Optofluidics Refractometers
Abstract
:1. Introduction
2. RI Sensing and Technologies
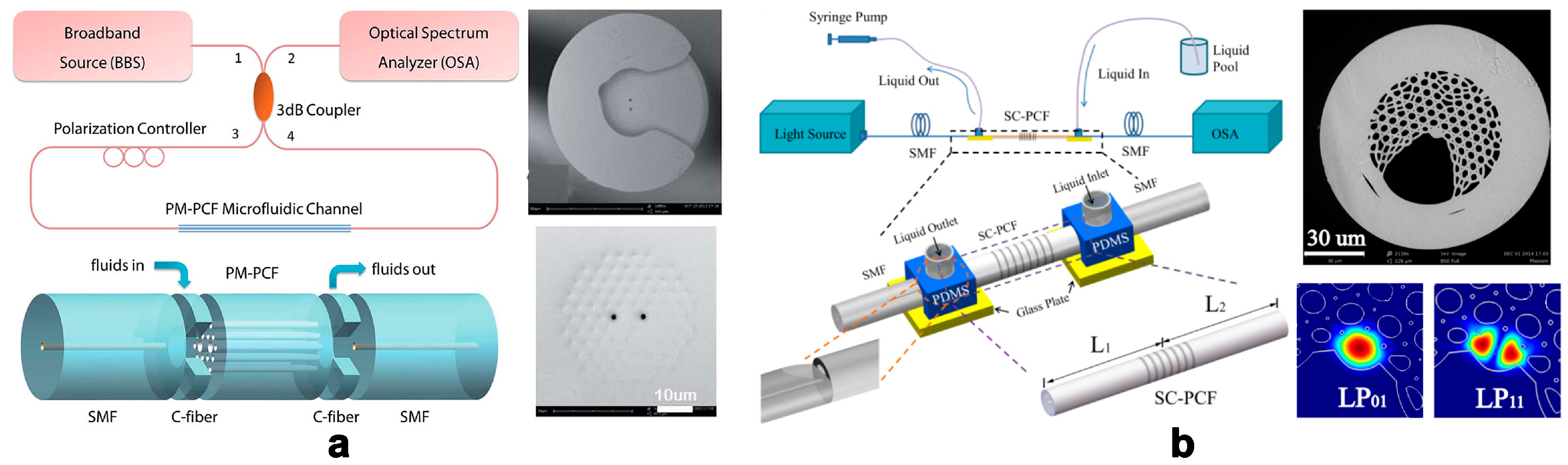
2.1. Photonic Crystal Fibers
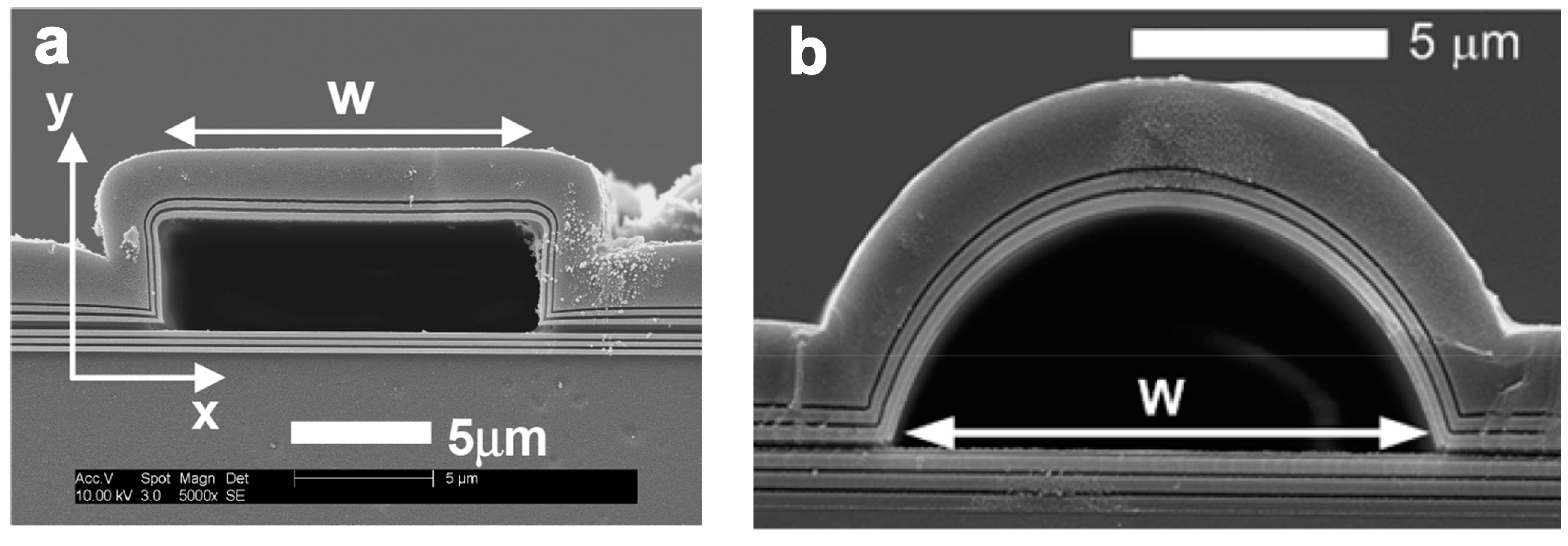
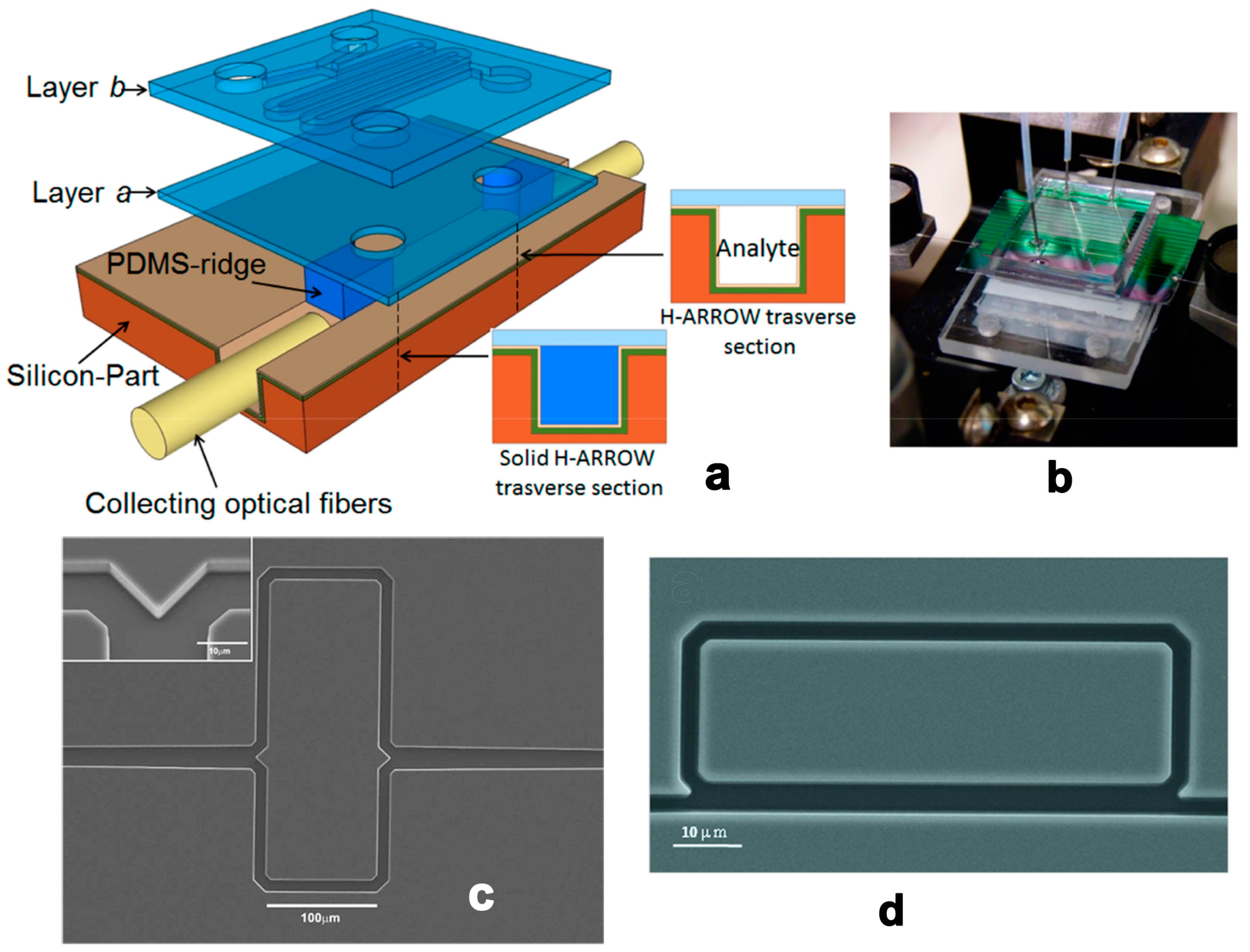
2.2. Planar Optical Waveguides
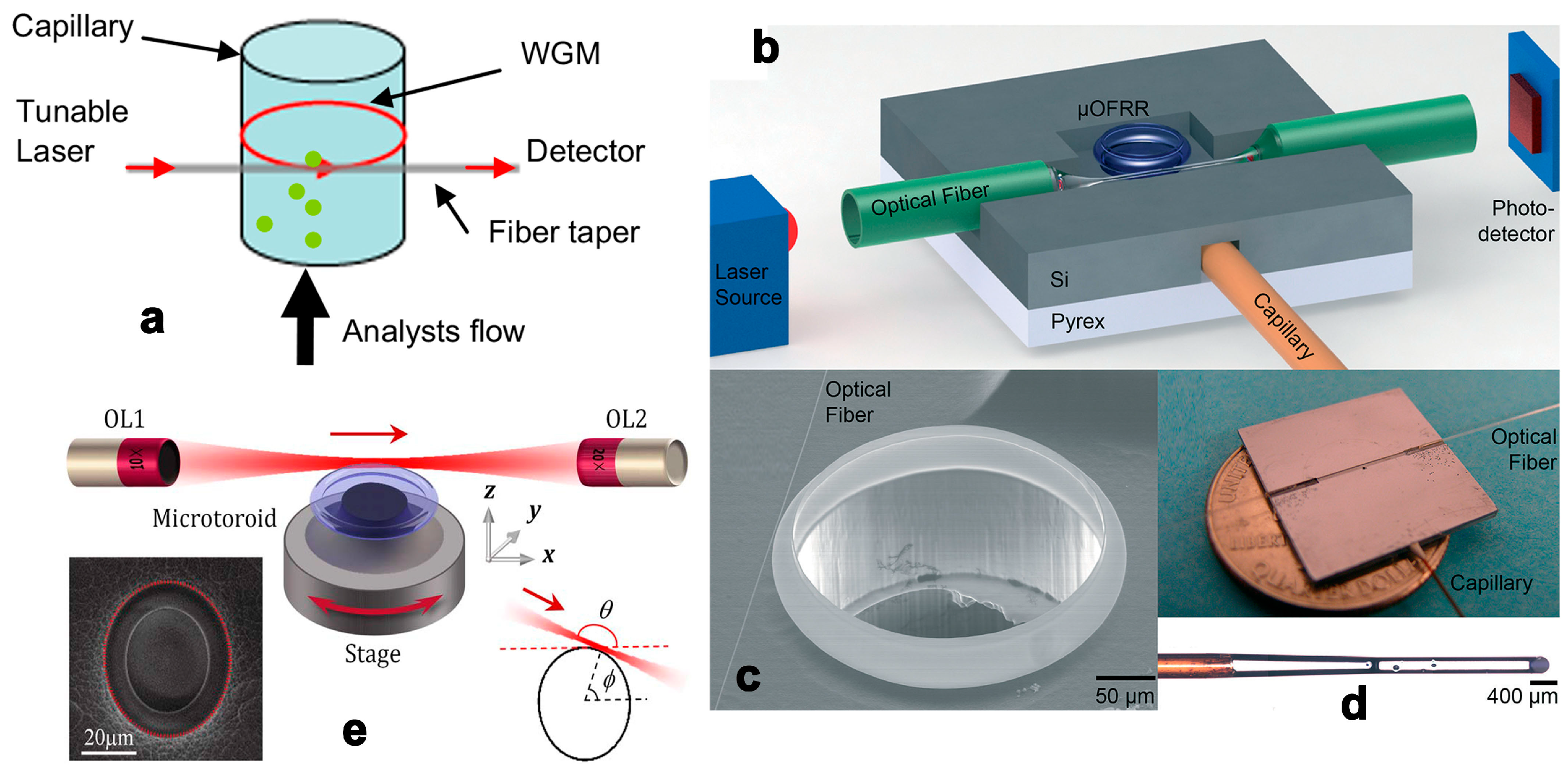
2.3. Whisper Gallery Mode
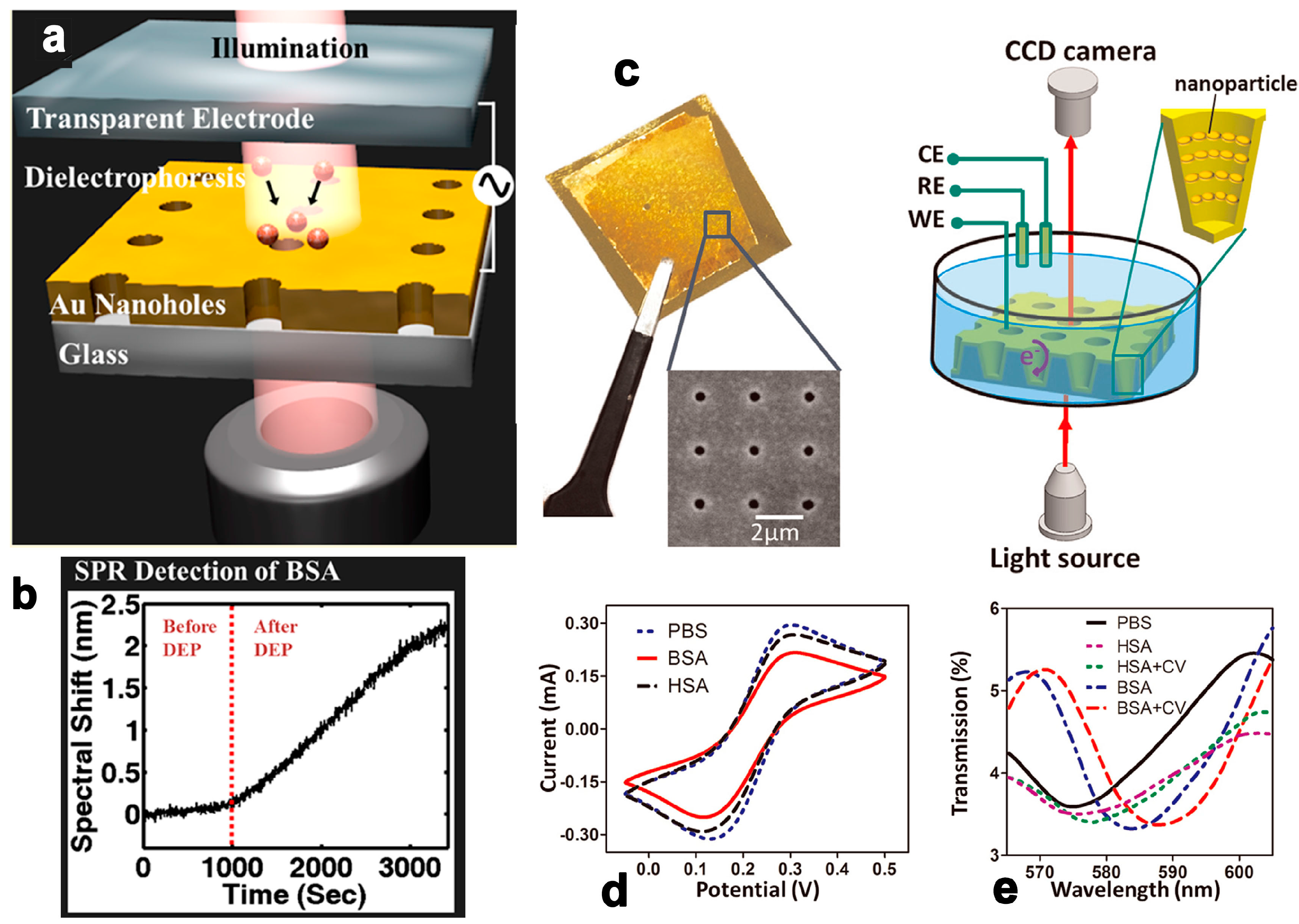
2.4. Surface Plasmon Resonance and Localized Surface Plasmon Resonance
3. Discussion and Outlook
3.1. Parameters for Sensor Characterization
3.2. New Areas for Exploration: Volume Sensing
3.3. Advanced Methods for Performance Enhancement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rotiroti, L.; De Stefano, L.; Rendina, I.; Moretti, L.; Rossi, A.M.; Piccolo, A. Optical microsensors for pesticides identification based on porous silicon technology. Biosens. Bioelectron. 2005, 20, 2136–2139. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Byun, J.-Y.; Yim, S.-Y.; Kim, M.-G. A localized surface plasmon resonance (LSPR)-based, simple, receptor-free and regeneratable Hg2+ detection system. J. Hazard. Mater. 2016, 307, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Melo, L.; Davies, B.; Fadaei, H.; Sinton, D.; Wild, P. Detecting supercritical CO2 in brine at sequestration pressure with an optical fiber sensor. Environ. Sci. Technol. 2012, 47, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Huang, W.; Chin, L.; Lei, L.; Lin, Z.; Ser, W.; Chen, H.; Ayi, T.; Yap, P.; Chen, C. Droplet optofluidic imaging for λ-bacteriophage detection via co-culture with host cell escherichia coli. Lab Chip 2014, 14, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chin, L.; Ser, W.; Ayi, T.; Yap, P.; Bourouina, T.; Leprince-Wang, Y. An optofluidic imaging system to measure the biophysical signature of single waterborne bacteria. Lab Chip 2014, 14, 4237–4243. