Self-Renewal and Differentiation of Adipose-Derived Stem Cells (ADSCs) Stimulated by Multi-Axial Tensile Strain in a Pneumatic Microdevice
Abstract
1. Introduction
2. Materials and Methods
2.1. Harvest and Isolation of Human ADSCs
2.2. Fabrication of the Microdevice Providing Multi-Axial Tensile Strain
2.3. ADSCs Cultured on the Microdevice
2.4. Investigation of mRNA Expressions
2.5. Quantification of Cell Proliferation
3. Results and Discussion
3.1. Investigation of the Membrane Deformation
3.2. mRNA Expressions of Cells after Mechanical Stretching Stimulation
3.3. Gene Expression Influenced by Individuals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cole, B.J.; ElAttrache, N.S.; Anbari, A. Arthroscopic rotator cuff repairs: An anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy 2007, 23, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, R.Z.; Hollins, A.M.; Kim, H.M.; Teefey, S.A.; Middleton, W.D.; Steger-May, K.; Galatz, L.M.; Yamaguchi, K. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am. J. Sports Med. 2010, 38, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Schnaser, E.; Bosley, J.; Lefebvre, Y.; Gobezie, R. Early structural and functional outcomes for arthroscopic double-row transosseous-equivalent rotator cuff repair. Am. J. Sport. Med. 2011, 39, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Kovacevic, D.; Ehteshami, J.R.; Dagher, E.; Packer, J.D.; Rodeo, S.A. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am. J. Sports Med. 2009, 37, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Overdevest, E.P.; Jakimowicz, J.J.; Oosterbos, C.J.; Schönberger, J.P.; Knape, J.T.; van Zundert, A. The use of autologous platelet-leukocyte gels to enhance the healing process in surgery, a review. Surg. Endosc. 2007, 21, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Evans, D.; Tonino, P.M.; Yong, S.; Callaci, J.J. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am. J. Sports Med. 2012, 40, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Lei, K.F.; Yeh, W.L. Development of a co-culture device for the study of human tenocytes in response to the combined stimulation of electric field and platelet rich plasma (PRP). Biomed. Microdevices 2017, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Blenman, P.R.; Beaupré, G.S. Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J. Orthop. Res. 1988, 6, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Liu, J.L.; Chang, C.H.; Lei, K.F.; Chen, A.C.Y. Investigation of osteogenic activity of primary rabbit periosteal cells stimulated by multi-axial tensile strain. Biomed. Microdevices 2017, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Brook, J.; Dauphinee, D.M.; Korpinen, J.; Rawe, I.M. Pulsed radiofrequency electromagnetic field therapy: A potential novel treatment of plantar fasciitis. J. Foot Ankle Surg. 2012, 51, 312–316. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, L.; Stanco, D.; Galliera, E.; Vigano, M.; Colombini, A.; Setti, S.; Vianello, E.; Corsi Romanelli, M.M.; Sansone, V. Low frequency pulsed electromagnetic field affects proliferation, tissue-specific gene expression, and cytokines release of human tendon cell. Cell Biochem. Biophys. 2013, 66, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, T.E. Electrical stimulation of human fracture healing by means of a slow pulsating, asymmetrical direct current. Clin. Orthop. Relat. Res. 1977, 124, 124–127. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, T.; Song, K.; Fan, X.; Ma, X.; Cui, Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem. Funct. 2008, 26, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A. The adipose-derived stem cell: Looking back and looking ahead. Mol. Biol. Cell 2010, 21, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Michalek, J.; Moster, R.; Lukac, L.; Proefrock, K.; Petrasovic, M.; Rybar, J.; Capkova, M.; Chaloupka, A.; Darinskas, A.; Michalek, J., Sr.; et al. Autologous adipose tissue-derived stromal vascular fraction cells application in patients with osteoarthritis. Cell Transplant. 2015. [Google Scholar] [CrossRef]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.J.O.; Breuls, R.G.M.; Schouten, T.E.; Jurgens, S.J.F.M.; Bontkes, H.Y.; Schuurhuis, G.J.; van Ham, S.M.; van Milligen, F.J. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007, 16, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Katz, A.J. Adipose-derived stem cells for the regeneration of damaged tissues. Expert Opin. Biol. Ther. 2006, 6, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Chowaniec, D.; Falcone, L.M.; Falcone, J.A.; Dugdale, E.M.; Deberardino, T.M.; Arciero, R.A.; Mazzocca, A.D. Rapid isolation of human stem cells (connective progenitor cells) from the distal femur during arthroscopic knee surgery. Arthroscopy 2012, 28, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Vangsness, C.T.; Farr, J.; Boyd, J.; Dellaero, D.T.; Mills, C.R.; LeRoux-Williams, M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: A randomized, double-blind, controlled study. J. Bone Jt. Surg. 2014, 96, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; Wulur, I.; Alfonso, Z.; Schreiber, R.E.; Fraser, J.K.; Hedrick, M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 2005, 54, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Watanabe, S.; Ju, Y.; Xu, B. Determination of optimal cyclic uniaxial stretches for stem cell-to-tenocyte differentiation under a wide range of mechanical stretch conditions by evaluating gene expression and protein synthesis levels. Acta Bioeng. Biomech. 2013, 15, 71–79. [Google Scholar] [PubMed]
- Altman, G.H.; Horan, R.L.; Martin, I.; Farhadi, J.; Stark, P.; Volloch, V.; Richmond, J.C.; Vunjak-Novakovic, G.; Kaplan, D.L. Cell differentiation by mechanical stress. FASEB J. 2002, 16, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Cao, Y.; Wu, Y.F.; Bais, A.J.; Gao, J.S.; Tang, J.B. Tendon healing in vivo: Gene expression and production of multiple growth factors in early tendon healing period. J. Hand Surg. 2008, 33, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, H.; Zhang, X.; Fu, Y.; Huang, Y.; Lui, P.P.; Tang, T.; Dai, K. Continuous cyclic mechanical tension inhibited Runx2 expression in mesenchymal stem cells through RhoA-ERK1/2 pathway. J. Cell Physiol. 2011, 226, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gong, P.; Lin, Y.; Zhang, L.; Li, X.; Yuan, Q.; Tan, Z.; Wang, Y.; Man, Y.; Tang, H. Cyclic tensile stretch modulates osteogenic differentiation of adipose-derived stem cells via the BMP-2 pathway. Arch. Med. Sci. 2010, 6, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Janmaleki, M.; Tafazzoli-Shadpour, M.; Teymoori, M.; Rezvaninejad, S. Effects of uniaxial cycle stretch loading on morphology of adipose derived stem cells. Tissue Eng. Regener. Med. 2016, 13, 396–402. [Google Scholar] [CrossRef]
