A Review of Fast Bubble-Driven Micromotors Powered by Biocompatible Fuel: Low-Concentration Fuel, Bioactive Fluid and Enzyme
Abstract
:1. Introduction
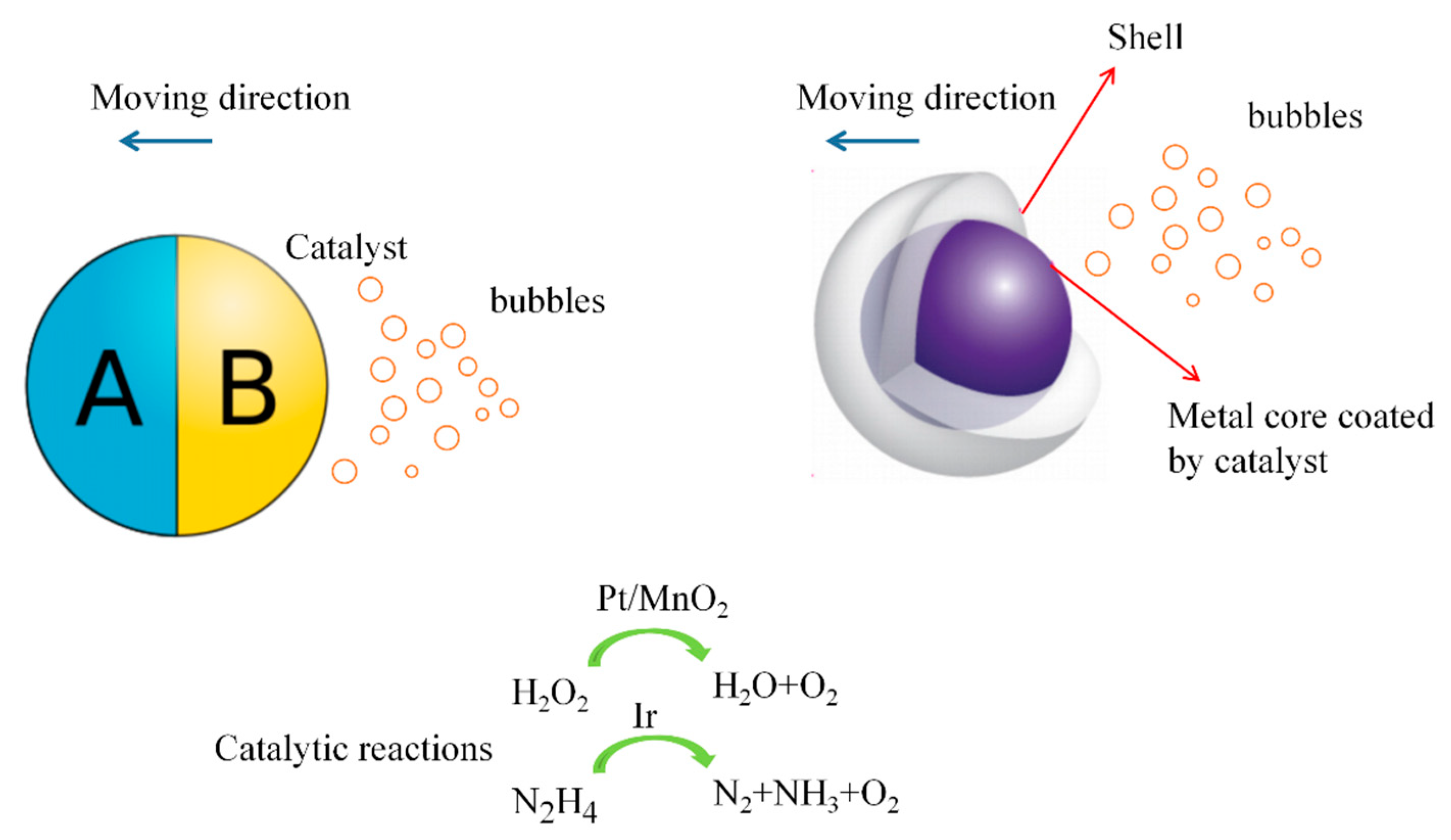
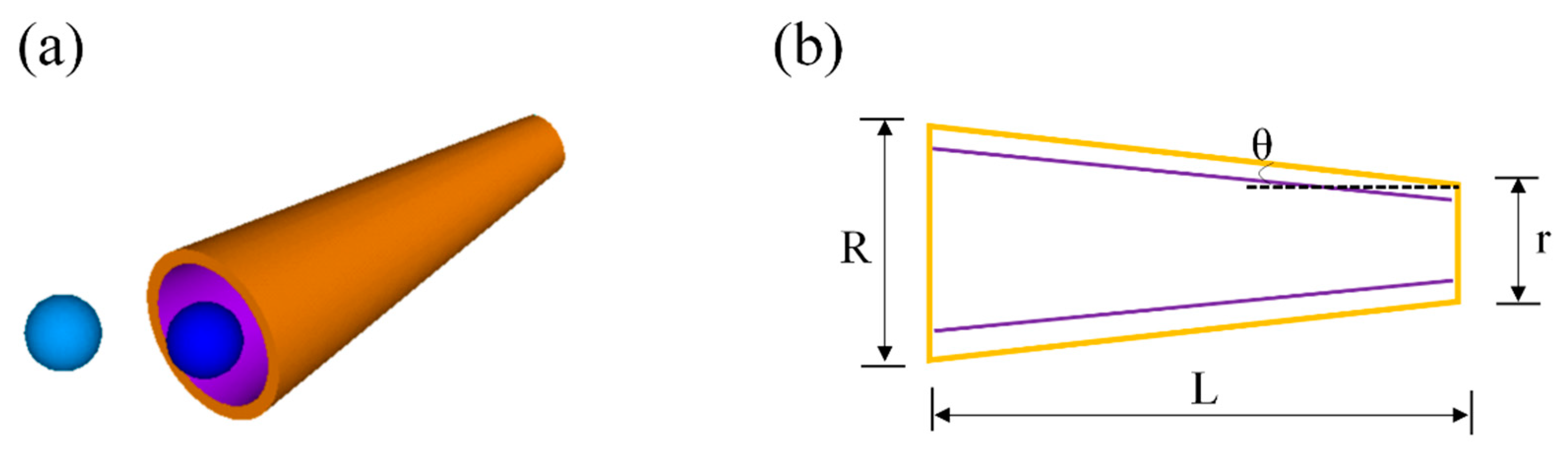
2. Low Concentration of Peroxide
2.1. Janus Micromotors
2.2. Tubular Micromotors
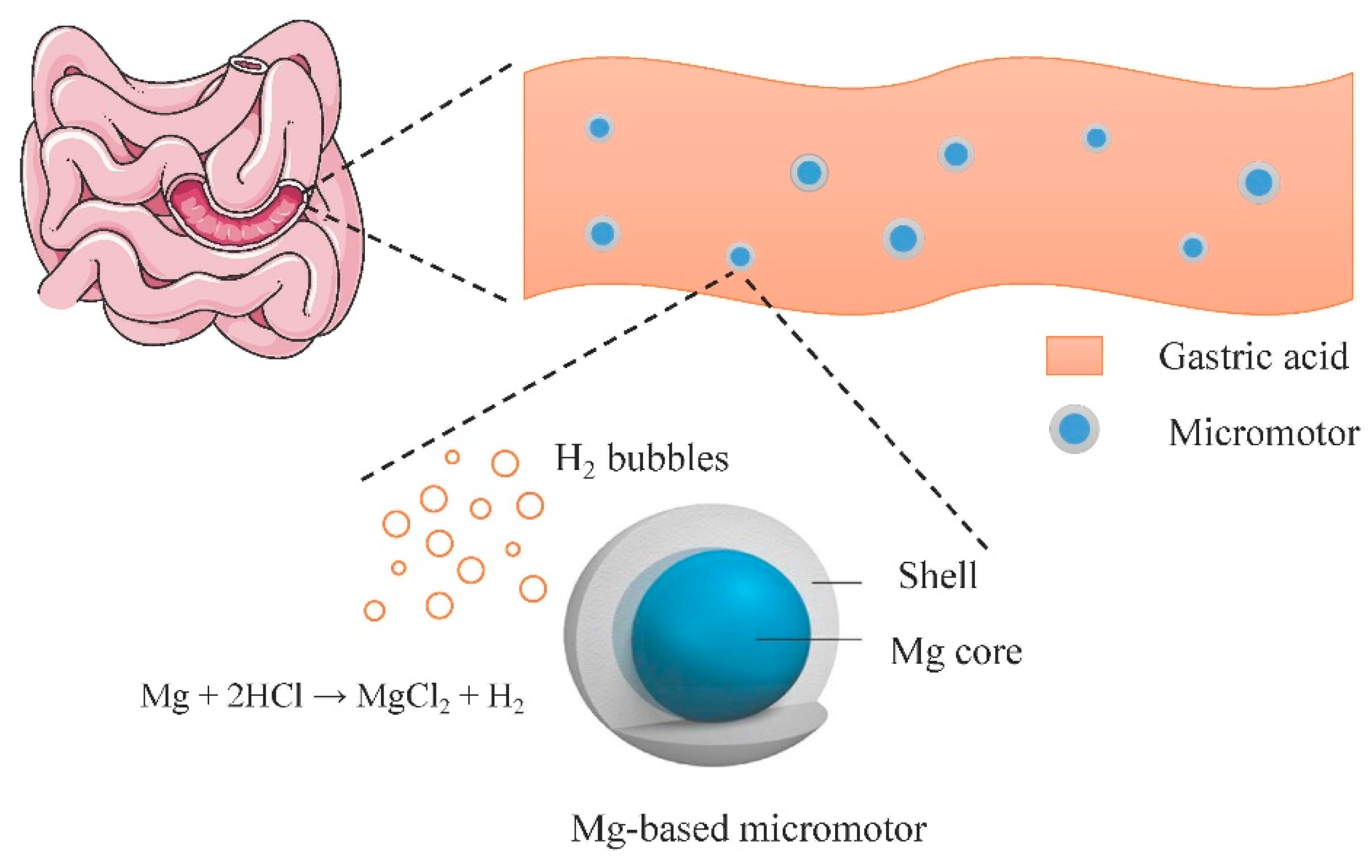
3. Bioactive Fluid as Fuel
3.1. Water
3.2. Native Acid
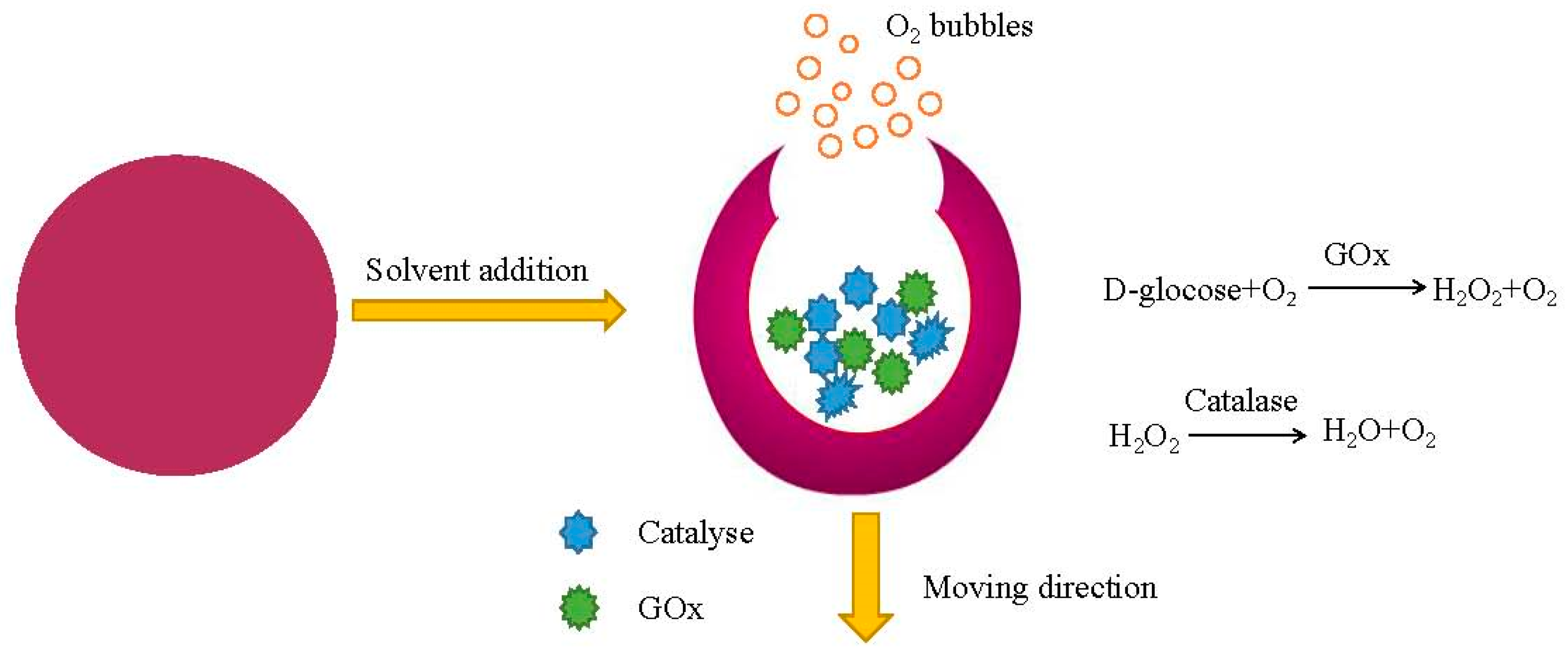
4. Enzyme-Driven Micromotors
4.1. Enzyme as a Fuel Source
4.2. Enzyme-Powered Micromotors
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Yu, X.; Xu, M.; Liu, W.; Sandraz, E.; Lan, H.; Wang, J.; Cohen, S.M. Metal-organic frameworks as micromotors with tunable engines and brakes. J. Am. Chem. Soc. 2017, 139, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mou, F.; Gong, H.; Luo, M.; Guan, J. Light-driven micro/nanomotors: From fundamentals to applications. Chem. Soc. Rev. 2017, 46, 6905–6926. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Uygun, A.; Wang, J. Hydrogen-bubble-propelled zinc-based microrockets in strongly acidic media. J. Am. Chem. Soc. 2012, 134, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Kagan, D.; Hu, C.M.; Campuzano, S.; Lobo-Castaãnon, M.J.; Lim, N.; Kang, D.Y.; Zimmerman, M.; Zhang, L.; Wang, J. Micromachine-enabled capture and isolation of cancer cells in complex media. Angew. Chemie 2011, 123, 4247–4250. [Google Scholar] [CrossRef]
- Yoshizumi, Y.; Okubo, K.; Yokokawa, M.; Suzuki, H. Programmed transport and release of cells by self-propelled micromotors. Langmuir 2016, 32, 9381–9388. [Google Scholar] [CrossRef] [PubMed]
- Estebanfernández, d.Á.B.; Angell, C.; Soto, F.; Lopezramirez, M.A.; Báez, D.F.; Xie, S.; Wang, J.; Chen, Y. Acoustically propelled nanomotors for intracellular siRNA delivery. ACS Nano 2016, 10, 4997–5005. [Google Scholar] [CrossRef] [PubMed]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; De, L.D.; Zhong, X.Y.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; Lafleur, M. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 2016, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Fei, P.; André, A.A.M.; Yongjun, M.; Mangala, S.; Daniela, A.W. Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano 2017, 11, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Feng, Y.; Wang, T.; Guan, J. Micro-/nanorobots at work in active drug delivery. Adv. Funct. Mater. 2018, 28, 1706100. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Guix, M.; Schmidt, O.G. Wastewater mediated activation of micromotors for efficient water cleaning. Nano Lett. 2015, 16, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dong, R.; Wu, Y.; Gao, W.; He, Z.; Ren, B. Light-driven Au-WO3@C Janus micromotors for rapid photodegradation of dye pollutants. ACS Appl. Mater. Interfaces 2017, 9, 4674–4683. [Google Scholar] [CrossRef] [PubMed]
