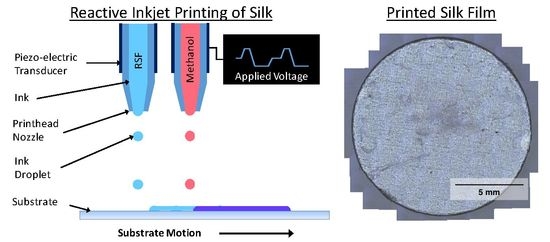
Reactive Inkjet Printing of Regenerated Silk Fibroin Films for Use as Dental Barrier Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silk Fibroin Extraction
2.2. Nano-Hydroxyapatite Synthesis
2.3. Ink Analysis
2.4. Inkjet Printing
2.5. Film Characterisation
3. Results
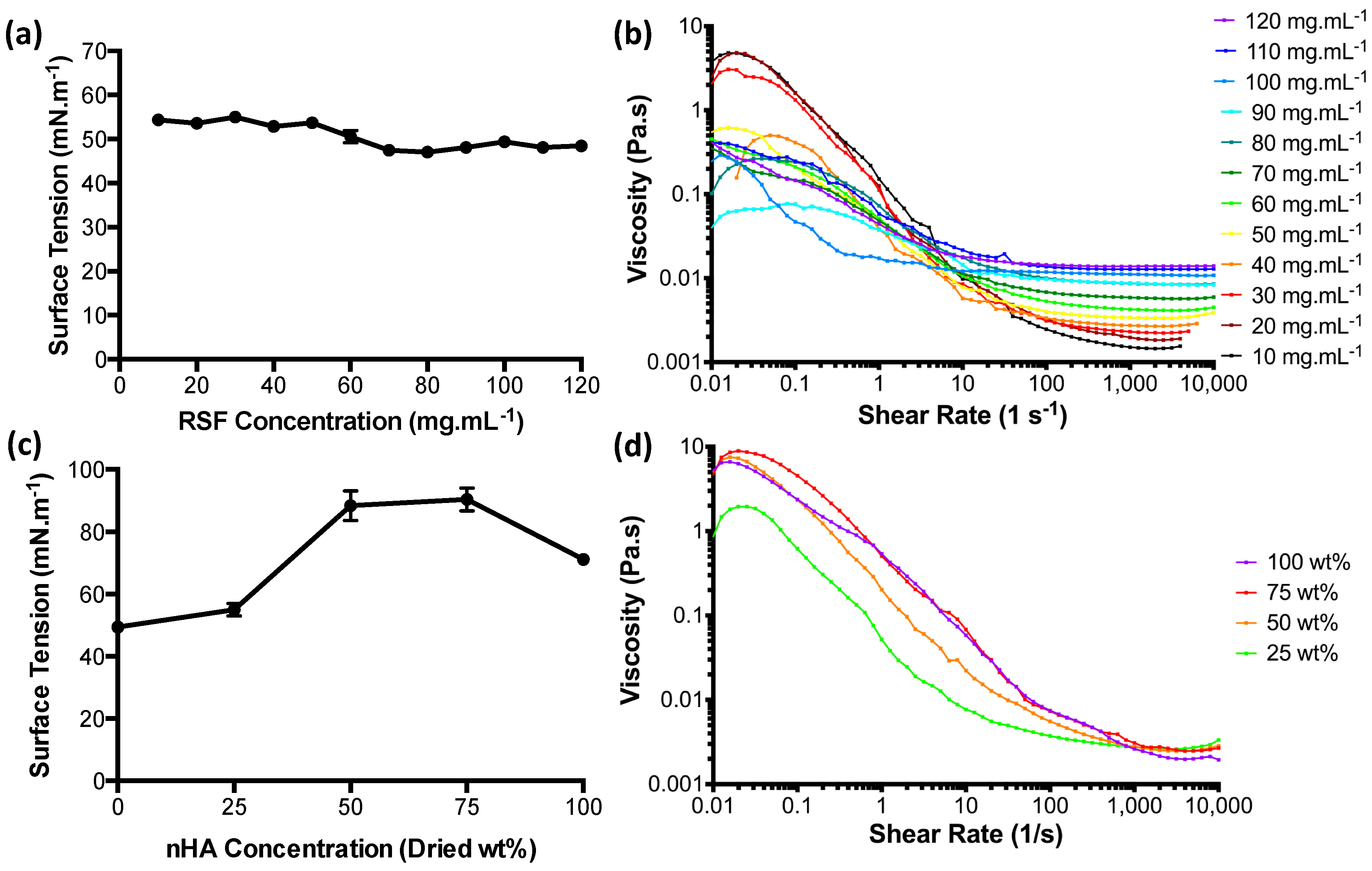
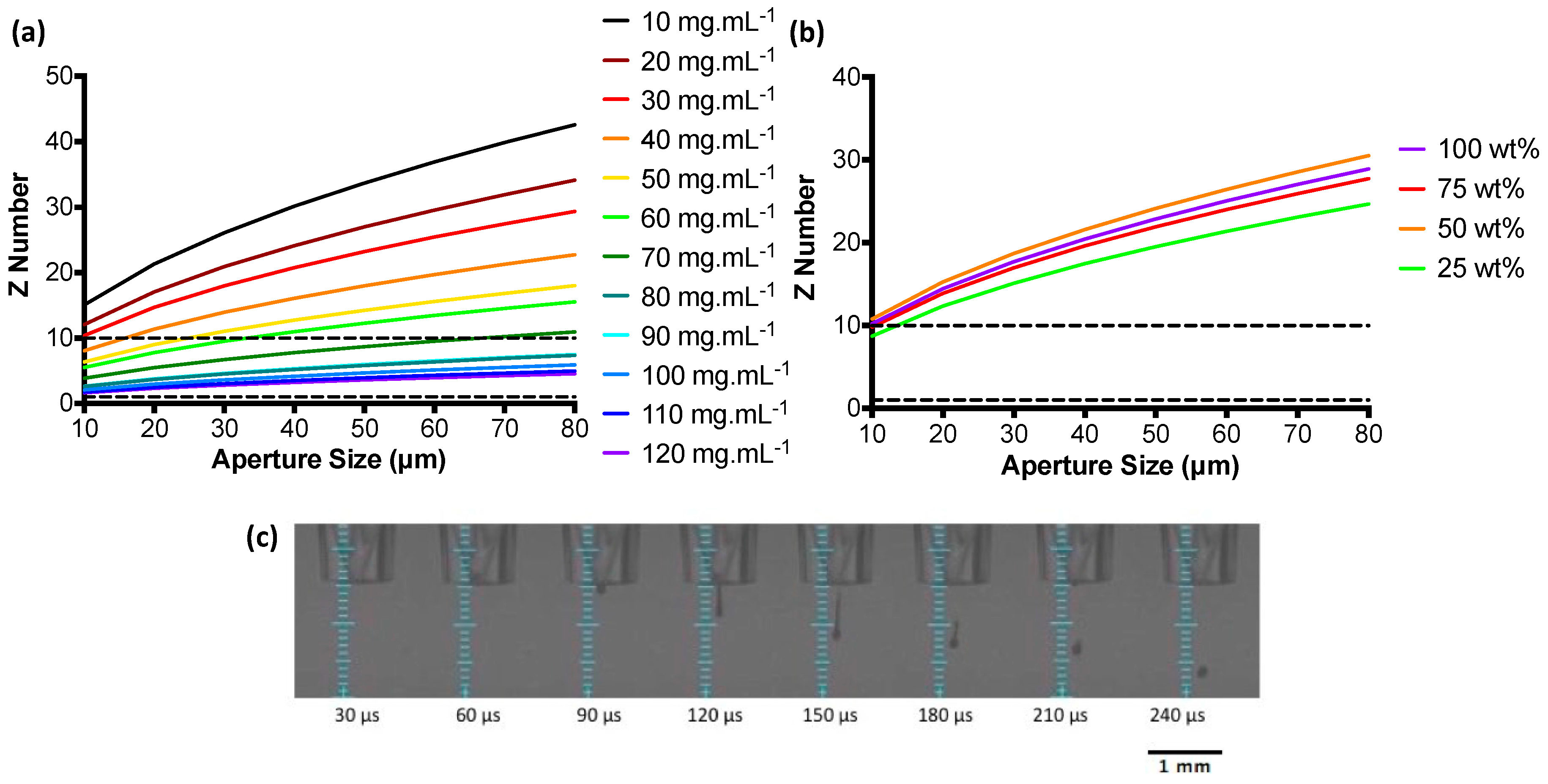
3.1. Ink Characterisation
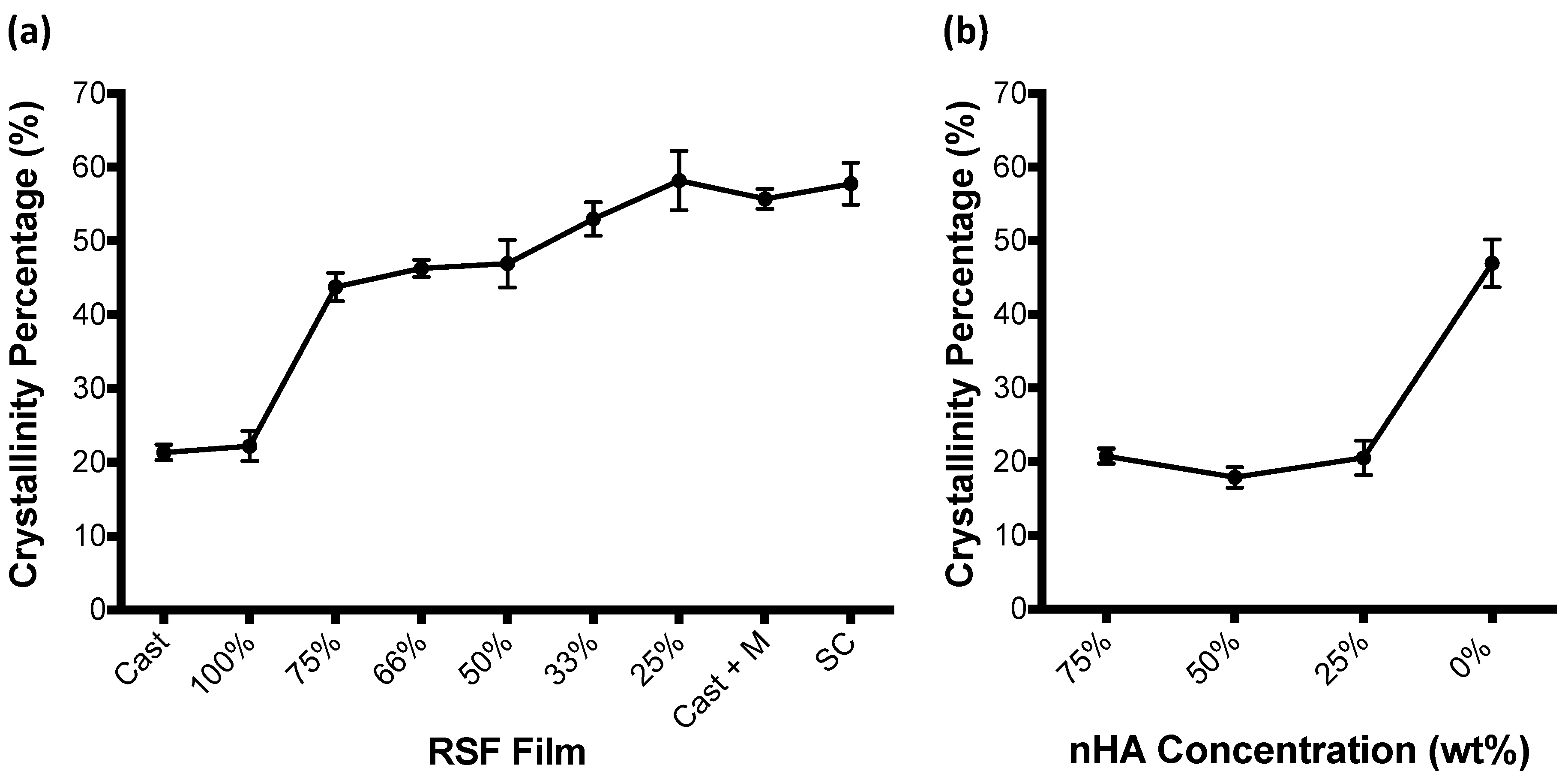
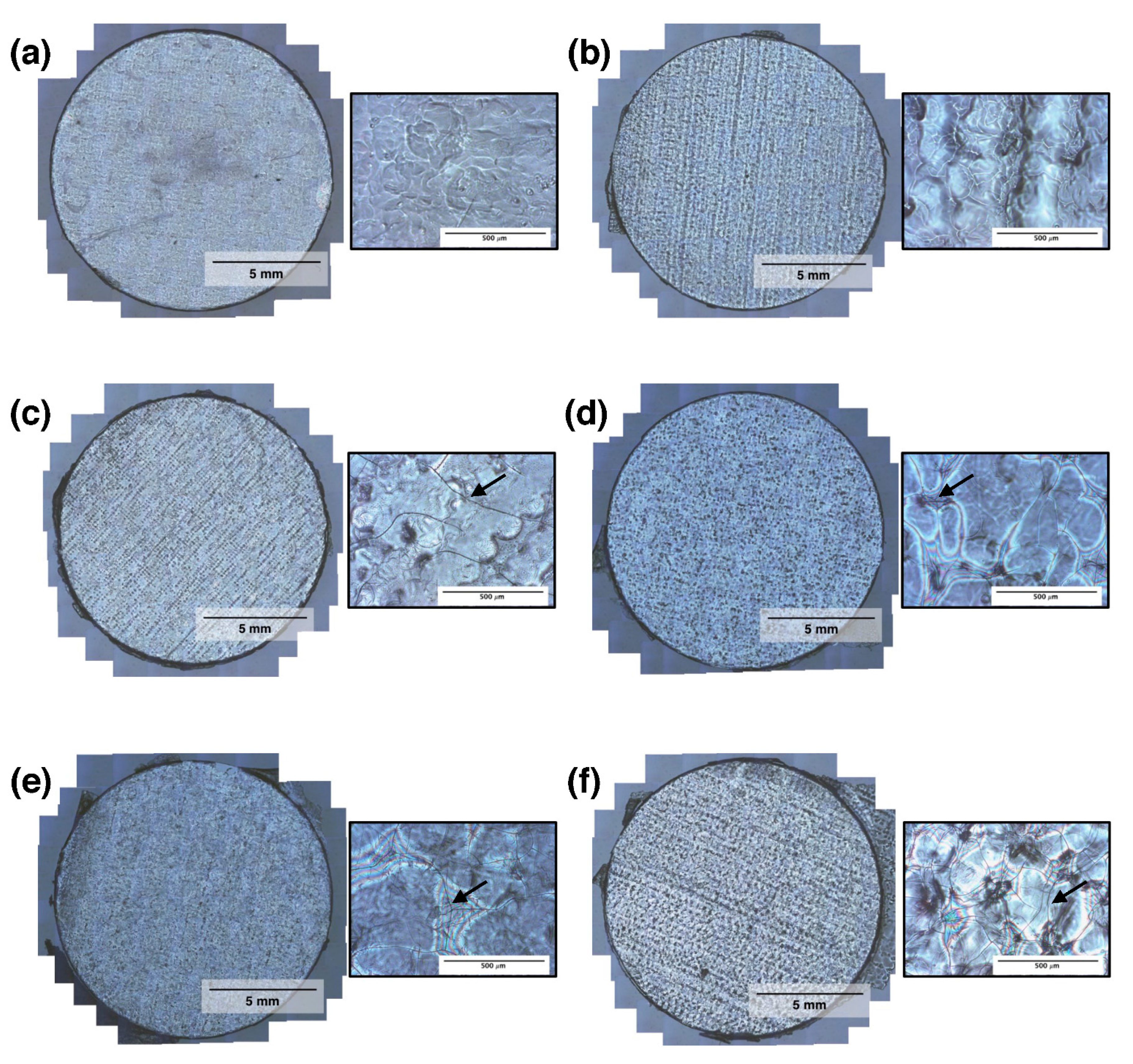
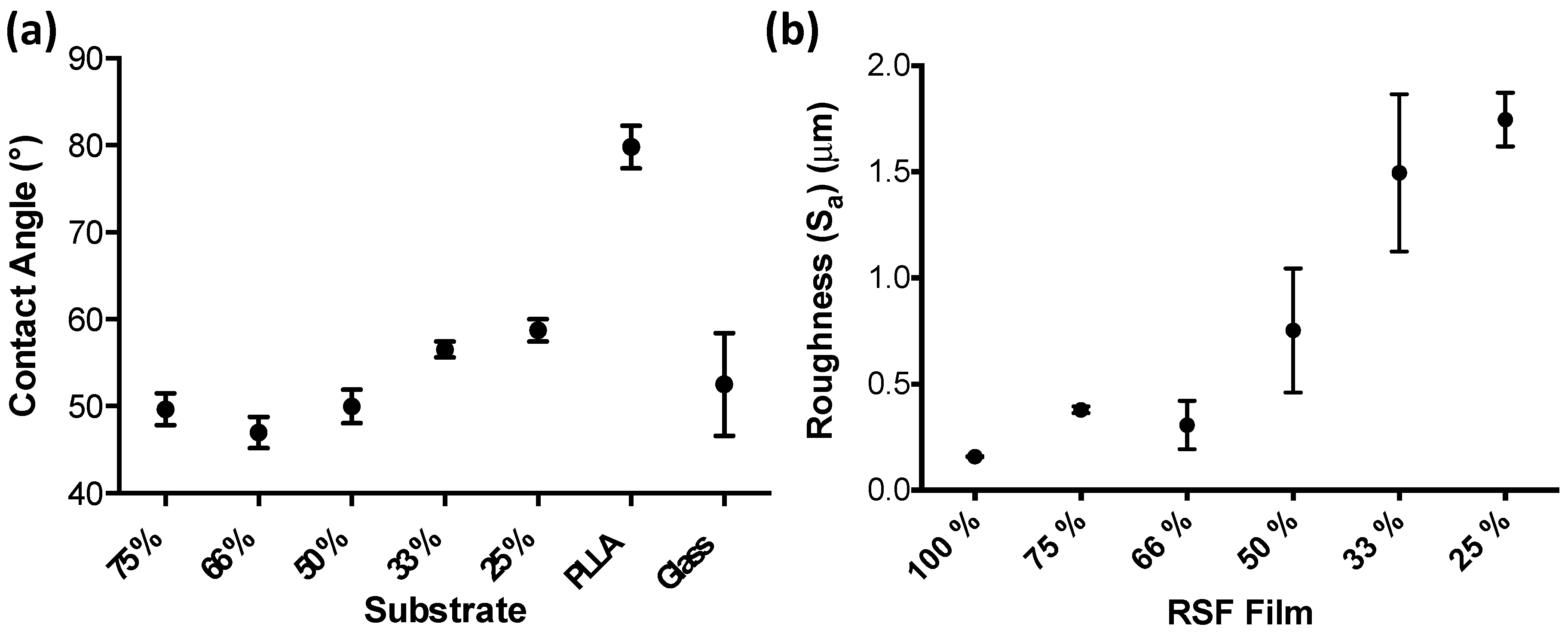
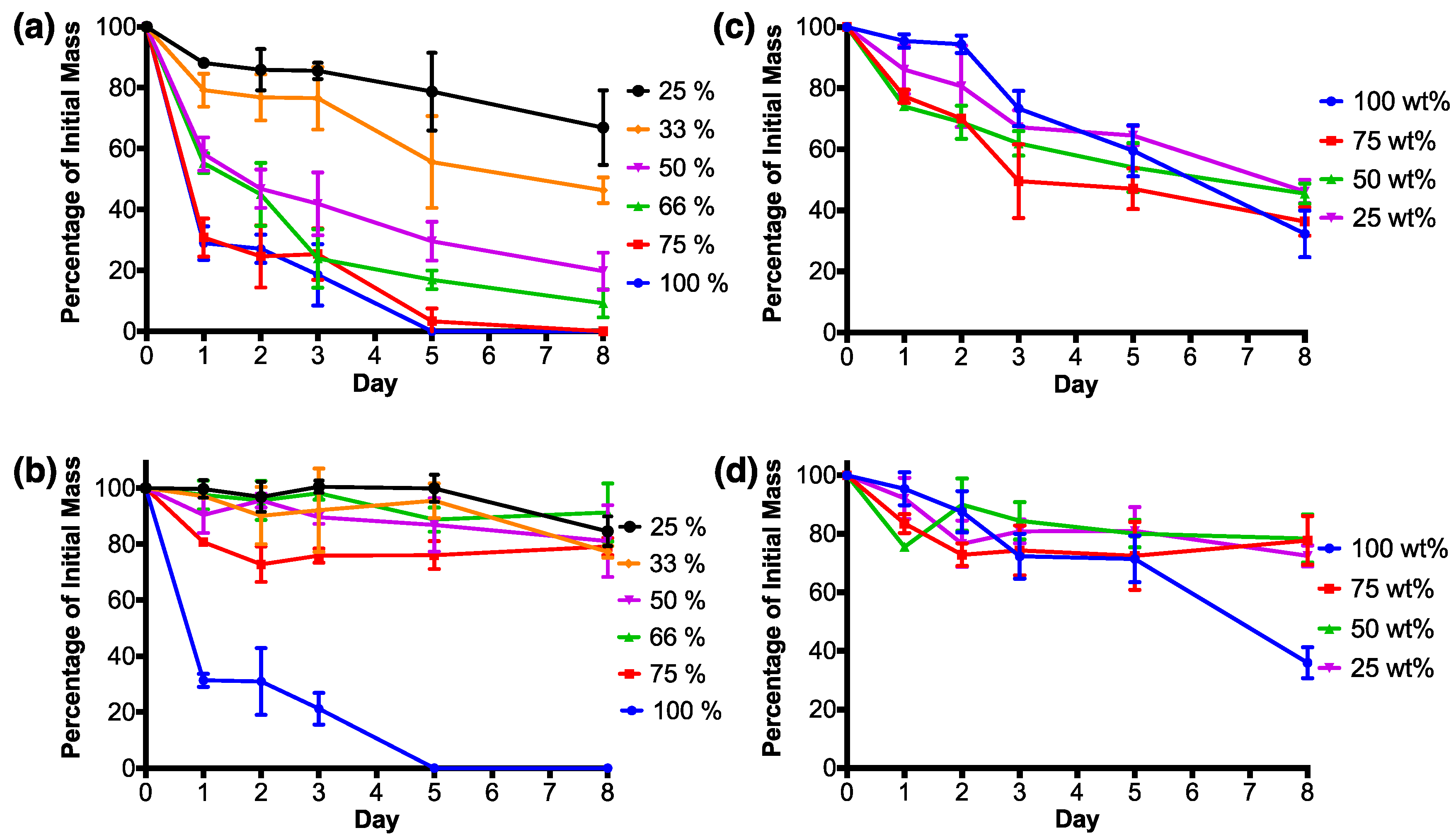
3.2. RSF Film Characterisation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Scantlebury, T.; Ambruster, J. The development of guided regeneration: Making the impossible possible and the unpredictable predictable. J. Evid. Dent. Pract. 2012, 12, 101–117. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Sharma, C.P. Review paper: Absorbable polymeric surgical sutures: Chemistry, production, properties, biodegradability, and performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ogiso, M.; Minoura, N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials 2003, 24, 357–365. [Google Scholar] [CrossRef]