- Hanson, A.D.; Marvel, S.W.; Bernackl, S.H.; Banes, A.J.; Van Aalst, J.; Loboa, E.G. Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann. Biomed. Eng. 2009, 37, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Grottkau, B.E.; Yang, X.; Zhang, L.; Ye, L.; Lin, Y. Comparison of effects of mechanical stretching on osteogenic potential of ASCs and BMSCs. Bone Res. 2013, 3, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kearney, E.M.; Farrell, E.; Prendergast, P.J.; Campbell, V.A. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann. Biomed. Eng. 2010, 38, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Ozawa, Y.; Yamaguchi, M.; Goseki, T.; Ohzeki, K.; Abiko, Y. Induction of COX-2 expression by mechanical tension force in human periodontal ligament cells. J. Periodontol. 1998, 69, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Tee, B.C. Molecular variations related to the regional differences in periosteal growth at the mandibular ramus. Bone Biol. 2011, 294, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoshimura, Y.; Deyama, Y.; Suzuki, K.; Kitagawa, Y. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int. J. Mol. Med. 2008, 21, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F. Microfluidic systems for diagnostic applications: A review. JALA 2012, 17, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F. Recent developments and patents on biological sensing using nanoparticles in microfluidic systems. Recent Pat. Nanotechnol. 2013, 7, 81–90. [Google Scholar] [PubMed]
- Lei, K.F. Review on impedance detection of cellular responses in micro/nano environment. Micromachines 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.R. A review on continuous-flow microfluidic PCR in droplets: Advances, challenges and future. Anal. Chim. Acta 2016, 914, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhang, Y.; Lin, S.; Wang, T.H.; Yang, S. Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnol. Adv. 2011, 29, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.C.