- Ávila, E.F.D.; Angsantikul, P.; Li, J.; Lopezramirez, M.A.; Ramírezherrera, D.E.; Thamphiwatana, S.; Chen, C.; Delezuk, J.; Samakapiruk, R.; Ramez, V. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 2017, 8, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.; Abdelghani, M.; Shen, G.; Cao, S.; Williams, D.S.; van Hest, J.C.M. Erythrocyte membrane modified Janus polymeric motors for thrombus therapy. ACS Nano 2018, 12, 4877–4885. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hernandez, R.M.; Bartlett, D.J.; Bingham, J.M.; Kline, T.R.; Ayusman, S.; Mallouk, T.E. Bipolar electrochemical mechanism for the propulsion of catalytic nanomotors in hydrogen peroxide solutions. Langmuir 2006, 22, 10451–10456. [Google Scholar]
- Gao, W.; D’Agostino, M.; Garcia-Gradilla, V.; Orozco, J.; Wang, J. Multi-fuel driven Janus micromotors. Small 2013, 9, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sattayasamitsathit, S.; Wang, J. Catalytically propelled micro-/nanomotors: How fast can they move? Chem. Rec. 2012, 12, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Guix, M.; Weiz, S.M.; Schmidt, O.G.; Medina-Sánchez, M. Self-propelled micro/nanoparticle motors. Part. Part. Syst. Charact. 2018, 35, 1700382. [Google Scholar] [CrossRef]
- Yang, F.; Manjare, M.; Zhao, Y.; Qiao, R. On the peculiar bubble formation, growth, and collapse behaviors in catalytic micro-motor systems. Microfluid. Nanofluid. 2017, 21, 6. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Yao, D.; Chen, Y.; Deng, Y.; Wang, C. Transportation and release of Janus micromotors by two-stage rocket hydrogel. J. Mater. Chem. A 2017, 5, 18442–18447. [Google Scholar] [CrossRef]
- Sanchez, S.; Ananth, A.N.; Fomin, V.M.; Viehrig, M.; Schmidt, O.G. Superfast motion of catalytic microjet engines at physiological temperature. J. Am. Chem. Soc. 2011, 133, 14860–14863. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, G.; Ye, M.; Li, M.; Liu, R.; Mei, Y. Dynamics of catalytic tubular microjet engines: Dependence on geometry and chemical environment. Nanoscale 2011, 3, 5083–5089. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.G.; Zhao, Y.P. Autonomously motile catalytic nanomotors by bubble propulsion. Appl. Phys. Lett. 2009, 94, 163104. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, G.; Pumera, M. Crucial role of surfactants in bubble-propelled microengines. J. Phys. Chem. C 2014, 118, 5268–5274. [Google Scholar] [CrossRef]
- Karshalev, E.; Ávila, B.E.d.; Wang, J. Micromotors for “chemistry-on-the-fly”. J. Am. Chem. Soc. 2018, 140, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Sánchez, B.; Pacheco, M.; Maria-Hormigos, R.; Escarpa, A. Perspectives on Janus micromotors: Materials and applications. Appl. Mater. Today 2017, 9, 407–418. [Google Scholar]
- Uygun, D.A.; Jurado-Sanchez, B.; Uygun, M.; Wang, J. Self-propelled chelation platforms for efficient removal of toxic metals. Environ. Sci. Nano 2016, 3, 559–566. [Google Scholar] [CrossRef]
- Hayakawa, M.; Onoe, H.; Nagai, K.; Takinoue, M. Influence of asymmetry and driving forces on the propulsion of bubble-propelled catalytic micromotors. Micromachines 2016, 7, 229. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Wang, J.; Escarpa, A. Ultrafast nanocrystals decorated micromotors for on-site dynamic chemical processes. ACS Appl. Mater. Interfaces 2016, 8, 19618–19625. [Google Scholar] [CrossRef] [PubMed]
