applications in microfluidics and drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Strong, L.E.; West, J.F. Thermally responsive polymer-nanoparticle composites for biomedical applications. WIREs Nanomed. Nanobiotechnol. 2011, 3, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Muddineti, O.S.; Ghosh, B.; Biswas, S. Current trends in using polymer coated gold nanoparticles for cancer therapy. Int. J. Pharm. 2015, 484, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, M.; Shapiro, B.; Smela, E. Characterization and modeling of PPy bilayer microactuators: Part 1. Curvature. Sens. Actuators B 2006, 115, 596–609. [Google Scholar] [CrossRef]
- Berry, K.R., Jr.; Russell, A.G.; Blake, P.A.; Roper, D.K. Gold nanoparticles reduced in situ and dispersed in polymer thin films: Optical and thermal properties. Nanotechnology 2012, 23, 375703. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, J.; Bi, Y.; Xu, X.; Zhou, H.; Gao, J.; Hu, Y.; Zhao, Y.; Chai, Z. Near-infrared light remote-controlled intracellular anti-cancer drug delivery using thermo/pH sensitive nanovehicle. Acta Biomater. 2015, 17, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.; Zink, J.I. Nanovalve-controlled cargo release activated by plasmonic heating. J. Am. Chem. Soc. 2012, 134, 7628–7631. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, L.L.; Peng, J.; Li, Q.-L.; Sun, Y.-L.; Zhang, J.; Ning, Y.-Q.; Yu, J.; Yang, Y.-W. Near-Infrared light-responsive supramolecular nanovalve based on mesoporous silica-coated gold nanorods. Chem. Soc. 2014, 5, 2804–2808. [Google Scholar] [CrossRef]








| Sample | Average Release Rate during Actuation (ng/min) | Average Release Rate during Diffusion (ng/min) |
|---|---|---|
| Sample A | 115.9 ± 23.3 | 5.65 ± 3.80 |
| Sample B | 46.34 ± 26.2 | 2.93 ± 1.95 |
| Sample C | 69.22 ± 22.2 | 2.20 ± 1.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, J.; Chen, A.; Zhang, H.; Burt, H.; Chiao, M. Design and Near-Infrared Actuation of a Gold Nanorod–Polymer Microelectromechanical Device for On-Demand Drug Delivery. Micromachines 2018, 9, 28. https://doi.org/10.3390/mi9010028
Jackson J, Chen A, Zhang H, Burt H, Chiao M. Design and Near-Infrared Actuation of a Gold Nanorod–Polymer Microelectromechanical Device for On-Demand Drug Delivery. Micromachines. 2018; 9(1):28. https://doi.org/10.3390/mi9010028
Chicago/Turabian StyleJackson, John, Aurora Chen, Hongbin Zhang, Helen Burt, and Mu Chiao. 2018. "Design and Near-Infrared Actuation of a Gold Nanorod–Polymer Microelectromechanical Device for On-Demand Drug Delivery" Micromachines 9, no. 1: 28. https://doi.org/10.3390/mi9010028