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Chin, L.K.; Ser, W.; Chen, H.F.; Hsieh, C.M.; Lee, C.H.; Sung, K.B.; Ayi, T.C.; Yap, P.H.; Liedberg, B.; et al. Cell refractive index for cell biology and disease diagnosis: Past, present and future. Lab Chip 2016, 16, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Kaler, K.V.; Prakash, R. Droplet microfluidics for chip-based diagnostics. Sensors 2014, 14, 23283–23306. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.B. Digital microfluidics: Is a true lab-on-a-chip possible? Microfluid. Nanofluid. 2007, 3, 245–281. [Google Scholar] [CrossRef]
- Yang, W.; Luo, C.; Lai, L.; Ouyang, Q. A novel microfluidic platform for studying mammalian cell chemotaxis in different oxygen environments under zero-flow conditions. Biomicrofluidics 2015, 9, 044121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Zhang, Z.; Fouladdel, S.; Deol, Y.; Ingram, P.N.; McDermott, S.P.; Azizi, E.; Wicha, M.S.; Yoon, E. Single cell dual adherent-suspension co-culture micro-environment for studying tumor–stromal interactions with functionally selected cancer stem-like cells. Lab Chip 2016, 16, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Cheng, Y.-H.; Kim, H.S.; Ingram, P.N.; Nor, J.E.; Yoon, E. Paired single cell co-culture microenvironments isolated by two-phase flow with continuous nutrient renewal. Lab Chip 2014, 14, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Chang, W.H.; Wang, C.H.; Wang, J.H.; Mai, J.D.; Lee, G.B. Nucleic acid amplification using microfluidic systems. Lab Chip 2013, 13, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, X.; Luo, S.; Liu, B.; Yang, C. Development of DNA-based signal amplification and microfluidic technology for protein assay: A review. TrAC Trends Anal. Chem. 2016, 80, 132–148. [Google Scholar] [CrossRef]