- Claussen, J.C.; Daniele, M.A.; Geder, J.; Pruessner, M.; Mäkinen, A.J.; Melde, B.J.; Twigg, M.; Verbarg, J.M.; Medintz, I.L. Platinum-paper micromotors: An urchin-like nanohybrid catalyst for green monopropellant bubble-thrusters. ACS Appl. Mater. Interfaces 2014, 6, 17837–17847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Pumera, M. Geometric asymmetry driven janus micromotors. Nanoscale 2014, 6, 11177–11180. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, S.; Nakai, Y.; Akiyama, M.; Komatsu, T. Self-propelled soft protein microtubes with a Pt nanoparticle interior surface. Chem.—A Eur. J. 2017, 23, 5044–5050. [Google Scholar] [CrossRef] [PubMed]
- Manjare, M.; Yang, B.; Zhao, Y.P. Bubble-propelled microjets: Model and experiment. J. Phys. Chem. C 2013, 117, 4657–4665. [Google Scholar] [CrossRef]
- Manjare, M.; Yang, B.; Zhao, Y.P. Bubble driven quasioscillatory translational motion of catalytic micromotors. Phys. Rev. Lett. 2012, 109, 128305. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Kong, L.; Chen, C.; Chen, Z.; Xu, L.; Guan, J. Light-controlled propulsion, aggregation and separation of water-fuelled TiO2/Pt Janus submicromotors and their “on-the-fly” photocatalytic activities. Nanoscale 2016, 8, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Moo, J.G.S.; Wang, H.; Pumera, M. Influence of pH on the motion of catalytic Janus particles and tubular bubble-propelled micromotors. Chem.—A Eur. J. 2016, 22, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Angsantikul, P.; Liu, W.; Thamphiwatana, S.; Xu, M.; Sandraz, E.; Wang, X.; Delezuk, J.; Gao, W. Micromotors spontaneously neutralize gastric acid for pH-responsive payload release. Angew. Chem. Int. Ed. Engl. 2017, 56, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Thamphiwatana, S.; Liu, W.; Ávila, E.F.D.; Angsantikul, P.; Sandraz, E.; Wang, J.; Xu, T.; Soto, F.; Ramez, V. Enteric micromotor can selectively position and spontaneously propel in the gastrointestinal tract. ACS Nano 2016, 10, 9536–9542. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Teora, S.P.; Hu, G.X.; Nijemeisland, M.; Wilson, D.A. High-throughput design of biocompatible enzyme-based hydrogel microparticles with autonomous movement. Angew. Chem. Int. Edit. 2018, 57, 9814–9817. [Google Scholar] [CrossRef] [PubMed]
- Solovev, A.A.; Sanchez, S.; Pumera, M.; Mei, Y.F.; Schmidt, O.G. Nanomotors: Magnetic control of tubular catalytic microbots for the transport, assembly, and delivery of micro-objects. Adv. Funct. Mater. 2010, 20, 2430–2435. [Google Scholar] [CrossRef]
- Sarkis, B.; Folio, D.; Ferreira, A. Catalytic Tubular Microjet Propulsion Model for Endovascular Navigation. In Proceedings of the IEEE International Conference on Robotics and Automation, Seattle, WA, USA, 26–30 May 2015; pp. 3537–3542. [Google Scholar]
- Yamamoto, D.; Shioi, A. Self-propelled nano/micromotors with a chemical reaction: Underlying physics and strategies of motion control. Powder Part. 2015, 32, 2–22. [Google Scholar] [CrossRef]
