Aluminum Patterned Electroplating from AlCl3–[EMIm]Cl Ionic Liquid towards Microsystems Application †
Abstract
:1. Introduction
2. Experimental Method
3. Results and Discussion
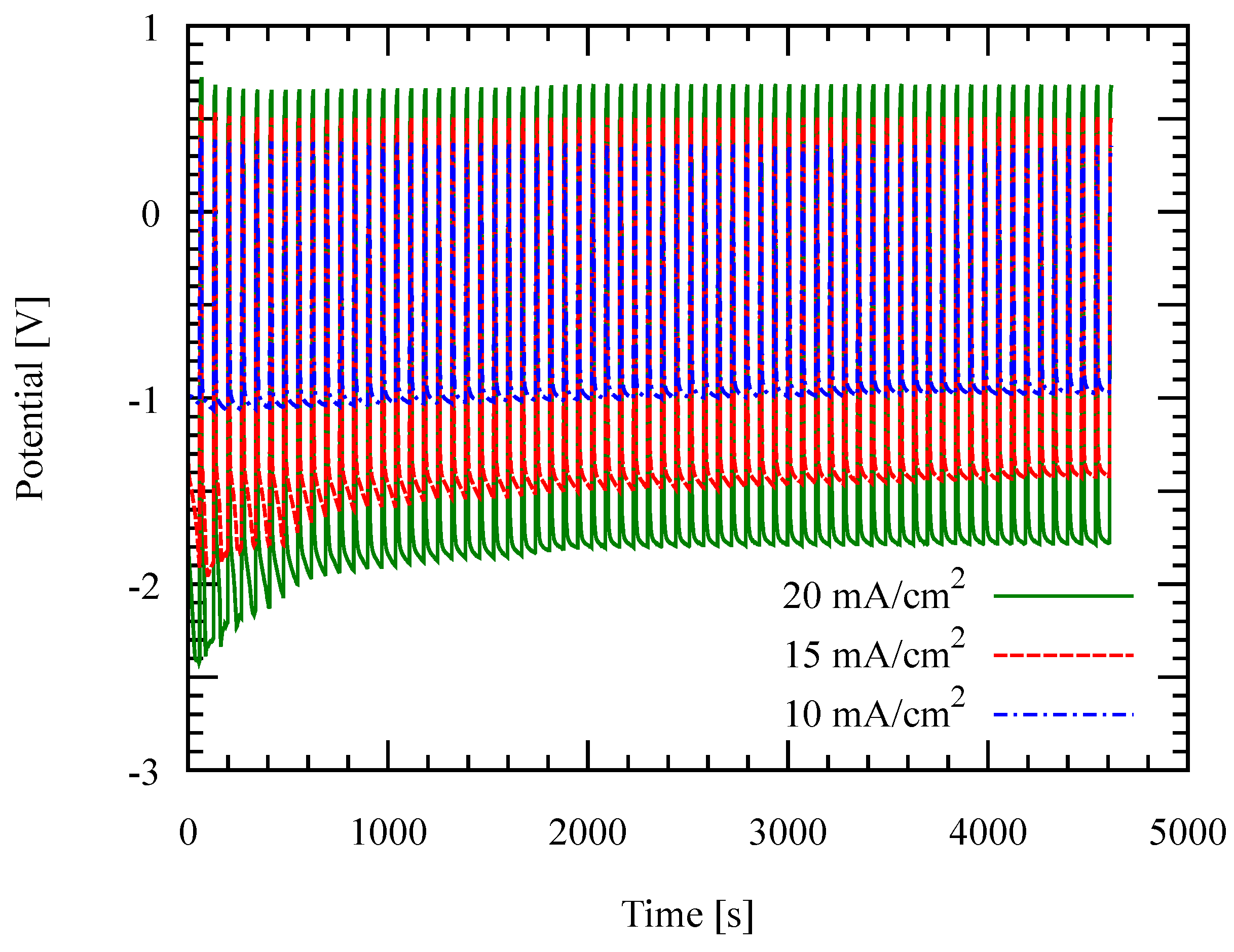
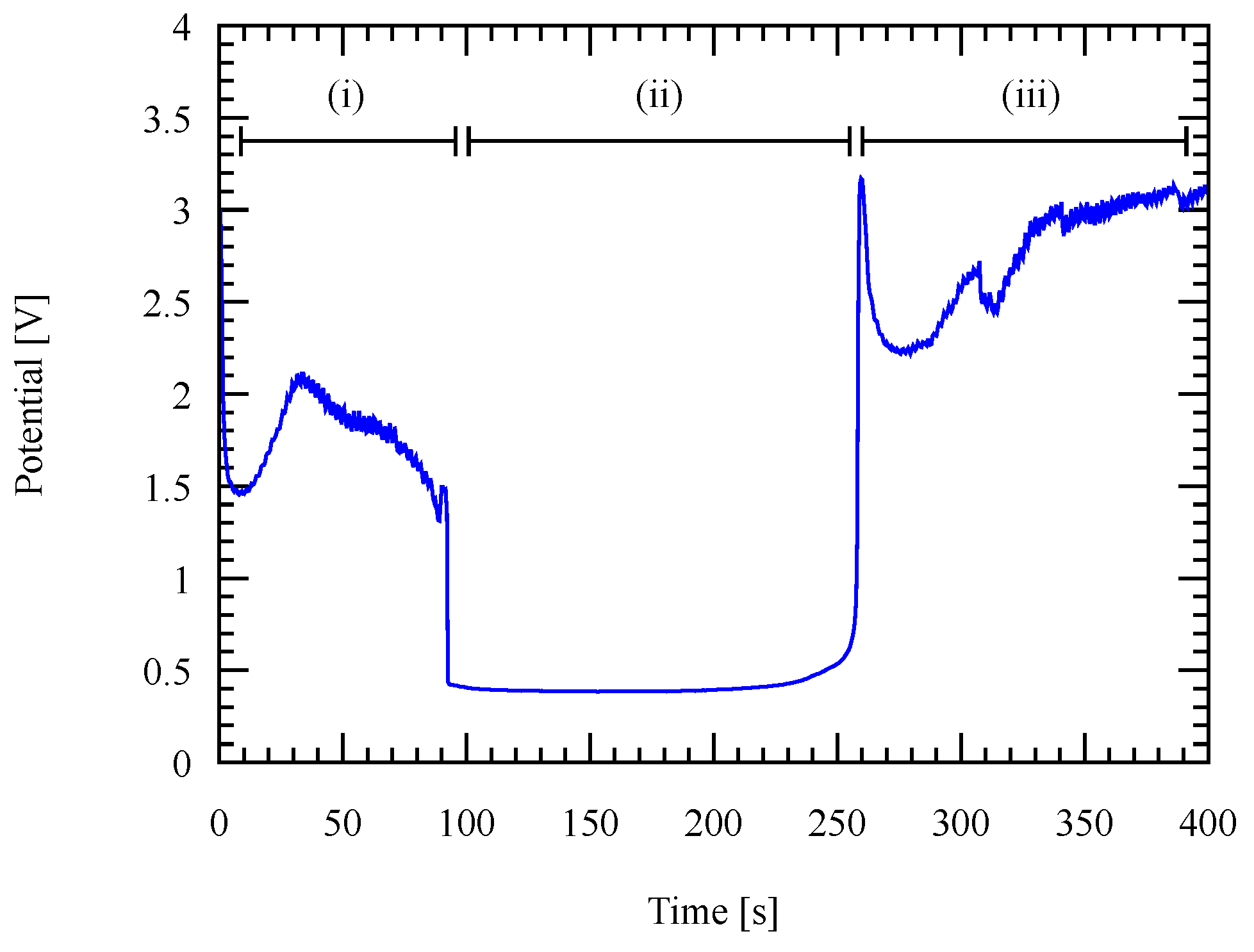
3.1. Recurrent Galvanic Pulse Electroplating
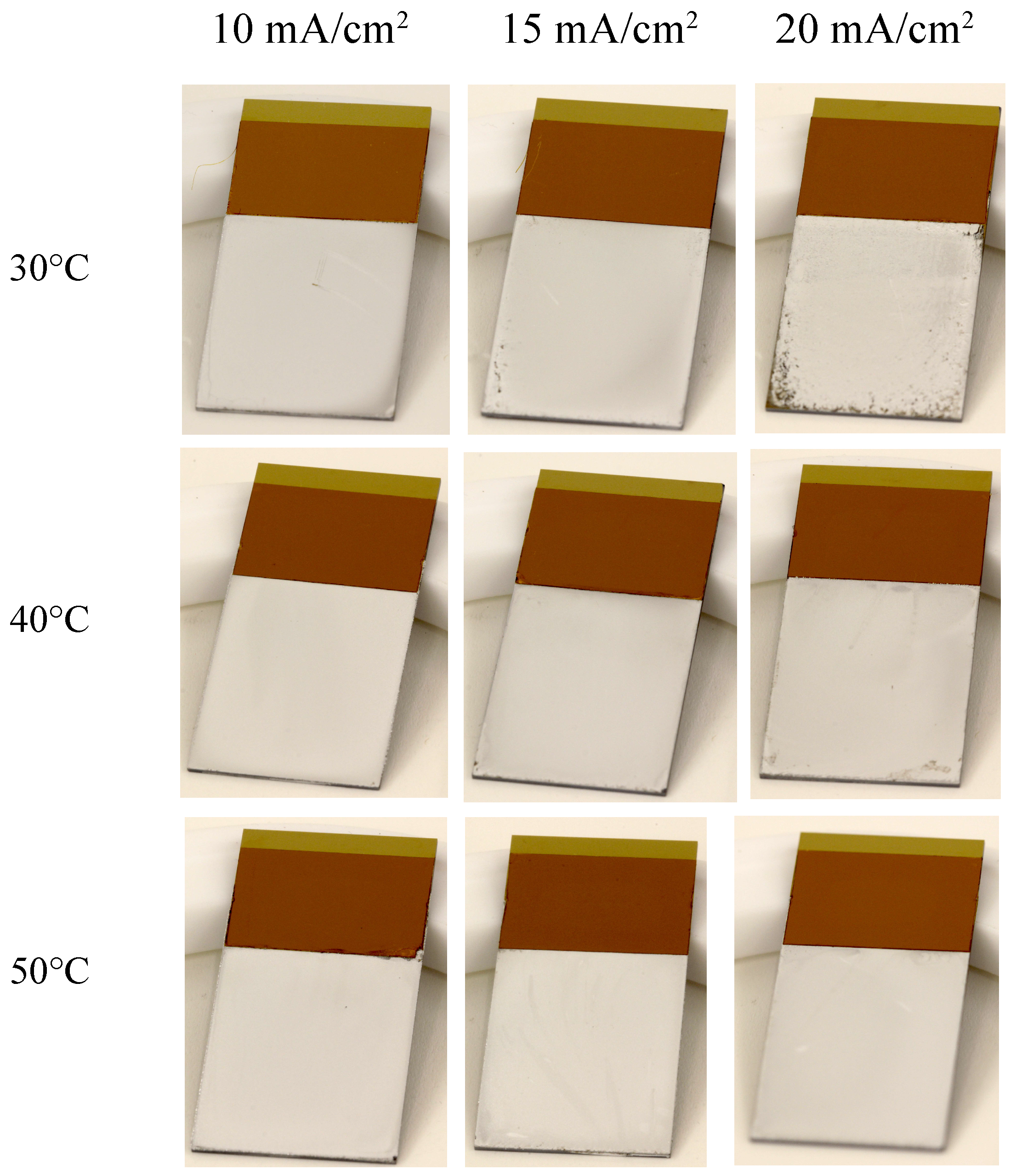
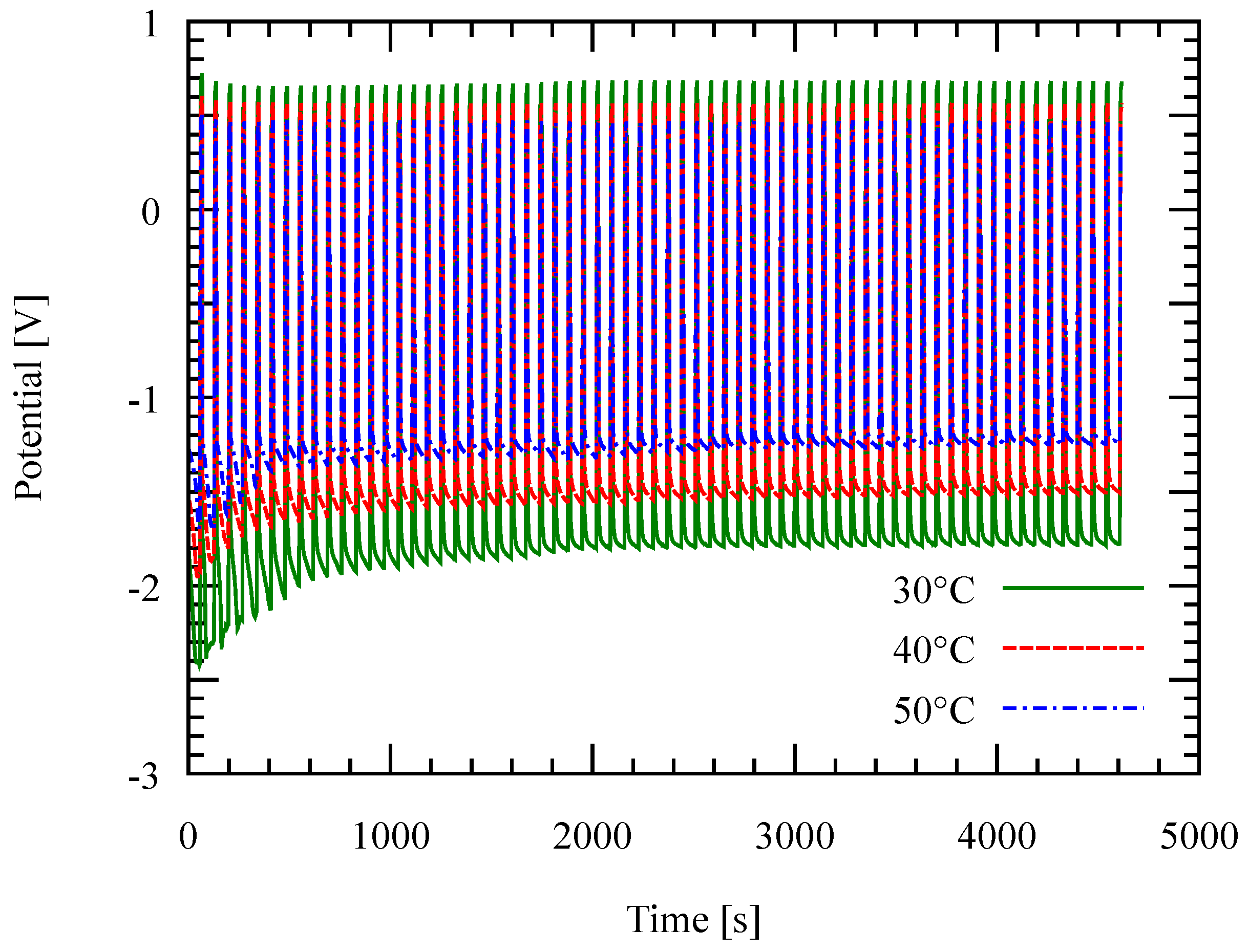
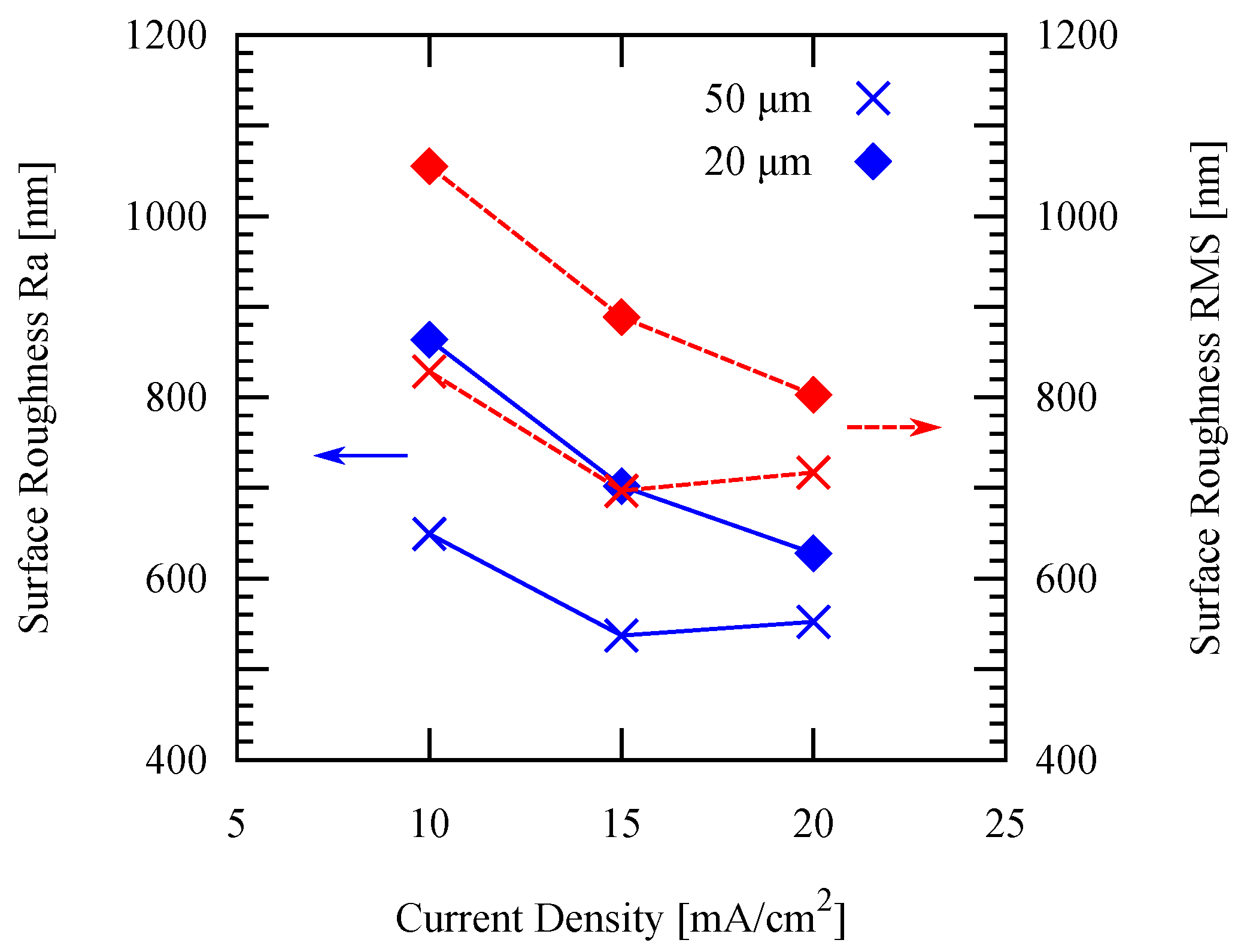
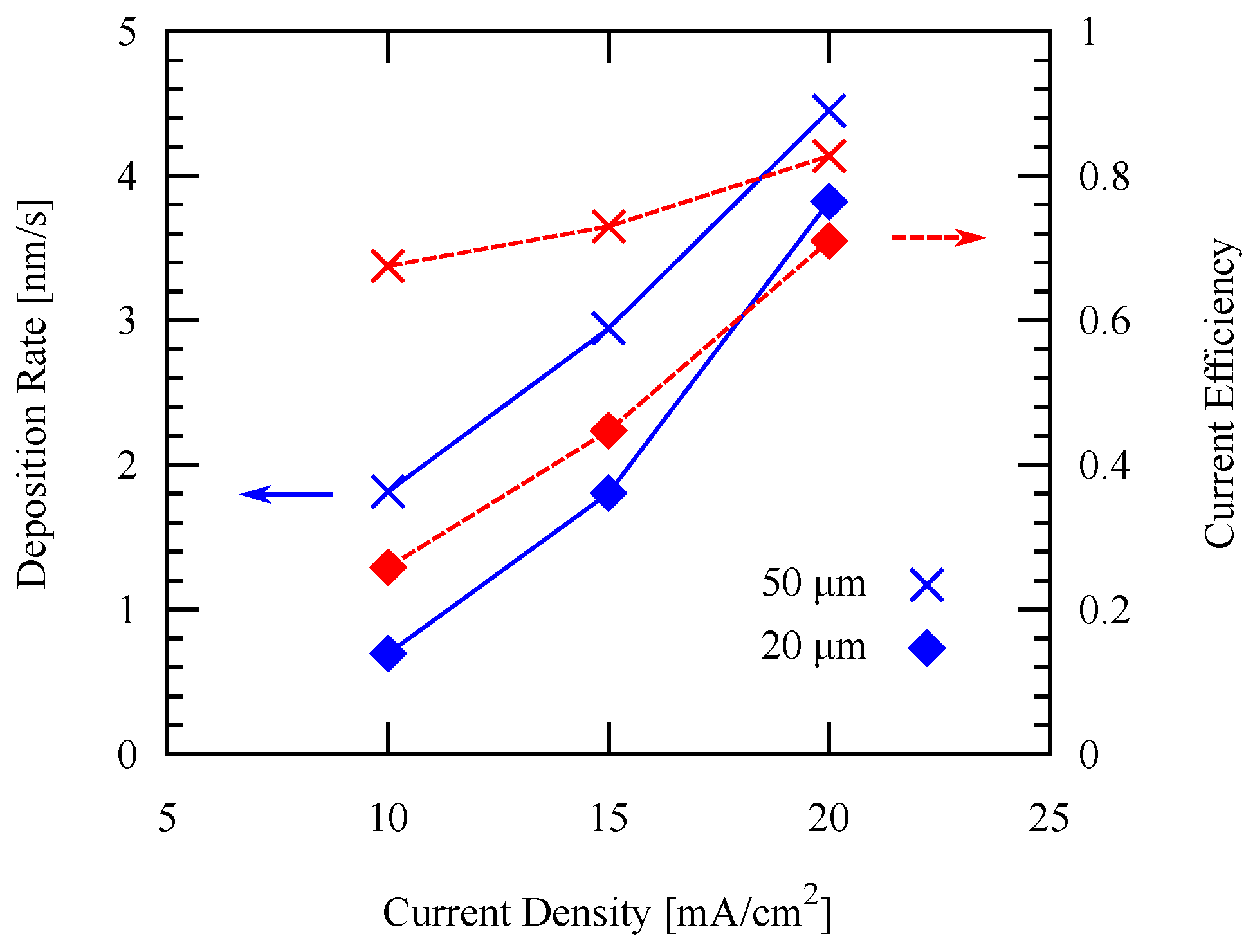
3.2. Effect of Temperature and Current Density
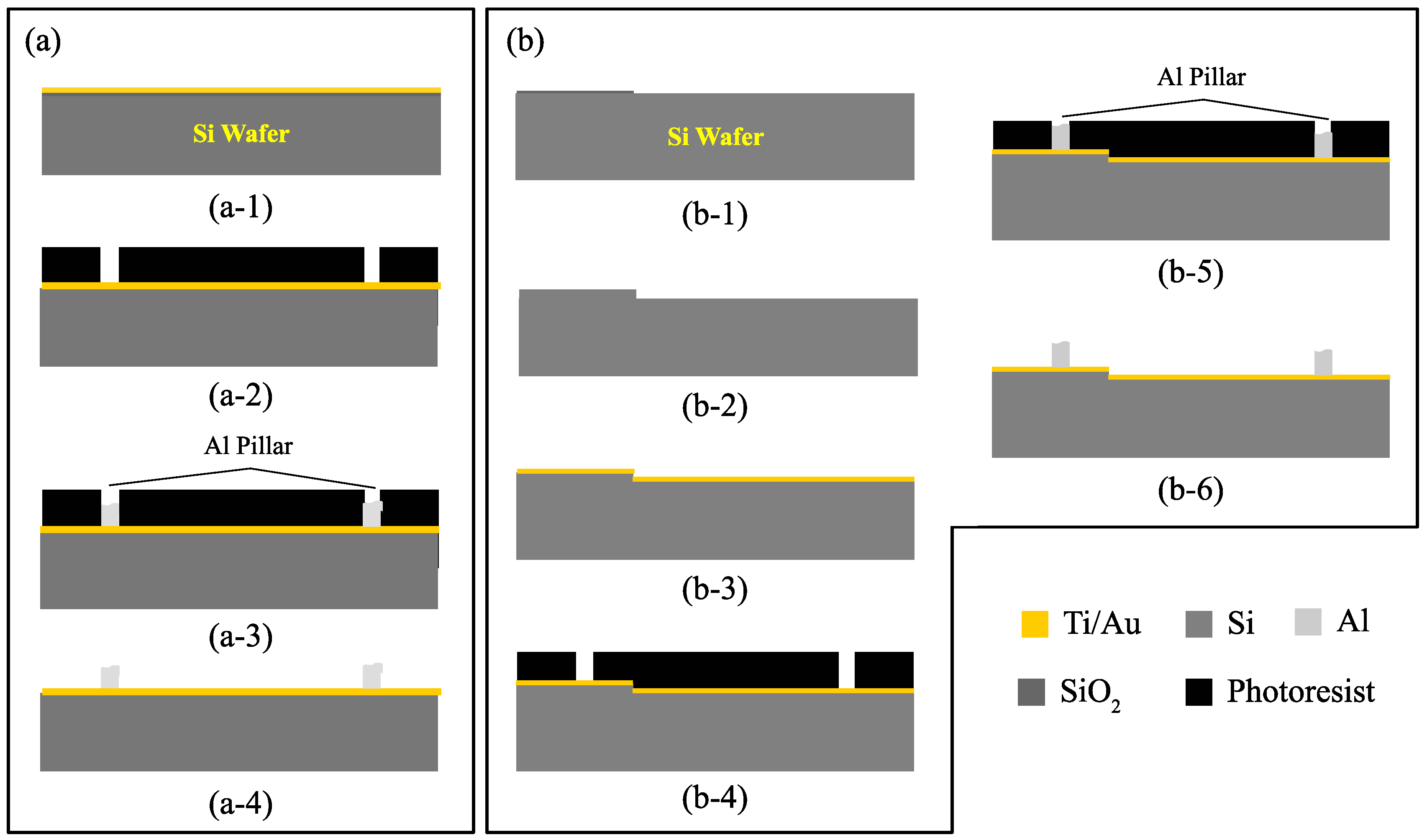
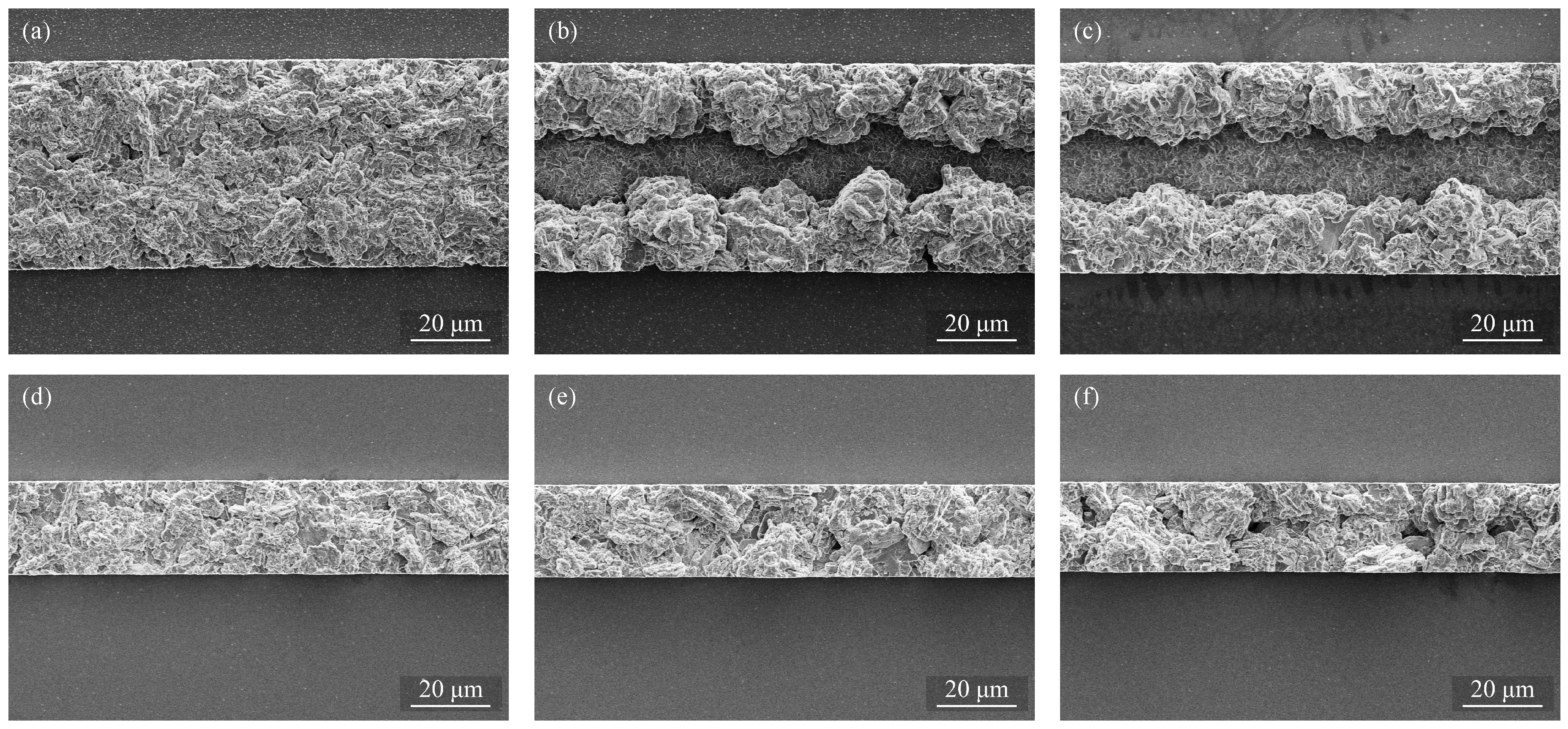
3.3. Patterned Electroplating
3.4. Al Seed Layer
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamamoto, S.; Higurashi, E.; Suga, T.; Sawada, R. Low-temperature hermetic packaging for microsystems using Au-Au surface-activated bonding at atmospheric pressure. J. Micromech. Microeng. 2012, 22, 055026. [Google Scholar] [CrossRef]
- Kurashima, Y.; Maeda, A.; Takagi, H. Room-temperature wafer scale bonding using smoothed Au seal ring surfaces for hermetic sealing. Jpn. J. Appl. Phys. 2016, 55, 016701. [Google Scholar] [CrossRef]
