Light Manipulation in Inhomogeneous Liquid Flow and Its Application in Biochemical Sensing
Abstract
:1. Introduction
2. Fundamental Concepts and Principles
2.1. The Reynolds Number
2.2. The Dean Number
2.3. The Convective–Diffusive Transport
2.4. The Péclet Number
3. Manipulation of the Liquid-Liquid Interface
3.1. Hydrodynamic Focusing
3.2. Dean Flow
4. Manipulation of the Gradient Refractive Index
4.1. Liquid Diffusion and Transformation Optics
4.2. Heat Conduction
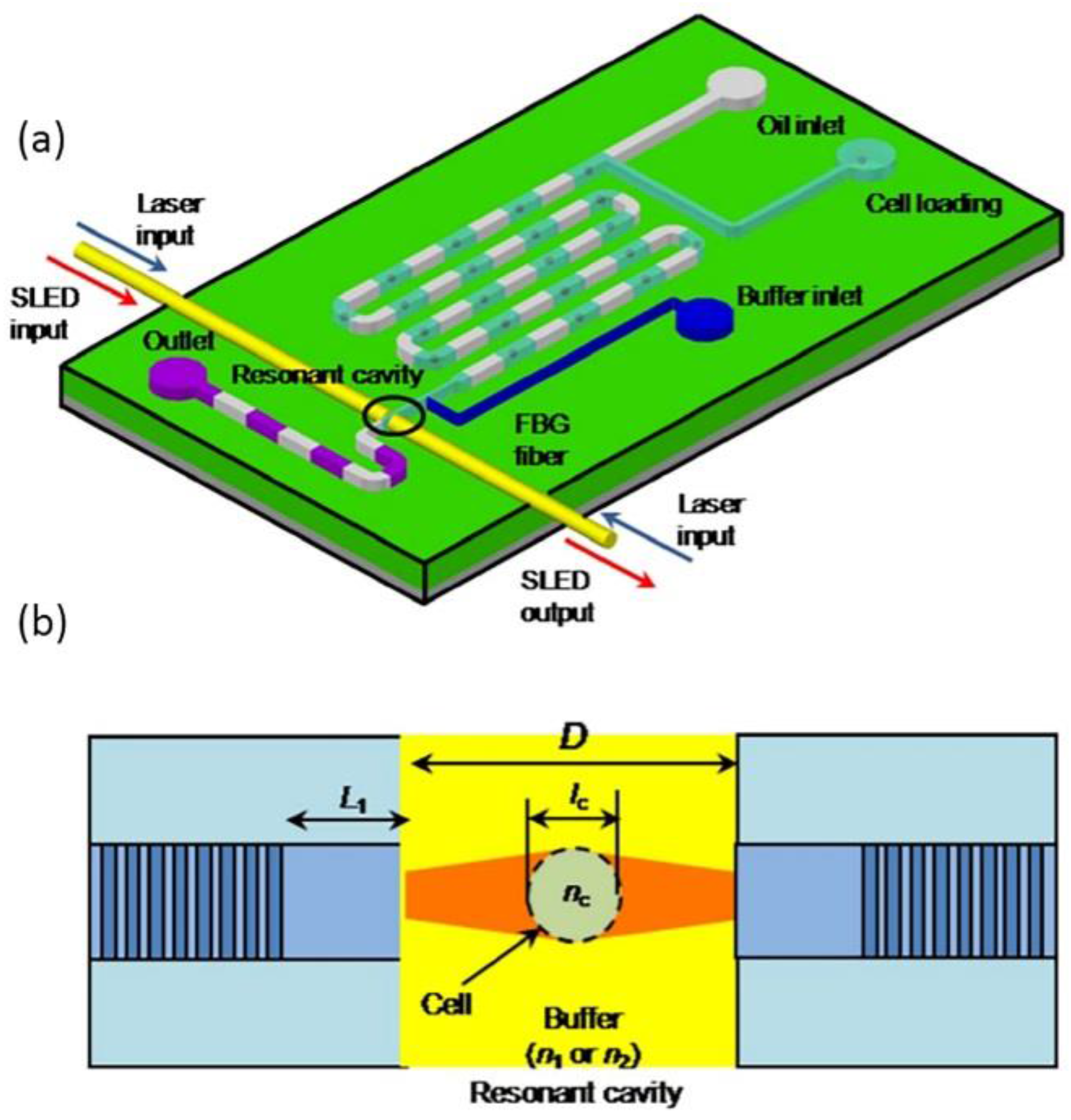
5. Application in Biochemical Sensing
6. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Erickson, D.; Heng, X.; Li, Z.Y.; Rockwood, T.; Emery, T.; Zhang, Z.Y.; Scherer, A.; Yang, C.H.; Psaltis, D. Optofluidics. Proc. SPIE 2005, 5908, 59080S-1. [Google Scholar]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.D.; Wang, B.J.; Wang, W. Biochemical sensing by nanofluidic crystal in a confined space. Lab Chip 2016, 16, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, X.M.; Wang, Y.; Yu, W.X.; Chan, H.L. Microfluidic reactors for photocatalytic water purification. Lab Chip 2014, 14, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.W.; Liu, J.; Yang, Q.J.; Liu, Y.; Zhu, Y.J.; Li, T.H.; Tsang, Y.H.; Zhang, X.M. Microfluidic chip-based one-step fabrication of artificial photosystem I for photocatalytic cofactor regeneration. RSC Adv. 2016, 6, 101974–101980. [Google Scholar] [CrossRef]
- Shui, L.L.; Pennathur, S.; Eijkel, J.C.T. Multiphase flow in lab on chip devices: A real tool for the future. Lab Chip 2008, 8, 1010–1014. [Google Scholar] [PubMed]
- Xie, Y.B.; Bos, D.; Vreede, L.J.D.; Boer, H.L.D.; Meulen, M.J.V.D.; Versluis, M.; Sprenkels, A.J.; Berg, A.V.D.; Eijkel, J.C.T. High-efficiency ballistic electrostatic generator using microdroplets. Nat. Commun. 2014, 5, 3575. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Tse, H.T.K.; Lee, W.; Amini, H.; Carlo, D.D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Tse, H.T.K.; Lee, S.A.; Ying, Y.; Lindgen, A.G.; Yang, O.O.; Rao, J.Y.; Clark, A.T.; Carlo, D.D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl. Acad. Sci. USA 2012, 109, 7630–7635. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, S.; Franze, K.; McClain, C.R.; Wylde, G.W.; Fisher, C.L.; Franklin, R.J.M.; Kabla, A.J.; Keyser, U.F.; Chalut, K.J. Auxetic nuclei in embryonic stem cells exiting pluripotency. Nat. Mater. 2014, 13, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Zilionis, R.; Nainys, J.; Veres, A.; Savova, V.; Zemmour, D.; Klein, A.M.; Mazutis, L. Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 2017, 12, 44–73. [Google Scholar] [CrossRef] [PubMed]
- Psaltis, D.; Quake, S.R.; Yang, C.H. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Minzioni, P.; Osellame, R.; Sada, C.; Zhao, S.; Omenetto, F.G.; Gylfason, K.B.; Haraldsson, T.; Zhang, Y.; Ozcan, A.; Wax, A.; et al. Roadmap for optofluidics. J. Opt. 2017, 19, 093003. [Google Scholar] [CrossRef]