- Zha, F.; Wang, T.; Luo, M.; Guan, J. Tubular micro/nanomotors: Propulsion mechanisms, fabrication techniques and applications. Micromachines 2018, 9, 78. [Google Scholar] [CrossRef]
- Guix, M.; Mayorgamartinez, C.C.; Merkoçi, A. Nano/micromotors in (bio)chemical science applications. Chem. Rev. 2014, 114, 6285–6322. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.V.; Wang, J. Nano/micromotors for security/defense applications. A review. Nanoscale 2015, 7, 19377–19389. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, T.; Chi, Q.; Wang, Z.; Xu, S.; Liu, Q.; Wang, Q.; Liu, L.; Bai, T.; Chi, Q. How to make a fast, efficient bubble-driven micromotor: A mechanical view. Micromachines 2017, 8, 267. [Google Scholar] [CrossRef]
- Wang, Z.; Chi, Q.; Liu, L.; Liu, Q.; Bai, T.; Wang, Q. A viscosity-based model for bubble-propelled catalytic micromotors. Micromachines 2017, 8, 198. [Google Scholar] [CrossRef]
- Li, J.; Gao, W.; Zhang, L.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef]
- Orozco, J.; Mercante, L.A.; Pol, R.; Merkoçi, A. Graphene-based Janus micromotors for the dynamic removal of pollutants. J. Mater. Chem. A 2016, 4, 3371–3378. [Google Scholar] [CrossRef] [Green Version]
- Ebbens, S.; Tu, M.H.; Howse, J.R.; Golestanian, R. Size dependence of the propulsion velocity for catalytic Janus-sphere swimmers. Phys. Rev. E 2012, 85, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Ebbens, S.; Gregory, D.A.; Dunderdale, G.; Howse, J.R.; Ibrahim, Y.; Liverpool, T.B.; Golestanian, R. Electrokinetic effects in catalytic platinum-insulator Janus swimmers. Epl-Europhys. Lett. 2014, 106, 58003. [Google Scholar] [CrossRef] [Green Version]
- Howse, J.R.; Jones, R.A.L.; Ryan, A.J.; Gough, T.; Vafabakhsh, R.; Golestanian, R. Self-motile colloidal particles: From directed propulsion to random walk. Phys. Rev. Lett. 2007, 99, 048102. [Google Scholar] [CrossRef] [PubMed]
- Guix, M.; Meyer, A.K.; Koch, B.; Schmidt, O.G. Carbonate-based Janus micromotors moving in ultra-light acidic environment generated by HeLa cells in situ. Sci. Rep. 2016, 6, 21701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; In, M.; Blanc, C.; Nobili, M.; Stocco, A. Enhanced active motion of Janus colloids at the water surface. Soft Matter 2015, 11, 7376–7384. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.-M.Y.; MacKintosh, F.C.; Dobrynin, A.V. Nonlinear elasticity: From single chain to networks and gels. Macromolecules 2013, 46, 3679–3692. [Google Scholar] [CrossRef]
- Jurado-Sanchez, B.; Sattayasamitsathit, S.; Gao, W.; Santos, L.; Fedorak, Y.; Singh, V.V.; Orozco, J.; Galarnyk, M.; Wang, J. Self-propelled activated carbon Janus micromotors for efficient water purification. Small 2015, 11, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, G.; Pumera, M. Beyond platinum: Bubble-propelled micromotors based on Ag and MnO2 catalysts. J. Am. Chem. Soc. 2014, 136, 2719–2722. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Pei, A.; Dong, R.; Wang, J. Catalytic iridium-based Janus micromotors powered by ultralow levels of chemical fuels. J. Am. Chem. Soc. 2014, 136, 2276–2279. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Manjare, M.; Zhao, Y. Catalytic nanoshell micromotors. J. Phys. Chem. C 2013, 117, 21590–21596. [Google Scholar] [CrossRef]