- Domachuk, P.; Littler, I.C.M.; Cronin-Golomb, M.; Eggleton, B.J. Compact resonant integrated microfluidic refractometer. Appl. Phys. Lett. 2006, 88, 093513. [Google Scholar] [CrossRef]
- Bitarafan, M.H.; DeCorby, R.G. On-chip high-finesse fabry-perot microcavities for optical sensing and quantum information. Sensors 2017, 17, 1748. [Google Scholar] [CrossRef] [PubMed]
- Trichet, A.A.; Foster, J.; Omori, N.E.; James, D.; Dolan, P.R.; Hughes, G.M.; Vallance, C.; Smith, J.M. Open-access optical microcavities for lab-on-a-chip refractive index sensing. Lab Chip 2014, 14, 4244–4249. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Chen, H.M.; Freeman, L.M.; Fainman, Y. Optofluidic devices and applications in photonics, sensing and imaging. Lab Chip 2012, 12, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Sinton, D.; Psaltis, D. Optofluidics for energy applications. Nat. Photonics 2011, 5, 583–590. [Google Scholar] [CrossRef]
- Schmidt, H.; Hawkins, A.R. The photonic integration of non-solid media using optofluidics. Nat. Photonics 2011, 5, 598–604. [Google Scholar] [CrossRef]
- Monat, C.; Domachuk, P.; Eggleton, B. Integrated optofluidics: A new river of light. Nat. Photonics 2007, 1, 106–114. [Google Scholar] [CrossRef]
- Song, W.; Zhang, X.; Liu, A.; Lim, C.; Yap, P.; Hosseini, H.M.M. Refractive index measurement of single living cells using on-chip fabry-pérot cavity. Appl. Phys. Lett. 2006, 89, 203901. [Google Scholar] [CrossRef]