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfludic large-scale integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Liu, X.; Krull, U.; Li, D. Electrokinetically controlled DNA hybridization microfluidic chip enabling rapid target analysis. Anal. Chem. 2004, 76, 7269–7277. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lee, G.B. Automatic bio-sampling chips integrated with micro-pumps and micro-valves for disease detection. Biosens. Bioelectron. 2005, 21, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Mehling, M.; Tay, S. Microfluidic cell culture. Curr. Opin. Biotechnol. 2014, 25, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.J.; Lee, P.J.; Sabounchi, P.; Aghdam, N.; Lin, R.; Lee, L.P. A novel high aspect ratio microfluidic design to provide a stable and uniform microenvironment for cell growth in a high throughput mammalian cell culture array. Lab Chip 2005, 5, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Kang, J.H.; Park, S.J.; Yoon, E.S.; Park, J.K. Microfluidic biomechanical device for compressive cell stimulation and lysis. Sens. Actuators B 2007, 128, 108–116. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Tong, H.; Lin, B.; Qin, J. A simple elastic membrane-based microfluidic chip for the proliferation and differentiation of mesenchymal stem cells under tensile stress. Electrophoresis 2011, 32, 3431–3436. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, J.L.; Rizen, M.; L’ltalien, G.J.; Benbrahim, A.; Megerman, J.; Gerstenfeld, L.C.; Gray, M.L. Device for the application of a dynamic biaxially uniform and isotropic strain to a flexible cell culture membrane. J. Orthop. Res. 1994, 12, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Kamble, H.; Barton, M.J.; Jun, M.; Park, S.; Nguyen, N.T. Cell stretching devices as research tools: Engineering and biological considerations. Lab Chip 2016, 16, 3193–3203. [Google Scholar] [CrossRef] [PubMed]
- Kamble, H.; Vadivelu, R.; Barton, M.; Shiddiky, M.J.A.; Nguyen, N.T. Pneumatically actuated cell-stretching array platform for engineering cell patterns in vitro. Lab Chip 2018, 18, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The homeoprotein NANOG is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional expression cloning of NANOG, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor SOX9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of SOX5 and SOX6. Gens. Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M. PPAR-γ: Adipogenic regulator and thiazolidinedione receptor. Diabetes 1998, 47, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef] [PubMed]