- Schmidt, H.; Hawkins, A.R. The photonic integration of non-solid media using optofluidics. Nat. Photonics 2011, 5, 598–604. [Google Scholar] [CrossRef]
- Erickson, D.; Sinton, D.; Psaltis, D. Optofluidics for energy applications. Nat. Photonics 2011, 5, 583–590. [Google Scholar] [CrossRef]
- Fan, X.D.; White, I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photonics 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Stratton, Z.S.; Guo, F.; Lapsley, M.L.; Chan, C.Y.; Lin, S.S.C.; Huang, T.J. Optofluidic imaging: Now and beyond. Lab Chip 2013, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, S.; Camposeo, A.; Polini, A.; Cingolani, R.; Pisignano, D. Electrospun light-emitting nanofibers as excitation source in microfluidic devices. Lab Chip 2009, 9, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, S.; Chimerel, C.; Langford, R.; Aarts, D.G.A.L.; Keyser, U.F. Parallel sub-micrometre channels with different dimensions for laser scattering detection. Lab Chip 2011, 11, 3365–3368. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kwon, D.; Choi, W.; Jung, G.Y.; Au, A.K.; Folch, A.; Jeon, S. 3D-Printed microfluidic device for the detection of pathogenic bacteria using size-based separation in helical channel with trapezoid cross-section. Sci. Rep. 2015, 5, 7717. [Google Scholar] [CrossRef] [PubMed]
- Cama, J.; Chimerel, C.; Pagliara, S.; Javer, A.; Keyser, U.F. A label-free microfluidic assay to quantitatively study antibiotic diffusion through lipid membranes. Lab Chip 2014, 14, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Aaron, R.; Hawkins, A.R. Optofluidic waveguides: I. Concepts and implementations. Microfluid. Nanofluid. 2008, 4, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; He, Z.; Zheng, C.; Chen, T.; Men, Y.; Huang, Y. Discretely tunable optofluidic compound microlenses. Lab Chip 2011, 11, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T. Micro-optofluidic Lenses: A review. Biomicrofluidics 2010, 4, 031501. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Liu, A.Q.; Chin, L.K.; Yang, Y. An optofluidic prism tuned by two laminar flows. Lab Chip 2011, 11, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.S.; Lin, M.S.; Yang, R.J. An in-plane optofluidic microchip for focal point control. Lab Chip 2013, 13, 3886–3892. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Q.; Yang, Y.; Liu, A.Q.; Chin, L.K.; Zhang, X.M. Microfluidic droplet grating for reconfigurable optical diffraction. Opt. Lett. 2010, 35, 1890–1892. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.K.; Liu, A.Q.; Soh, Y.C.; Lim, C.S.; Lin, C.L. A reconfigurable optofluidic Michelson interferometer using tunable droplet grating. Lab Chip 2010, 10, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.C.; Lim, S.P.; Lee, H.P. Tunable optofluidic switch via hydrodynamic control of laminar flow rate. Appl. Phys. Lett. 2009, 95, 114105. [Google Scholar] [CrossRef]