- Numata, K.; Cebe, P.; Kaplan, D.L. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials 2010, 31, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Park, J.; Karageorgiou, V.; Kim, U.J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Water-stable silk films with reduced β-sheet content. Adv. Funct. Mater. 2005, 15, 1241–1247. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, B.; Li, M.; Zuo, B.; Kaplan, D.L.; Huang, Y.; Zhu, H. Degradation mechanism and control of silk fibroin. Biomacromolecules 2011, 12, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Zichner, L.; Langer, R.; Kaplan, D.L.; Vunjak-Novakovic, G. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnol. Bioeng. 2004, 88, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Kirker-Head, C.; McCool, J.; Gronowicz, G.; Zichner, L.; Langer, R.; Vunjak-Novakovic, G.; Kaplan, D.L. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005, 26, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Römer, L.M.; Scheibel, T.R. Polymeric materials based on silk proteins. Polymer 2008, 49, 4309–4327. [Google Scholar] [CrossRef] [Green Version]
- Magoshi, J.; Magoshi, Y.; Nakamura, S. Physical properties and structure of silk. VII. Crystallization of amorphous silk fibroin induced by immersion in methanol. J. Polym. Sci. Polym. Phys. 1981, 19, 185–186. [Google Scholar] [CrossRef]
- Huemmerich, D.; Slotta, U.; Scheibel, T. Processing and modification of films made from recombinant spider silk proteins. Appl. Phys. A 2006, 82, 219–222. [Google Scholar] [CrossRef]
- Greving, I.; Cai, M.; Vollrath, F.; Schniepp, H.C. Shear-induced self-assembly of native silk proteins into fibrils studied by atomic force microscopy. Biomacromolecules 2012, 13, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kaplan, D.L.; Cebe, P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Rider, P.; Zhang, Y.; Tse, C.C.W.; Zhang, Y.; Jayawardane, D.; Stringer, J.; Callaghan, J.; Brook, I.M.; Miller, C.A.; Zhao, X.; et al. Biocompatible silk fibroin scaffold prepared by reactive inkjet printing. J. Mater. Sci. 2016, 51, 8625–8630. [Google Scholar] [CrossRef]