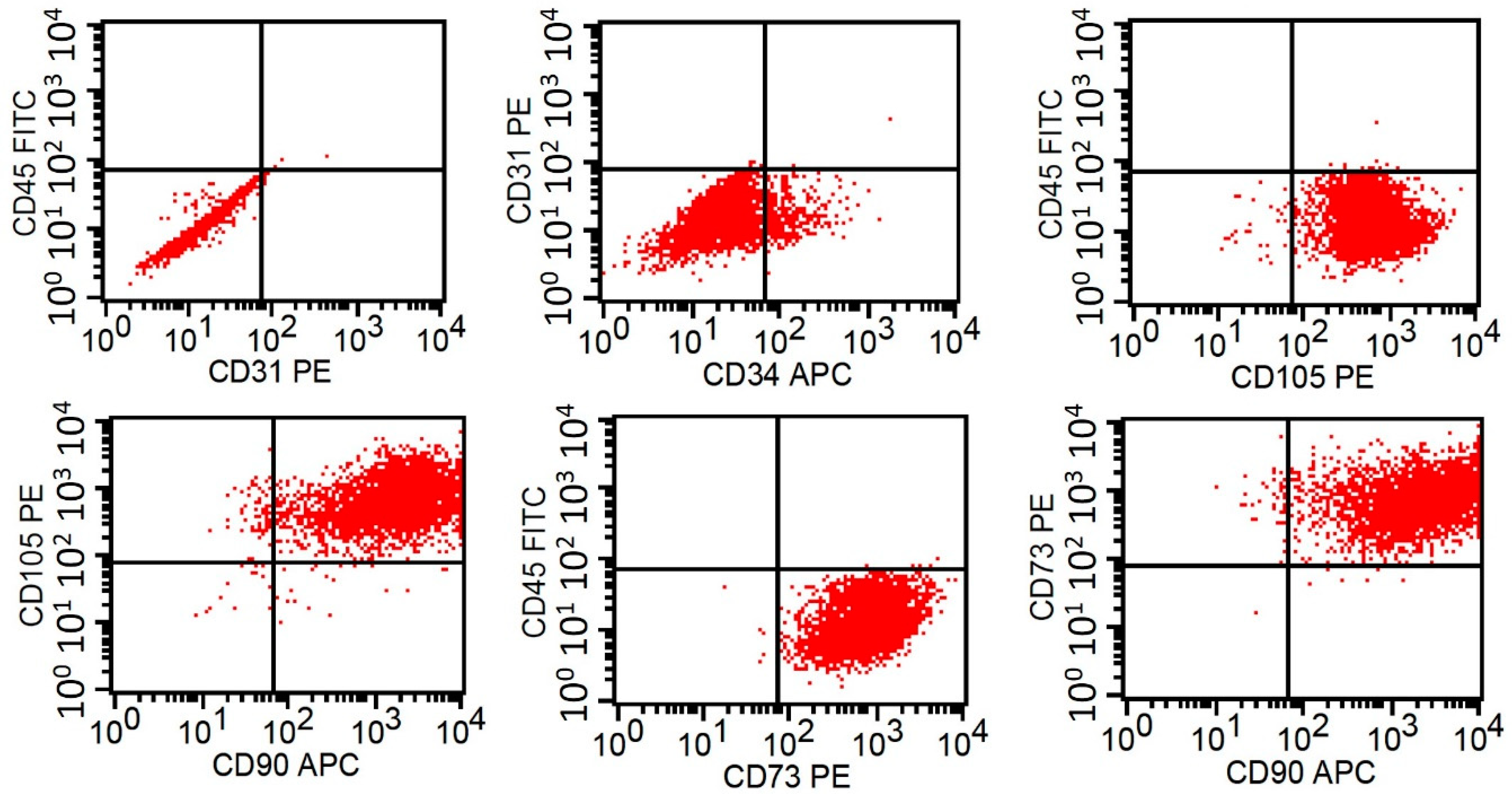
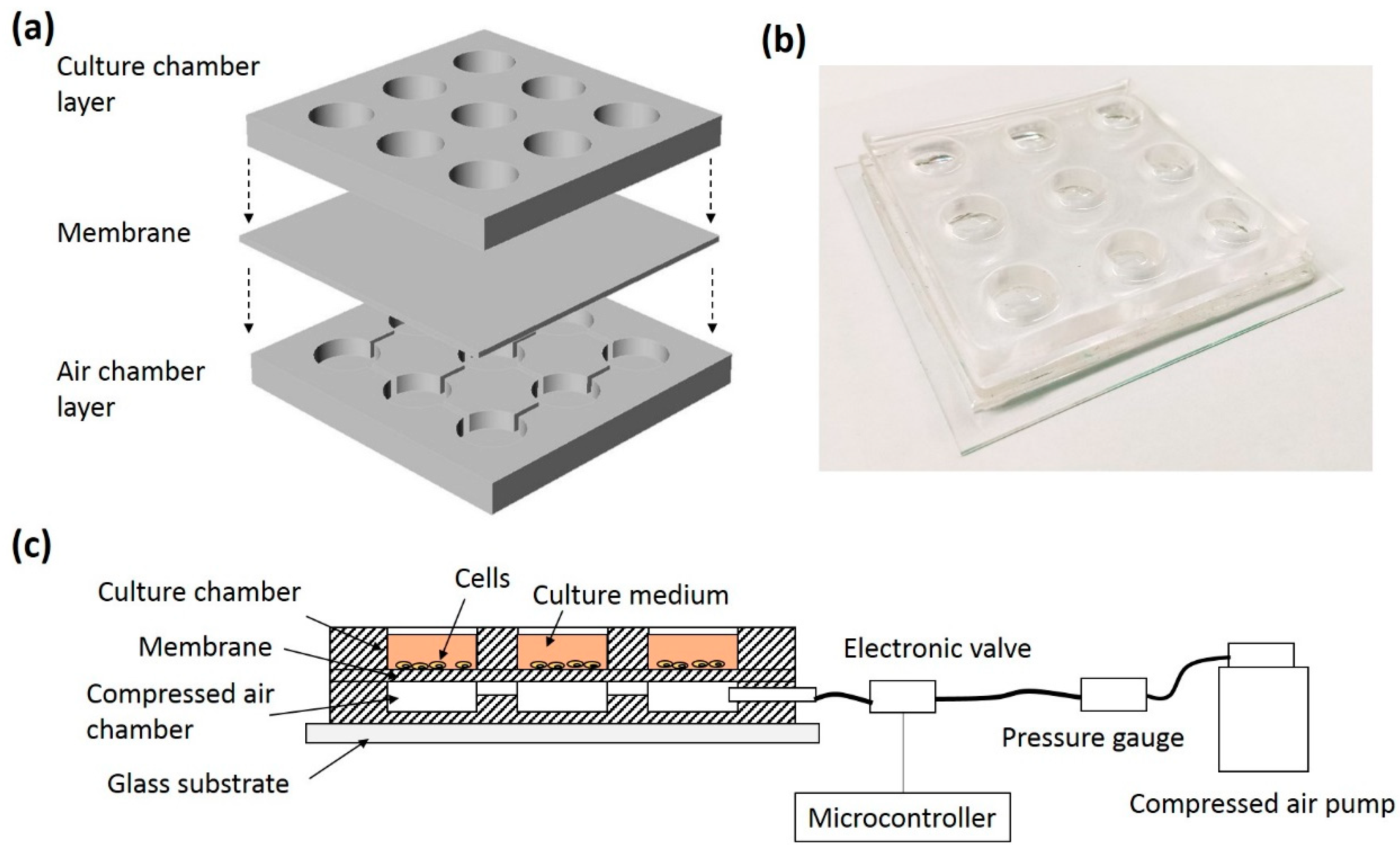





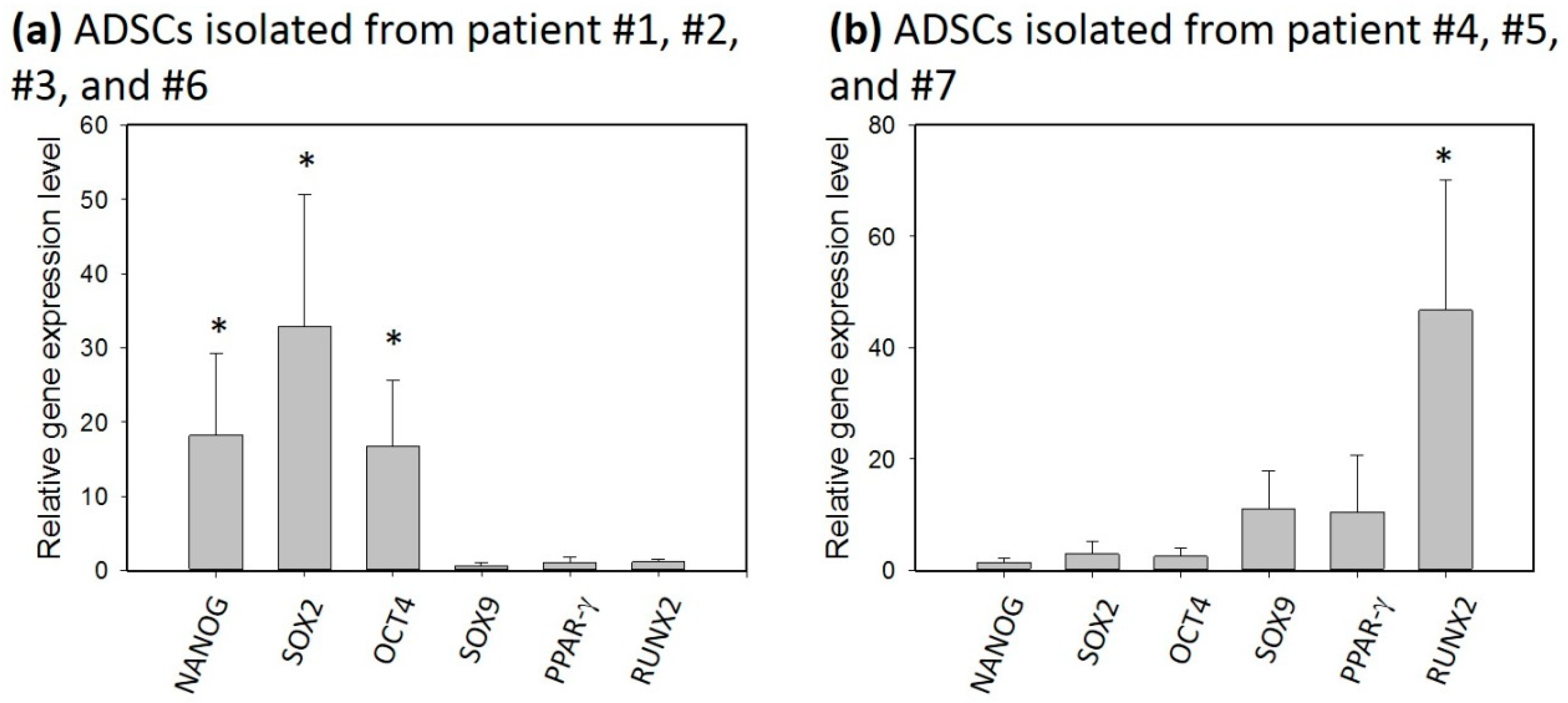
| Patient Number | Age | Gender |
|---|---|---|
| 1 | 83 | Female |
| 2 | 77 | Male |
| 3 | 69 | Female |
| 4 | 78 | Male |
| 5 | 78 | Female |
| 6 | 63 | Female |
| 7 | 70 | Female |
| Gene | Assay Number (ThermoFisher Scientific, Waltham, MA, USA) |
|---|---|
| GAPDH | Hs03929097_g1 |
| NANOG | Hs02387400_g1 |
| SOX2 | Hs00602736_s1 |
| OCT4 | Hs01895061_u1 |
| SOX9 | Hs00165814_m1 |
| PPAR-γ | Hs01115513_m1 |
| RUNX2 | Hs00231692_m1 |
| Applied Pressure (kPa) | Axial Elongation (mm) | Axial Strain (%) |
|---|---|---|
| 0 | 10.00 | 0 |
| 2 | 10.38 | 3.8 |
| 4 | 10.53 | 5.3 |
| 6 | 10.87 | 8.7 |
| 8 | 11.00 | 10 |
| 10 | 11.20 | 12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-H.; Tong, Y.-W.; Yeh, W.-L.; Lei, K.F.; Chen, A.C.-Y. Self-Renewal and Differentiation of Adipose-Derived Stem Cells (ADSCs) Stimulated by Multi-Axial Tensile Strain in a Pneumatic Microdevice. Micromachines 2018, 9, 607. https://doi.org/10.3390/mi9110607
Chiu C-H, Tong Y-W, Yeh W-L, Lei KF, Chen AC-Y. Self-Renewal and Differentiation of Adipose-Derived Stem Cells (ADSCs) Stimulated by Multi-Axial Tensile Strain in a Pneumatic Microdevice. Micromachines. 2018; 9(11):607. https://doi.org/10.3390/mi9110607
Chicago/Turabian StyleChiu, Chih-Hao, Yun-Wen Tong, Wen-Ling Yeh, Kin Fong Lei, and Alvin Chao-Yu Chen. 2018. "Self-Renewal and Differentiation of Adipose-Derived Stem Cells (ADSCs) Stimulated by Multi-Axial Tensile Strain in a Pneumatic Microdevice" Micromachines 9, no. 11: 607. https://doi.org/10.3390/mi9110607
APA StyleChiu, C.-H., Tong, Y.-W., Yeh, W.-L., Lei, K. F., & Chen, A. C.-Y. (2018). Self-Renewal and Differentiation of Adipose-Derived Stem Cells (ADSCs) Stimulated by Multi-Axial Tensile Strain in a Pneumatic Microdevice. Micromachines, 9(11), 607. https://doi.org/10.3390/mi9110607