- Itoh, T.; Okada, H.; Takagi, H.; Maeda, R.; Suga, T. Room temperature vacuum sealing using surface activated bonding method. In Proceedings of the 12th International Conference on Solid-State Sensors, Actuators and Microsystems. Digest of Technical Papers (Cat. No.03TH8664), Boston, MA, USA, 8–12 June 2003; Volume 2, pp. 1828–1831. [Google Scholar]
- Decharat, A.; Yu, J.; Boers, M.; Stemme, G.; Niklaus, F. Room-temperature sealing of microcavities by cold metal welding. J. Microelectromec. Syst. 2009, 18, 1318–1325. [Google Scholar] [CrossRef]
- Wang, X.; Bleiker, S.J.; Antelius, M.; Stemme, G.; Niklaus, F. Wafer-Level Vacuum Packaging Enabled by Plastic Deformation and Low-Temperature Welding of Copper Sealing Rings with a Small Footprint. J. Microelectromec. Syst. 2017, 26, 357–365. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Hirano, H.; Frömel, J.; Tanaka, S. Wafer-level hermetic thermo-compression bonding using electroplated gold sealing frame planarized by fly-cutting. J. Micromech. Microeng. 2017, 27, 015029. [Google Scholar] [CrossRef]
- Liu, C.; Hirano, H.; Froemel, J.; Tanaka, S. Wafer-level vacuum sealing using AgAg thermocompression bonding after fly-cut planarization. Sens. Actuators A Phys. 2017, 261, 210–218. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Hirano, H.; Tanaka, S. Low-temperature hermetic thermo-compression bonding using electroplated copper sealing frame planarized by fly-cutting for wafer-level MEMS packaging. Sens. Actuators A Phys. 2018, 279, 671–679. [Google Scholar] [CrossRef]
- Malik, N.; Poppe, E.; Schjølberg-Henriksen, K.; Taklo, M.; Finstad, T. Hermeticity and reliability of Al-Al thermocompression wafer bonding. ECS Trans. 2014, 64, 149–160. [Google Scholar] [CrossRef]
- Taklo, M.M.V.; Storås, P.; Schjølberg-Henriksen, K.; Hasting, H.K.; Jakobsen, H. Strong, high-yield and low-temperature thermocompression silicon wafer-level bonding with gold. J. Micromech. Microeng. 2004, 14, 884–890. [Google Scholar] [CrossRef]
- Tan, C.S.; Fan, J.; Lim, D.F.; Chong, G.Y.; Li, K.H. Low temperature wafer-level bonding for hermetic packaging of 3D microsystems. J. Micromech. Microeng. 2011, 21, 075006. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Hertel, S.; Wiemer, M.; Otto, T. Investigation of aluminum patterned electrodeposition process from AlCl3–[EMIm]Cl ionic liquid for microsystems application. In Proceedings of the 2018 International Conference on Electronic Packaging and iMAPS All Asia Conference, Kuwana, Japan, 17–21 April 2018; pp. 415–418. [Google Scholar]
- Que, L. Thermal Actuation. In Comprehensive Microsystems; Elsevier: Amsterdam, The Netherlands, 2008; pp. 69–100. [Google Scholar]
- Enikov, E.T.; Lazarov, K. PCB-integrated metallic thermal micro-actuators. Sens. Actuators A Phys. 2003, 105, 76–82. [Google Scholar] [CrossRef]
- Carlin, R.T. Aluminum Anodization in a Basic Ambient Temperature Molten Salt. J. Electrochem. Soc. 1989, 136, 1409. [Google Scholar] [CrossRef]
- Endres, F. Ionic liquids: Solvents for the electrodeposition of metals and semiconductors. ChemPhysChem 2002, 3, 144–154. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Carlin, R.T.; Crawford, W.; Bersch, M. Nucleation and Morphology Studies of Aluminum Deposited from an Ambient-Temperature Chloroaluminate Molten Salt. J. Electrochem. Soc. 1992, 139, 2720–2727. [Google Scholar] [CrossRef]