- Yang, M.; Shuai, Y.; Zhou, G.; Mandal, N.; Zhu, L. Nucleation of hydroxyapatite on Antheraea pernyi (A. pernyi) silk fibroin film. Bio-med. Mater. Eng. 2014, 24, 731–740. [Google Scholar]
- Novotna, K.; Zajdlova, M.; Suchy, T.; Hadraba, D.; Lopot, F.; Zaloudkova, M.; Douglas, T.E.L.; Munzarova, M.; Juklickova, M.; Stranska, D.; et al. Polylactide nanofibers with hydroxyapatite as growth substrates for osteoblast-like cells. J. Biomed. Mater. Res. A 2014, 102, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Sah, M.K.; Pramanik, K. Regenerated silk fibroin from B. mori silkcocoon for tissue engineering applications. Int. J. Environ. Sci. Dev. 2010, 1, 404–408. [Google Scholar] [CrossRef]
- Ryabenkova, Y.; Pinnock, A.; Quadros, P.A.; Goodchild, R.L.; Möbus, G.; Crawford, A.; Hatton, P.V.; Miller, C.A. The relationship between particle morphology and rheological properties in injectable nano-hydroxyapatite bone graft substitutes. Mate. Sci. Eng. C 2017, 75, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Dias, M.; Da Silva, V. Production Method for Calcium Phosphate Nano-Particles with High Purity and Their Use. U.S. Patent 20,090,263,497, 22 October 2009. [Google Scholar]