- Song, W.Z.; Psaltis, D. Pneumatically tunable optofluidic 2 × 2 switch for reconfigurable optical circuit. Lab Chip 2011, 11, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Z.; Vasdekis, A.E.; Li, Z.Y.; Psaltis, D. Low-order distributed feedback optofluidic dye laser with reduced threshold. Appl. Phys. Lett. 2009, 94, 051117. [Google Scholar] [CrossRef]
- Chen, Y.; Lei, L.; Zhang, K.; Shi, L.; Wang, L.; Li, H.; Zhang, X.M.; Wang, Y.; Chan, H.L. Optofluidic microcavities: Dye-lasers and biosensors. Biomicrofluidics 2010, 4, 043002. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Mala, G.M.; Li, D. Flow characteristics of water in microtubes. Int. J. Heat Fluid Flow 1999, 20, 142–148. [Google Scholar] [CrossRef]
- Carlo, D.D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Perumal, M.; Ranga Raju, K.G. Approximate convection-diffusion equations. J. Hydrol. Eng. 1999, 4, 160–164. [Google Scholar] [CrossRef]
- Pan, M.; Kim, M.; Kuiper, S.; Tang, S.K.T. Actuating fluid–fluid interfaces for the reconfiguration of light. IEEE J. Sel. Top. Quantum Electron. 2015, 21, 9100612. [Google Scholar] [CrossRef]
- Lee, G.B.; Chang, C.C.; Huang, S.B.; Yang, R.J. The hydrodynamic focusing effect inside rectangular microchannels. J. Micromech. Microeng. 2006, 16, 1024–1032. [Google Scholar] [CrossRef]
- Wu, Z.G.; Nguyen, N.T. Hydrodynamic focusing in microchannels under consideration of diffusive dispersion: Theories and experiments. Sens. Actuators B 2005, 107, 965–974. [Google Scholar] [CrossRef]
- Knight, J.B.; Vishwanath, A.; Brody, J.P.; Austin, R.H. Hydrodynamic focusing on a silicon chip: Mixing nanoliters in microseconds. Phys. Rev. Lett. 1998, 80, 3863–3866. [Google Scholar] [CrossRef]
- Wolfe, D.B.; Conroy, R.S.; Garstecki, P.; Mayers, B.T.; Fischbach, M.A.; Paul, K.E.; Prentiss, M.; Whitesides, G.M. Dynamic control of liquid-core/liquid-cladding optical waveguides. Proc. Natl. Acad. Sci. USA 2004, 101, 12434–12438. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.K.Y.; Stan, C.A.; Whitesides, G.M. Dynamically reconfigurable liquid-core liquid-cladding lens in a microfluidic channel. Lab Chip 2008, 8, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.C.; Liu, A.Q.; Chin, L.K.; Li, X.C.; Huang, H.J.; Cheng, T.H.; Zhou, X.Q. Different curvatures of tunable liquid microlens via the control of laminar flow rate. Appl. Phys. Lett. 2008, 93, 084101. [Google Scholar] [CrossRef]
- Song, C.L.; Nguyen, N.T.; Asundi, A.K.; Low, C.L.N. Biconcave micro-optofluidic lens with low-refractive-index liquids. Opt. Lett. 2009, 34, 3622–3624. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.L.; Waldeisen, J.R.; Huang, T.J. “Microfluidic drifting’’—Implementing three-dimensional hydrodynamic focusing with a single-layer planar microfluidic device. Lab Chip 2007, 7, 1260–1262. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, S.B.; Lee, K.H.; Sung, H.J.; Kim, S.S. Three-dimensional microfluidic liquid-core/liquid-cladding waveguide. Appl. Phys. Lett. 2010, 97, 021109. [Google Scholar] [CrossRef]