- Yue, G.; Lu, X.; Zhu, Y.; Zhang, X.; Zhang, S. Surface morphology, crystal structure and orientation of aluminium coatings electrodeposited on mild steel in ionic liquid. Chem. Eng. J. 2009, 147, 79–86. [Google Scholar] [CrossRef]
- Bardi, U.; Caporali, S.; Craig, M.; Giorgetti, A.; Perissi, I.; Nicholls, J.R. Electrodeposition of aluminium film on P90 Li-Al alloy as protective coating against corrosion. Surf. Coat. Technol. 2009, 203, 1373–1378. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.K.; Chen, S.Y.; Tsai, W.T.; Deng, M.J.; Sun, I.W. Electrodeposition of aluminum on magnesium alloy in aluminum chloride (AlCl3)-1-ethyl-3-methylimidazolium chloride (EMIC) ionic liquid and its corrosion behavior. Electrochem. Commun. 2007, 9, 1602–1606. [Google Scholar] [CrossRef]
- Sheng, P.F.; Chen, B.; Shao, H.B.; Wang, J.M.; Zhang, J.Q.; Cao, C.N. Electrodeposition and corrosion behavior of nanocrystalline aluminum from a chloroaluminate ionic liquid. Mater. Corros. 2015, 66, 1338–1343. [Google Scholar] [CrossRef]