- Daerr, A.; Mogne, A. Pendent_drop: An imagej plugin to measure the surface tension from an image of a pendent drop. J. Open Res. Softw. 2016, 4, e3. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Stalder, A.F.; Kulik, G.; Sage, D.; Barbieri, L.; Hoffmann, P. A snake-based approach to accurate determination of both contact points and contact angles. Colloids Surf. A 2006, 286, 92–103. [Google Scholar] [CrossRef]
- Pritchard, E.M.; Hu, X.; Finley, V.; Kuo, C.K.; Kaplan, D.L. Effect of silk protein processing on drug delivery from silk films. Macromol. Biosci. 2013, 13, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Petrova, T.; Dooley, R.B. Revised release on surface tension of ordinary water substance. In Proceedings of the International Association for the Properties of Water and Steam, Moscow, Russia, 23–27 June 2014. [Google Scholar]
- Derby, B. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet printing as a deposition and patterning tool for polymers and inorganic particles. Soft Matter 2008, 4, 703–713. [Google Scholar] [CrossRef]
- Won, Y.S.; Chung, D.K.; Mills, A.F. Density, viscosity, surface tension, and carbon dioxide solubility and diffusivity of methanol, ethanol, aqueous propanol, and aqueous ethylene glycol at 25 °C. J. Chem. Eng. Data 1981, 26, 140–141. [Google Scholar] [CrossRef]
- Rider, P.; Brook, I.M.; Smith, P.J.; Miller, C.A. Chapter 7: Reactive inkjet printing of silk barrier membranes for dental applications. In Reactive Inkjet Printing; Smith, P.J., Morrin, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 147–168. [Google Scholar]
- Dong, H.; Carr, W.W.; Morris, J.F. An experimental study of drop-on-demand drop formation. Phys. Fluids 2006, 18, 072102. [Google Scholar] [CrossRef]
- Bhat, P.P.; Appathurai, S.; Harris, M.T.; Pasquali, M.; McKinley, G.H.; Basaran, O.A. Formation of beads-on-a-string structures during break-up of viscoelastic filaments. Nat. Phys. 2010, 6, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Vadillo, D.C.; Tuladhar, T.R.; Mulji, A.C.; Jung, S.; Hoath, S.D.; Mackley, M.R. Evaluation of the inkjet fluid’s performance using the “Cambridge Trimaster” filament stretch and break-up device. J. Rheol. 