- Mao, X.L.; Waldeisen, J.R.; Juluri, B.K.F.; Huang, T.J. Hydrodynamically tunable optofluidic cylindrical microlens. Lab Chip 2007, 7, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, A.Q.; Lei, L.; Chin, L.K.; Ohl, C.D.; Wang, Q.J.; Yoon, H.S. A tunable 3D optofluidic waveguide dye laser via two centrifugal Dean flow streams. Lab Chip 2011, 11, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Rosenauer, M.; Vellekoop, M.J. 3D fluidic lens shaping—A multiconvex hydrodynamically adjustable optofluidic microlens. Lab Chip 2009, 9, 1040–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, X.Q.; Liang, L.; Zuo, Y.F.; Xu, Y.S.; Yang, Y.; Yuan, Y.J.; Huang, Q.Q. Switchable 3D optofluidic Y-branch waveguides tuned by Dean flows. Sci. Rep. 2016, 6, 38338. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhu, X.Q.; Liu, H.L.; Shi, Y.; Yang, Y. A switchable 3D liquid–liquid biconvex lens withenhanced resolution using Dean flow. Lab Chip 2017, 17, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D.B.; Vezenov, D.V.; Mayers, B.T.; Whitesides, G.M.; Conroy, R.S.; Prentiss, M.G. Diffusion-controlled optical elements for optofluidics. Appl. Phys. Lett. 2005, 87, 181105. [Google Scholar] [CrossRef]
- Mao, X.L.; Steven Lin, S.Z.; Lapsley, M.I.; Shi, J.J.; Juluri, B.K.; Huang, T.J. Tunable liquid gradient refractive index (L-GRIN) lens with two degrees of freedom. Lab Chip 2009, 9, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.T.; Yang, Y.; Chin, L.K.; Chen, H.F.; Zhu, W.M.; Zhang, J.B.; Yap, P.H.; Liedberg, B.; Wang, K.; Wang, G.; et al. Optofluidic lens with low spherical and low field curvature aberrations. Lab Chip 2016, 16, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liang, L.; Zhu, X.Q.; Zhang, X.M.; Yang, Y. Tunable self-imaging effect using hybrid optofluidic waveguides. Lab Chip 2015, 15, 4398–4403. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chan, C.T.; Sheng, P. Transformation optics and metamaterials. Nat. Mater. 2010, 9, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.A.; Rahm, M.; Pendry, J.B.; Smith, D.R. Transformation-optical design of sharp waveguide bends and corners. Appl. Phys. Lett. 2008, 93, 251111. [Google Scholar] [CrossRef]
- Huangfu, J.T.; Xi, S.; Kong, F.M.; Zhang, J.J.; Chen, H.S.; Wang, D.X.; Wu, B.L.; Ran, L.X.; Kong, J.A. Application of coordinate transformation in bent waveguides. J. Appl. Phys. 2008, 104, 014502. [Google Scholar] [CrossRef]
- Li, J.S.; Pendry, J.B. Hiding under the carpet: A new strategy for cloaking. Phys. Rev. Lett. 2008, 101, 203901. [Google Scholar] [CrossRef] [PubMed]