- Pradhan, D.; Reddy, R.G. Dendrite-free aluminum electrodeposition from AlCl3-1-ethyl-3-methyl-imidazolium chloride ionic liquid electrolytes. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2012, 43, 519–531. [Google Scholar] [CrossRef]
- Lai, P.K.; Skyllas-Kazacos, M. Electrodeposition of aluminium in aluminium chloride/1-methyl-3-ethylimidazolium chloride. J. Electroanal. Chem. 1988, 248, 431–440. [Google Scholar] [CrossRef]
- Zein El Abedin, S.; Moustafa, E.M.; Hempelmann, R.; Natter, H.; Endres, F. Electrodeposition of nano- and macrocrystalline aluminium in three different air and water stable ionic liquids. ChemPhysChem 2006, 7, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Reddy, R.G. Mechanistic study of Al electrodeposition from EMIC-AlCl3 and BMIC-AlCl3 electrolytes at low temperature. Mater. Chem. Phys. 2014, 143, 564–569. [Google Scholar] [CrossRef]
- Jiang, T.; Chollier Brym, M.J.; Dubé, G.; Lasia, A.; Brisard, G.M. Electrodeposition of aluminium from ionic liquids: Part I-electrodeposition and surface morphology of aluminium from aluminium chloride (AlCl3)-1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) ionic liquids. Surf. Coat. Technol. 2006, 201, 1–9. [Google Scholar] [CrossRef]