- Gregory, D.A.; Campbell, A.I.; Ebbens, S.J. Effect of catalyst distribution on spherical bubble swimmer trajectories. J. Phys. Chem. C 2015, 119, 15339–15348. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Lin, X.; He, Q.; Li, J. Autonomous movement of controllable assembled Janus capsule motors. ACS Nano 2012, 6, 10910–10916. [Google Scholar] [CrossRef] [PubMed]
- Solovev, A.A.; Xi, W.; Gracias, D.H.; Harazim, S.M.; Deneke, C.; Sanchez, S.; Schmidt, O.G. Self-propelled nanotools. ACS Nano 2012, 6, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.; Huang, G.; An, Z.; Chen, G.; Zhang, J.; Li, M.; Liu, R.; Mei, Y. Hierarchical nanoporous microtubes for high-speed catalytic microengines. Npg Asia Mater. 2014, 6, e94. [Google Scholar] [CrossRef]
- Jiang, C.; Huang, G.S.; Ding, S.J.; Dong, H.L.; Men, C.L.; Mei, Y.F. Atomic layer deposition of Pt nanoparticles for microengine with promoted catalytic motion. Nanoscale Res. Lett. 2016, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Ma, G.; Kang, J.; Sun, H.; Wang, S. Pt-free microengines at extremely low peroxide levels. Chem. Commun. 2018, 54, 4653–4656. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Self-propelled nanojets via template electrodeposition. Nanoscale 2013, 5, 1319–1324. [Google Scholar]
- Li, J.; Zhang, J.; Gao, W.; Huang, G.; Di, Z.; Liu, R.; Wang, J.; Mei, Y. Dry-released nanotubes and nanoengines by particle-assisted rolling. Adv. Mater. 2013, 25, 3715–3721. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Karshalev, E.; Guan, J.; Wang, J. Magnesium-based micromotors: Water-powered propulsion, multifunctionality, and biomedical and environmental applications. Small 2018, 8, 1704252. [Google Scholar] [CrossRef] [PubMed]
- Mariahormigos, R.; Juradosanchez, B.; Vazquez, L.; Escarpa, A. Carbon allotrope nanomaterials based catalytic micromotors. Chem. Mater. 2016, 28, 8962–8970. [Google Scholar] [CrossRef]
- Wang, S.; Wu, N. Selecting the swimming mechanisms of colloidal particles: Bubble propulsion versus self-diffusiophoresis. Langmuir ACS J. Surfaces Colloids 2014, 30, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, U.; Soler, L.; Gibbs, J.G.; Sanchez, S.; Fischer, P. Surface roughness-induced speed increase for active Janus micromotors. Chem. Commun. 2015, 51, 8660–8663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Sattayasamitsathit, S.; Orozco, J.; Wang, J. Highly efficient catalytic microengines: Template electrosynthesis of polyaniline/platinum microtubes. J. Am. Chem. Soc. 2011, 133, 11862–11864. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Solovev, A.A.; Mei, Y.F.; Schmidt, O.G. Dynamics of biocatalytic microengines mediated by variable friction control. J. Am. Chem. Soc. 2010, 132, 13144–13145. [Google Scholar] [CrossRef] [PubMed]