- Ergin, T.; Stenger, N.; Brenner, P.; Pendry, J.B.; Wegener, M. Three-dimensional invisibility cloak at optical wavelengths. Science 2010, 328, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ji, C.; Mock, J.J.; Chin, J.Y.; Cui, T.J.; Smith, D.R. Broadband ground-plane cloak. Science 2009, 323, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Rahm, M.; Cummer, S.A.; Schurig, D.; Pendry, J.B.; Smith, D.R. Optical design of reflectionless complex media by finite embedded coordinate transformations. Phys. Rev. Lett. 2008, 100, 063903. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, A.Q.; Chin, L.K.; Zhang, X.M.; Tsai, D.P.; Lin, C.L.; Lu, C.; Wang, G.P.; Zheludev, N.I. Optofluidic waveguide as a transformation optics device for lightwave bending and manipulation. Nature Commun. 2012, 3, 651. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chin, L.K.; Tsai, J.M.; Tsai, D.P.; Zheludev, N.I.; Liu, A.Q. Transformation optofluidics for large-angle light bending and tuning. Lab Chip 2012, 12, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Zhu, X.Q.; Liang, L.; Zhang, X.M.; Yang, Y. Tunable transformation optical waveguide bends in liquid. Optica 2017, 4, 839–846. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Liang, L.; Zuo, Y.F.; Zhang, X.M.; Yang, Y. Tunable visible cloaking using liquid diffusion. Laser Photonics Rev. 2017, 11, 1700066. [Google Scholar] [CrossRef]
- Tang, S.K.T.; Mayers, B.T.; Vezenov, D.V.; Whitesides, G.M. Optical waveguiding using thermal gradients across homogeneous liquids in microfluidic channels. Appl. Phys. Lett. 2006, 88, 061112. [Google Scholar] [CrossRef]
- Chen, Q.M.; Jian, A.Q.; Li, Z.H.; Zhang, X.M. Optofluidic tunable lenses using laser-induced thermal gradient. Lab Chip 2016, 16, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Shi, Y.; Liang, L.; Li, L.; Guo, S.S.; Yin, L.; Yang, Y. A liquid thermal gradient refractive index lens and sing it to trap single living cell in flowing environments. Lab Chip 2017, 17, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Özbakır, Y.; Jonáš, A.; Kiraz, A.; Erkey, C. Total internal reflection-based optofluidic waveguides fabricated in aerogels. J. Sol-Gel Sci. Technol. 2017, 84, 522–534. [Google Scholar] [CrossRef]
- Liu, P.Y.; Chin, L.K.; Ser, W.; Chen, H.F.; Hsieh, C.M.; Lee, C.H.; Sung, K.B.; Ayi, T.C.; Yap, P.H.; Liedberg, B.; et al. Cell refractive index for cell biology and disease diagnosis: Past, present and future. Lab Chip 2016, 16, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Godin, J.M.; Chen, C.H.; Qiao, W.; Lee, H.; Lo, Y.H. Review Article: Recent advancements in optofluidic flow cytometer. Biomicrofluidics 2010, 4, 043001. [Google Scholar] [CrossRef] [PubMed]
- Domachuk, P.; Cronin-Golomb, M.; Eggleton, B.J. Application of optical trapping to beam manipulation in optofluidics. Opt. Express 2005, 13, 7265–7275. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.H.; Moore, S.D.; Schmidt, B.S.; Klug, M.; Lipson, M.; Erickson, D. Optical manipulation of nanoparticles and biomolecules in sub-wavelength slot waveguides. Nature 2009, 457, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zuo, Y.F.; Wu, W.; Zhu, X.Q.; Yang, Y. Optofluidic restricted imaging, spectroscopy and counting of nanoparticles by evanescent wave using immiscible liquids. Lab Chip 