- Jiang, T.; Chollier Brym, M.J.; Dubé, G.; Lasia, A.; Brisard, G.M. Electrodeposition of aluminium from ionic liquids: Part II—Studies on the electrodeposition of aluminum from aluminum chloride (AICl3)— trimethylphenylammonium chloride (TMPAC) ionic liquids. Surf. Coat. Technol. 2006, 201, 10–18. [Google Scholar] [CrossRef]
- Li, B.; Fan, C.; Chen, Y.; Lou, J.; Yan, L. Pulse current electrodeposition of Al from an AlCl3-EMIC ionic liquid. Electrochim. Acta 2011, 56, 5478–5482. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Kain, V.; Hubli, R.C. Stirring effects on aluminium coatings electrodeposited in ionic liquids. Surf. Eng. 2014, 30, 562–567. [Google Scholar] [CrossRef]
- Berretti, E.; Giaccherini, A.; Martinuzzi, S.M.; Innocenti, M.; Schubert, T.J.S.; Stiemke, F.M.; Caporali, S. Aluminium electrodeposition from ionic liquid: Effect of deposition temperature and sonication. Materials 2016, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Q.; Chen, B.; Lu, X.; Zhang, S. Electrodeposition of Bright Al Coatings from 1-Butyl-3-Methylimidazolium Chloroaluminate Ionic Liquids with Specific Additives. J. Electrochem. Soc. 2015, 162, 320–324. [Google Scholar] [CrossRef]
- Zangari, G. Microelectromechanical Systems. In Modern Electroplating; Schlesinger, M., Paunovic, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Chapter 28; pp. 113–116. [Google Scholar]
- Bakkar, A.; Neubert, V. A new method for practical electrodeposition of aluminium from ionic liquids. Electrochem. Commun. 2015, 51, 113–116. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Farisi, M.S.; Hertel, S.; Wiemer, M.; Otto, T. Aluminum Patterned Electroplating from AlCl3–[EMIm]Cl Ionic Liquid towards Microsystems Application. Micromachines 2018, 9, 589. https://doi.org/10.3390/mi9110589
Al Farisi MS, Hertel S, Wiemer M, Otto T. Aluminum Patterned Electroplating from AlCl3–[EMIm]Cl Ionic Liquid towards Microsystems Application. Micromachines. 2018; 9(11):589. https://doi.org/10.3390/mi9110589
Chicago/Turabian StyleAl Farisi, Muhammad Salman, Silvia Hertel, Maik Wiemer, and Thomas Otto. 2018. "Aluminum Patterned Electroplating from AlCl3–[EMIm]Cl Ionic Liquid towards Microsystems Application" Micromachines 9, no. 11: 589. https://doi.org/10.3390/mi9110589