2010, 54, 261. [Google Scholar] [CrossRef]
- Yang, Y.; Dicko, C.; Bain, C.D.; Gong, Z.; Jacobs, R.M.J.; Shao, Z.; Terry, A.E.; Vollrath, F. Behavior of silk protein at the air-water interface. Soft Matter 2012, 8, 9705–9712. [Google Scholar] [CrossRef]
- Reis, N.; Ainsley, C.; Derby, B. Ink-jet delivery of particle suspensions by piezoelectric droplet ejectors. J. Appl. Phys. 2005, 97, 094903. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, C. Generation of inkjet droplet of non-Newtonian fluid. Rheol. Acta 2013, 52, 313–325. [Google Scholar] [CrossRef]
- Rider, P.M. Reactive Inkjet Printing of Novel Silk Dental Barrier Membranes. Ph.D. Thesis, The University of Sheffield, March, UK, September 2017. [Google Scholar]
- Yamane, T.; Umemura, K.; Nakazawa, Y.; Asakura, T. Molecular dynamics simulation of conformational change of poly(ala-gly) from silk I to silk II in relation to fiber formation mechanism of bombyxmorisilk fibroin. Macromolecules 2003, 36, 6766–6772. [Google Scholar] [CrossRef]
- Wilson, D.; Valluzzi, R.; Kaplan, D.L. Conformational transitions in model silk peptides. Biophys. J. 2000, 78, 2690–2701. [Google Scholar] [CrossRef]
- Ha, S.-W.; Gracz, H.S.; Tonelli, A.E.; Hudson, S.M. Structural study of irregular amino acid sequences in the heavy chain of bombyxmorisilk fibroin. Biomacromolecules 2005, 6, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rider, P.M.; Brook, I.M.; Smith, P.J.; Miller, C.A. Reactive Inkjet Printing of Regenerated Silk Fibroin Films for Use as Dental Barrier Membranes. Micromachines 2018, 9, 46. https://doi.org/10.3390/mi9020046
Rider PM, Brook IM, Smith PJ, Miller CA. Reactive Inkjet Printing of Regenerated Silk Fibroin Films for Use as Dental Barrier Membranes. Micromachines. 2018; 9(2):46. https://doi.org/10.3390/mi9020046
Chicago/Turabian StyleRider, Patrick M., Ian. M. Brook, Patrick J. Smith, and Cheryl A. Miller. 2018. "Reactive Inkjet Printing of Regenerated Silk Fibroin Films for Use as Dental Barrier Membranes" Micromachines 9, no. 2: 46. https://doi.org/10.3390/mi9020046