- Bates, K.E.; Lu, H. Optics-integrated microfluidic platforms for biomolecular analyses. Biophys. J. 2016, 110, 1684–1697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Stratton, Z.S.; Guo, F.; Lapsley, M.I.; Chan, C.Y.; Lin, S.C.; Huang, T.J. Optofluidic imaging: Now and beyond. Lab Chip 2013, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.C.; Lim, S.P.; Lee, H.P. Optofluidic variable-focus lenses for light manipulation. Lab Chip 2012, 12, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- La Notte, M.; Troia, B.; Muciaccia, T.; Campanella, C.E.; De Leonardis, F.; Passaro, V.M. Recent advances in gas and chemical detection by vernier effect-based photonic sensors. Sensors 2014, 14, 4831–4855. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photonics 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Cubillas, A.M.; Unterkofler, S.; Euser, T.G.; Etzold, B.J.; Jones, A.C.; Sadler, P.J.; Wasserscheid, P.; Russell, P.S.J. Photonic crystal fibres for chemical sensing and photochemistry. Chem. Soc. Rev. 2013, 42, 8629–8648. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tian, F.; Zhu, Y.; Lavlinskaia, N.; Du, H. Long-period gratings in photonic crystal fiber as an optofluidic label-free biosensor. Biosens. Bioelectron. 2011, 26, 4774–4778. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tse, M.L.; Liu, Z.; Guan, B.O.; Zhang, A.P.; Lu, C.; Tam, H.Y. In-line microfluidic integration of photonic crystal fibres as a highly sensitive refractometer. Analyst 2014, 139, 5422–5429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Humbert, G.; Wu, Z.; Li, K.; Shum, P.P.; Zhang, N.M.; Cui, Y.; Auguste, J.L.; Dinh, X.Q.; Wei, L. In-line optofluidic refractive index sensing in a side-channel photonic crystal fiber. Opt. Express 2016, 24, 27674–27682. [Google Scholar] [CrossRef] [PubMed]