- Manesh, K.M.; Cardona, M.; Yuan, R.; Clark, M.; Kagan, D.; Balasubramanian, S.; Wang, J. Template-assisted fabrication of salt-independent catalytic tubular microengines. ACS Nano 2010, 4, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2015, 46, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Baumgaertner, A. Crawling of a driven adherent membrane. J. Chem. Phys. 2012, 137, 144906. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Ma, M.; Huang, M.; Duan, R.; Wang, H.; Sun, L.; Zhu, M. Enhanced hydrogen generation properties of MgH2-based hydrides by breaking the magnesium hydroxide passivation layer. Energies 2015, 8, 4237–4252. [Google Scholar] [CrossRef]
- Gao, W.; Pei, A.; Wang, J. Water-driven micromotors. ACS Nano 2012, 6, 8432–8438. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, J.; Li, T.; Gao, W.; He, Q.; Zhang, L.; Wang, J. Water-powered cell-mimicking Janus micromotor. Adv. Funct. Mater. 2016, 25, 7497–7501. [Google Scholar] [CrossRef]
- Mou, F.; Chen, C.; Ma, H.; Yin, Y.; Wu, Q.; Guan, J. Self-propelled micromotors driven by the magnesium-water reaction and their hemolytic properties. Angew. Chem. Int. Edit. 2013, 52, 7208–7212. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Chen, C.; Zhong, Q.; Yin, Y.; Ma, H.; Guan, J. Autonomous motion and temperature-controlled drug delivery of Mg/Pt-poly(N-isopropylacrylamide) Janus micromotors driven by simulated body fluid and blood plasma. ACS Appl. Mater. Interfaces 2014, 6, 9897–9903. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Singh, V.V.; Sattayasamitsathit, S.; Orozco, J.; Kaufmann, K.; Dong, R.; Gao, W.; Jurado-Sanchez, B.; Fedorak, Y.; Wang, J. Water-driven micromotors for rapid photocatalytic degradation of biological and chemical warfare agents. ACS Nano 2014, 8, 11118–11125. [Google Scholar] [CrossRef] [PubMed]
- Delezuk, J.A.; Ramírez-Herrera, D.E.; Esteban-Fernández, d.Á.B.; Wang, J. Chitosan-based water-propelled micromotors with strong antibacterial activity. Nanoscale 2017, 9, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Llorente, C.; Jepsen, P.; Inamine, T.; Wang, L.; Bluemel, S.; Wang, H.J.; Loomba, R.; Bajaj, J.S.; Schubert, M.L.; Sikaroodi, M. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat. Commun. 2017, 8, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damaghi, M.; Tafreshi, N.K.; Lloyd, M.C.; Sprung, R.; Estrella, V.; Wojtkowiak, J.W.; Morse, D.L.; Koomen, J.M.; Bui, M.M.; Gatenby, R.A. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 2015, 6, 8752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Zhang, J.; Yang, F.; Zhu, J.; Tian, X.; Chen, X. In vitro degradation and cell response of calcium carbonate composite ceramic in comparison with other synthetic bone substitute materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 50, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, E.S.; Amna, T.; Jang, Y.; Dong, H.P.; Kim, B.S. Effects of heat-treatment on surface morphologies, mechanical properties of nanofibrous poly(propylene carbonate) biocomposites and its cell culture. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 138–143. [Google Scholar] [CrossRef]