2016, 16, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A. Optical trapping and manipulation of neutral particles using lasers. Proc. Natl. Acad. Sci. USA 1997, 94, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Padgett, M.; Leonardo, R.D. Holographic optical tweezers and their relevance to lab on chip devices. Lab Chip 2011, 11, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Grier, D.G. A revolution in optical manipulation. Nature 2003, 424, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, S.; Dettmer, S.L.; Keyser, U.F. Channel-facilitated diffusion boosted by particle binding at the channel entrance. Phys. Rev. Lett. 2014, 113, 048102. [Google Scholar] [CrossRef] [PubMed]
- Padgett, M.; Bowman, R. Tweezers with a twist. Nat. Photonics 2011, 5, 343–348. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, X.Q.; Zuo, Y.F.; Liang, L.; Zhang, S.P.; Zhang, X.M.; Yang, Y. Precise sorting of gold nanoparticles in a flowing system. ACS Photonics 2016, 3, 2497–2504. [Google Scholar] [CrossRef]
- Song, W.Z.; Liu, A.Q.; Swaminathan, S.; Lim, C.S.; Yap, P.H.; Ayi, T.C. Determination of single living cell’s dry/water mass using optofluidic chip. Appl. Phys. Lett. 2007, 91, 223902. [Google Scholar] [CrossRef]
- Song, W.Z.; Zhang, X.M.; Liu, A.Q.; Lim, C.S.; Yap, P.H.; Hosseini, H.M.M. Refractive index measurement of single living cells using on-chip Fabry-Pérot cavity. Appl. Phys. Lett. 2006, 89, 203901. [Google Scholar] [CrossRef]
- Chin, L.K.; Liu, A.Q.; Lim, C.S.; Zhang, X.M.; Ng, J.H.; Hao, J.Z.; Takahashi, S. Differential single living cell refractometry using grating resonant cavity with optical trap. Appl. Phys. Lett. 2007, 91, 243901. [Google Scholar] [CrossRef]
- Zhu, J.M.; Shi, Y.; Zhu, X.Q.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; Han, X.T. Optofluidic marine phosphate detection with enhanced absorption using a Fabry–Pérot resonator. Lab Chip 2017, 17, 4025–4030. [Google Scholar] [CrossRef] [PubMed]










| Technology | σ () | |
|---|---|---|
| Optofluidics | 1 | 10−3 |
| Liquid crystal | 10−1 | 10−3 |
| Injection current | 10−2 | 10−9 |
| Temperature | 10−2 | 1 |
| Photorefractive | 10−3 | 10−1–10−5 |
| Electro-optic (10 kV/cm) | 10−3 | 10−12 |
| Photoelastic/Acousto-optic (10 W) | 10−4 | 10−6–10−7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Y.; Zhu, X.; Shi, Y.; Liang, L.; Yang, Y. Light Manipulation in Inhomogeneous Liquid Flow and Its Application in Biochemical Sensing. Micromachines 2018, 9, 163. https://doi.org/10.3390/mi9040163
Zuo Y, Zhu X, Shi Y, Liang L, Yang Y. Light Manipulation in Inhomogeneous Liquid Flow and Its Application in Biochemical Sensing. Micromachines. 2018; 9(4):163. https://doi.org/10.3390/mi9040163
Chicago/Turabian StyleZuo, Yunfeng, Xiaoqiang Zhu, Yang Shi, Li Liang, and Yi Yang. 2018. "Light Manipulation in Inhomogeneous Liquid Flow and Its Application in Biochemical Sensing" Micromachines 9, no. 4: 163. https://doi.org/10.3390/mi9040163