- Kozma, P.; Kehl, F.; Ehrentreich-Forster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Schmidt, H.; Barber, J.P.; Lunt, E.J.; Hawkins, A.R. Optical characterization of arch-shaped arrow waveguides with liquid cores. Opt. Express 2005, 13, 10564–10570. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Persichetti, G.; Sarro, P.M.; Bernini, R. A hybrid silicon-pdms optofluidic platform for sensing applications. Biomed. Opt. Express 2014, 5, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Persichetti, G.; Bernini, R. Liquid core arrow waveguides: A promising photonic structure for integrated optofluidic microsensors. Micromachines 2016, 7, 47. [Google Scholar] [CrossRef]
- Testa, G.; Huang, Y.; Sarro, P.M.; Zeni, L.; Bernini, R. High-visibility optofluidic mach-zehnder interferometer. Opt. Lett. 2010, 35, 1584–1586. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Huang, Y.; Sarro, P.M.; Zeni, L.; Bernini, R. Integrated silicon optofluidic ring resonator. Appl. Phys. Lett. 2010, 97, 131110. [Google Scholar] [CrossRef]
- Dolatabady, A.; Asgari, S.; Granpayeh, N. Tunable mid-infrared nanoscale graphene-based refractive index sensor. IEEE Sens. J. 2017, 18, 569–574. [Google Scholar] [CrossRef]
- Tan, Y.; He, R.; Cheng, C.; Wang, D.; Chen, Y.; Chen, F. Polarization-dependent optical absorption of MoS2 for refractive index sensing. Sci. Rep. 2014, 4, 7523. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Braun, D.; Libchaber, A.; Khoshsima, M.; Teraoka, I.; Arnold, S. Protein detection by optical shift of a resonant microcavity. Appl. Phys. Lett. 2002, 80, 4057–4059. [Google Scholar] [CrossRef]
- Zhi, Y.; Yu, X.C.; Gong, Q.; Yang, L.; Xiao, Y.F. Single nanoparticle detection using optical microcavities. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, X.; Xu, M.; Chen, Z.; Fan, X. Optofluidic glucose detection by capillary-based ring resonators. Opt. Laser Technol. 2014, 56, 12–14. [Google Scholar] [CrossRef]