- Qian, W.Y.; Sun, D.M.; Zhu, R.R.; Du, X.L.; Liu, H.; Wang, S.L. pH-sensitive strontium carbonate nanoparticles as new anticancer vehicles for controlled etoposide release. Int. J. Nanomed. 2012, 7, 5781. [Google Scholar]
- Qi, C.; Zhu, Y.J.; Lu, B.Q.; Zhao, X.Y.; Zhao, J.; Chen, F.; Wu, J. ATP-stabilized amorphous calcium carbonate nanospheres and their application in protein adsorption. Small 2014, 10, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Baylis, J.R.; Ju, H.Y.; Thomson, M.H.; Kazerooni, A.; Wang, X.; John, A.E.S.; Lim, E.B.; Chien, D.; Lee, A.; Zhang, J.Q. Self-propelled particles that transport cargo through flowing blood and halt hemorrhage. Sci. Adv. 2015, 1, e1500379. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Dong, R.; Thamphiwatana, S.; Li, J.; Gao, W.; Zhang, L.; Wang, J. Artificial micromotors in the mouse’s stomach: A step towards in vivo use of synthetic motors. ACS Nano 2015, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.K.; Zhao, X.; Tansi, B.M.; Méndez-Ortiz, W.J.; Córdova-Figueroa, U.M.; Golestanian, R.; Sen, A. Micromotors powered by enzyme catalysis. Nano Lett. 2015, 15, 8311–8315. [Google Scholar] [CrossRef] [PubMed]
- Schattling, P.S.; Ramosdocampo, M.A.; Salgueiriño, V.; Städler, B. Double-fueled Janus swimmers with magnetotactic behavior. ACS Nano 2017, 11, 3973–3983. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hortelao, A.C.; Patiño, T.; Sanchez, S. Enzyme catalysis to power micro/nanomachines. ACS Nano 2016, 10, 9111–9122. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jannasch, A.; Albrecht, U.R.; Hahn, K.; Miguel-López, A.; Schäffer, E.; Sánchez, S. Enzyme-powered hollow mesoporous Janus nanomotors. Nano Lett. 2015, 15, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Schattling, P.; Bo, T.; Städler, B. Enhanced diffusion of glucose-fueled Janus particles. Chem. Mater. 2015, 27, 7412–7418. [Google Scholar] [CrossRef]
- Abdelmohsen, L.K.; Nijemeisland, M.; Pawar, G.M.; Janssen, G.J.; Nolte, R.J.; van Hest, J.C.; Wilson, D.A. Dynamic loading and unloading of proteins in polymeric stomatocytes: Formation of an enzyme-loaded supramolecular nanomotor. ACS Nano 2016, 10, 2652–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubert, B.; Boutigny, D.; Gaillard, J.M.; Hicheur, A.; Karyotakis, Y.; Lees, J.P.; Robbe, P.; Tisserand, V.; Palano, A.; Pompili, A. Modification with hemeproteins increases the diffusive movement of nanorods in dilute hydrogen peroxide solutions. Chem. Commun. 2013, 49, 8803–8805. [Google Scholar]
- Ma, X.; Sánchez, S. Bio-catalytic mesoporous Janus nano-motors powered by catalase enzyme. Tetrahedron 2017, 73, 4883–4886. [Google Scholar] [CrossRef]
- Simmchen, J.; Baeza, A.; Ruiz, D.; Esplandiu, M.J.; Vallet-Regí, M. Asymmetric hybrid silica nanomotors for capture and cargo transport: Towards a novel motion-based DNA sensor. Small 2012, 8, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Nolte, R.J.M.; Hest, J.C.M.V. Autonomous movement of platinum-loaded stomatocytes. Nat. Chem. 2012, 4, 268. [Google Scholar] [CrossRef] [PubMed]
- Orozco, J.; Garcíagradilla, V.; D’Agostino, M.; Gao, W.; Cortés, A.; Wang, J. Artificial enzyme-powered microfish for water-quality testing. ACS Nano 2013, 7, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.V.; Kaufmann, K.; Esteban-Fernández, d.Á.B.; Uygun, M.; Wang, J. Nanomotors responsive to nerve-agent vapor plumes. Chem. Commun. 2016, 52, 3360–3363. [Google Scholar] [CrossRef] [PubMed]
- Sattayasamitsathit, S.; Kaufmann, K.; Galarnyk, M.; Vazquezduhalt, R.; Wang, J. Dual-enzyme natural motors incorporating decontamination and propulsion capabilities. RSC Adv. 2014, 4, 27565–27570. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, X.; Zou, X.; Sun, J.; He, Q. Biodegradable protein-based rockets for drug transportation and light-triggered release. ACS Appl. Mater. Interfaces 2015, 7, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.G.; Li, J.X.; de Avila, B.E.F.; Li, T.L.; Gao, W.W.; He, Q.; Zhang, L.F.; Wang, J. Water-powered cell-mimicking janus micromotor. Adv. Funct. Mater. 2015, 25, 7497–7501. [Google Scholar] [CrossRef]

| Type | Specific Type | Methods | Speeds (μm/s/bl/s), Diameter/Length (μm), Fuel Concentrations |
|---|---|---|---|
| Low concentration of peroxide | Janus micromotors | Slowing down the rotational diffusion Microporous large carbon motors Nanoshell motors multilayer hollow capsules | 140,000/3111, 45, 5% [33] 190/3.17, 60, 2% [55] 25/1.25, 20, 0.1% [56] 20/4.22, 4.74, 0.001% [57] 140/17.5, 8, 3% [60] |
| Tubular micromotors | Increased solution temperature Hierarchical nanoporous walls Atomic layer deposition Embedding nanoparticles Enlarging rougher surface | >400/4, 100, 5% [21] 100–1000/6.45–38.76, 15.5–25.8, 5% [32] 10,000/200, 50, 5% [20] 120/6, 20, 0.2% [62] 1000/20, 50, 5% [63] 183/1.22, 150, 5% [73] | |
| Bioactive fluid | Water | Al-Ga Janus micromotors RBC-Mg Janus micromotors Pt microspheres exposing a Mg core Light-activated TiO2/Au/Mg micromotor | 3000/150, 20 [71] 172/8.6, 20 [109] 75.7/3.785, 20 [80] 72.6/3.63, 20 [83] |
| Native acid | Carbonate-based materials Zn/Mg-based motor | 15,000/1500, 10 [90] 60/4, 15 [37] 120/6, 20 [12] | |
| Enzyme-driven micromotors | Catalase Glucose oxidase Catalase and peroxidase Supramolecular stomatocytes | 60/182, 0.33, 111 mM [100] 226.1/10, 22.6, 1.5% [72] 5000–28,000/0.71–4, 7000, <1% [107] 59/2.95, 20, 2% [108] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, Q.; Wang, Z.; Tian, F.; You, J.; Xu, S. A Review of Fast Bubble-Driven Micromotors Powered by Biocompatible Fuel: Low-Concentration Fuel, Bioactive Fluid and Enzyme. Micromachines 2018, 9, 537. https://doi.org/10.3390/mi9100537
Chi Q, Wang Z, Tian F, You J, Xu S. A Review of Fast Bubble-Driven Micromotors Powered by Biocompatible Fuel: Low-Concentration Fuel, Bioactive Fluid and Enzyme. Micromachines. 2018; 9(10):537. https://doi.org/10.3390/mi9100537
Chicago/Turabian StyleChi, Qingjia, Zhen Wang, Feifei Tian, Ji’an You, and Shuang Xu. 2018. "A Review of Fast Bubble-Driven Micromotors Powered by Biocompatible Fuel: Low-Concentration Fuel, Bioactive Fluid and Enzyme" Micromachines 9, no. 10: 537. https://doi.org/10.3390/mi9100537