- Scholten, K.; Fan, X.; Zellers, E.T. A microfabricated optofluidic ring resonator for sensitive, high-speed detection of volatile organic compounds. Lab Chip 2014, 14, 3873–3880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Wang, L.; Li, Z.Y.; Li, Y.; Gong, Q.; Xiao, Y.F. Free-space coupling efficiency in a high-q deformed optical microcavity. Opt. Lett. 2016, 41, 4437–4440. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Roh, S.; Park, J. Current status of micro-and nano-structured optical fiber sensors. Opt. Fiber Technol. 2009, 15, 209–221. [Google Scholar] [CrossRef]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Otto, L.M.; Yoo, D.; Jose, J.; Johnson, T.W.; Oh, S.H. Dielectrophoresis-enhanced plasmonic sensing with gold nanohole arrays. Nano Lett. 2014, 14, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lehman, S.E.; Schulmerich, M.V.; Le, A.P.; Lee, T.W.; Gray, S.K.; Bhargava, R.; Nuzzo, R.G. Refractive index sensing and surface-enhanced raman spectroscopy using silver-gold layered bimetallic plasmonic crystals. Beilstein J. Nanotechnol. 2017, 8, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lu, Y.; Jiang, J.; Zhang, Q.; Yao, Y.; Wang, P.; Chen, B.; Cheng, Q.; Liu, G.L.; Liu, Q. Nanoplasmonic biosensor: Coupling electrochemistry to localized surface plasmon resonance spectroscopy on nanocup arrays. Biosens. Bioelectron. 2015, 67, 237–242. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; Fan, X. On the performance quantification of resonant refractive index sensors. Opt. Express 2008, 16, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.J.; Chang, S.-H.; Schatz, G.C.; Van Duyne, R.P.; Wiley, B.J.; Xia, Y. Localized surface plasmon resonance spectroscopy of single silver nanocubes. Nano Lett. 2005, 5, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Jian, A.; Zhang, X.; Zhu, W.; Yu, M. Optofluidic refractometer using resonant optical tunneling effect. Biomicrofluidics 2010, 4, 043008. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.; Liu, A.; Lim, C.; Lin, C.; Ayi, T.; Yap, P. An optofluidic volume refractometer using fabry–pérot resonator with tunable liquid microlenses. Biomicrofluidics 2010, 4, 024107. [Google Scholar] [CrossRef] [PubMed]
- St-Gelais, R.; Masson, J.; Peter, Y.-A. All-silicon integrated fabry–pérot cavity for volume refractive index measurement in microfluidic systems. Appl. Phys. Lett. 2009, 94, 243905. [Google Scholar] [CrossRef]
- Trichet, A.A.; Dolan, P.R.; James, D.; Hughes, G.M.; Vallance, C.; Smith, J.M. Nanoparticle trapping and characterization using open microcavities. Nano Lett. 2016, 16, 6172–6177. [Google Scholar] [CrossRef] [PubMed]
- Mader, M.; Reichel, J.; Hänsch, T.W.; Hunger, D. A scanning cavity microscope. Nat. Commun. 2015, 6, 7249. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.K.; Liu, A.Q.; Lim, C.S.; Zhang, X.M.; Ng, J.H.; Hao, J.Z.; Takahashi, S. Differential single living cell refractometry using grating resonant cavity with optical trap. Appl. Phys. Lett. 2007, 91, 243901. [Google Scholar] [CrossRef]
- Chin, L.K.; Liu, A.Q.; Lim, C.S.; Lin, C.L.; Ayi, T.C.; Yap, P.H. An optofluidic volume refractometer using fabry-perot resonator with tunable liquid microlenses. Biomicrofluidics 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dostalek, J.; Knoll, W. Magnetic nanoparticle-enhanced biosensor based on grating-coupled surface plasmon resonance. Anal. Chem. 2011, 83, 6202–6207. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; Liu, L.; Fan, X.; Xu, L. Self-referencing optofluidic ring resonator sensor for highly sensitive biomolecular detection. Anal. Chem. 2013, 85, 9328–9332. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-F.; He, L.; Zhu, J.; Yang, L. Electromagnetically induced transparency-like effect in a single polydimethylsiloxane-coated silica microtoroid. Appl. Phys. Lett. 2009, 94, 231115. [Google Scholar] [CrossRef]
- Lu, H.; Liu, X.; Mao, D.; Wang, G. Plasmonic nanosensor based on Fano resonance in waveguide-coupled resonators. Opt. Lett. 2012, 37, 3780–3782. [Google Scholar] [CrossRef] [PubMed]
| Working Principle | Sensitivity | Q Factor | FOM | Detection Limit | Analyte | Reference |
|---|---|---|---|---|---|---|
| PCF | 8699 nm/RIU | - | - | 4.0 × 10−6 RIU | - | [30] |
| PCF | 1145 nm/RIU | - | - | - | - | [31] |
| POW | 260 nm/RIU | 800 | - | - | - | [37] |
| POW | 1920 nm/RIU | - | - | 5.2 × 10−7 RIU | - | [38] |
| WGM | 1.84 pm/mM | 4 × 105 | - | 0.035 mM | Glucose | [42] |
| WGM | 0.018 pm/mg m−3 | 11,500 | - | 6.9 ppm | Benzene | [43] |
| SPR | - | - | - | 1 pM | BSA | [47] |
| SPR | ~104 nm/RIU | - | - | - | - | [49] |
| FPC | 960 nm/RIU | 600 | 18.79 | 0.01 RIU | - | [53] |
| FPC | 907 nm/RIU | 400 | 9 | 1.7 × 10−5 RIU | - | [54] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Bai, G.; Zhang, Y.; Zhang, M.; Jian, A. Optofluidics Refractometers. Micromachines 2018, 9, 136. https://doi.org/10.3390/mi9030136
Li C, Bai G, Zhang Y, Zhang M, Jian A. Optofluidics Refractometers. Micromachines. 2018; 9(3):136. https://doi.org/10.3390/mi9030136
Chicago/Turabian StyleLi, Cheng, Gang Bai, Yunxiao Zhang, Min Zhang, and Aoqun Jian. 2018. "Optofluidics Refractometers" Micromachines 9, no. 3: 136. https://doi.org/10.3390/mi9030136





