Open Design 3D-Printable Adjustable Micropipette that Meets the ISO Standard for Accuracy
Abstract
:1. Introduction
2. Materials and Methods
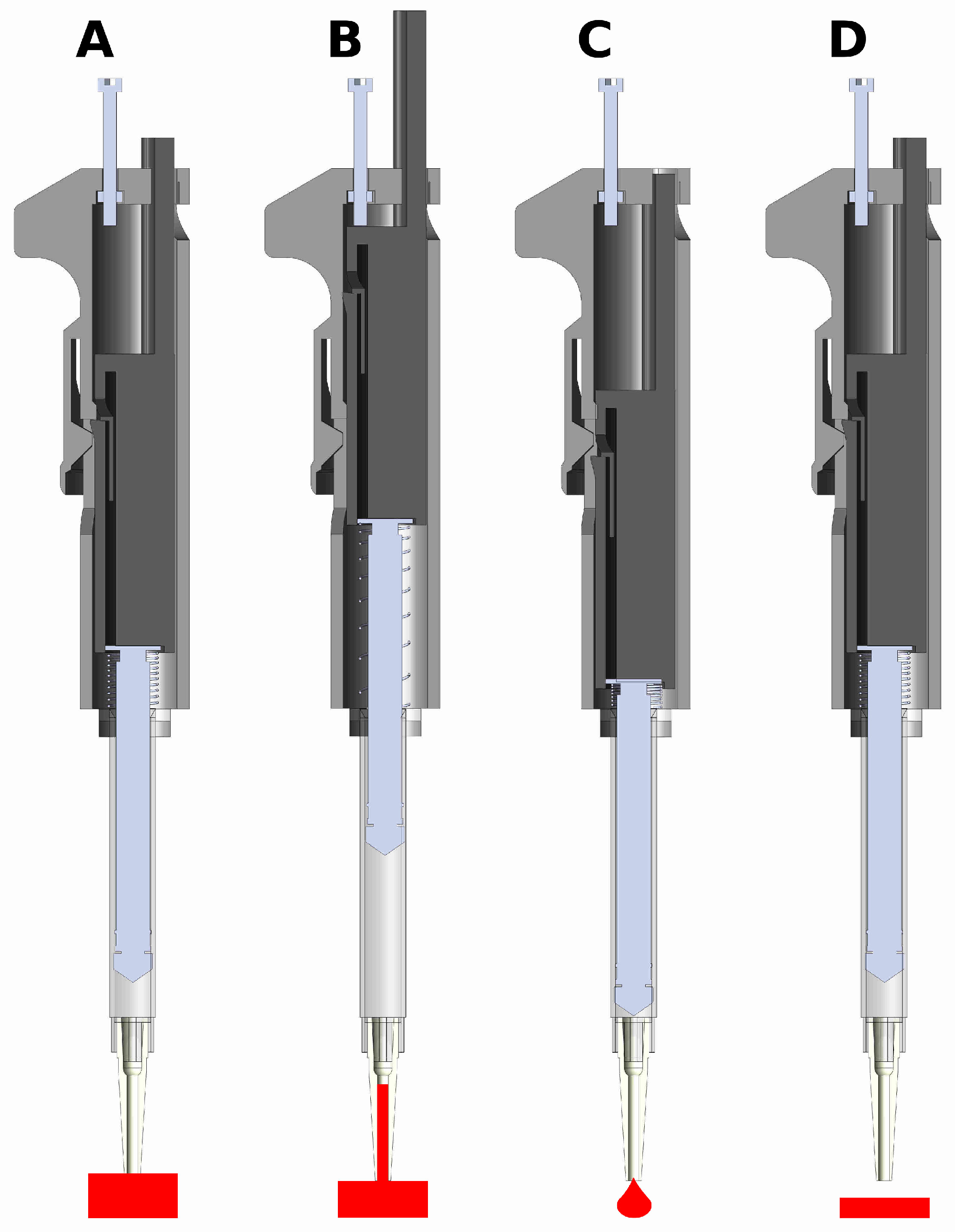
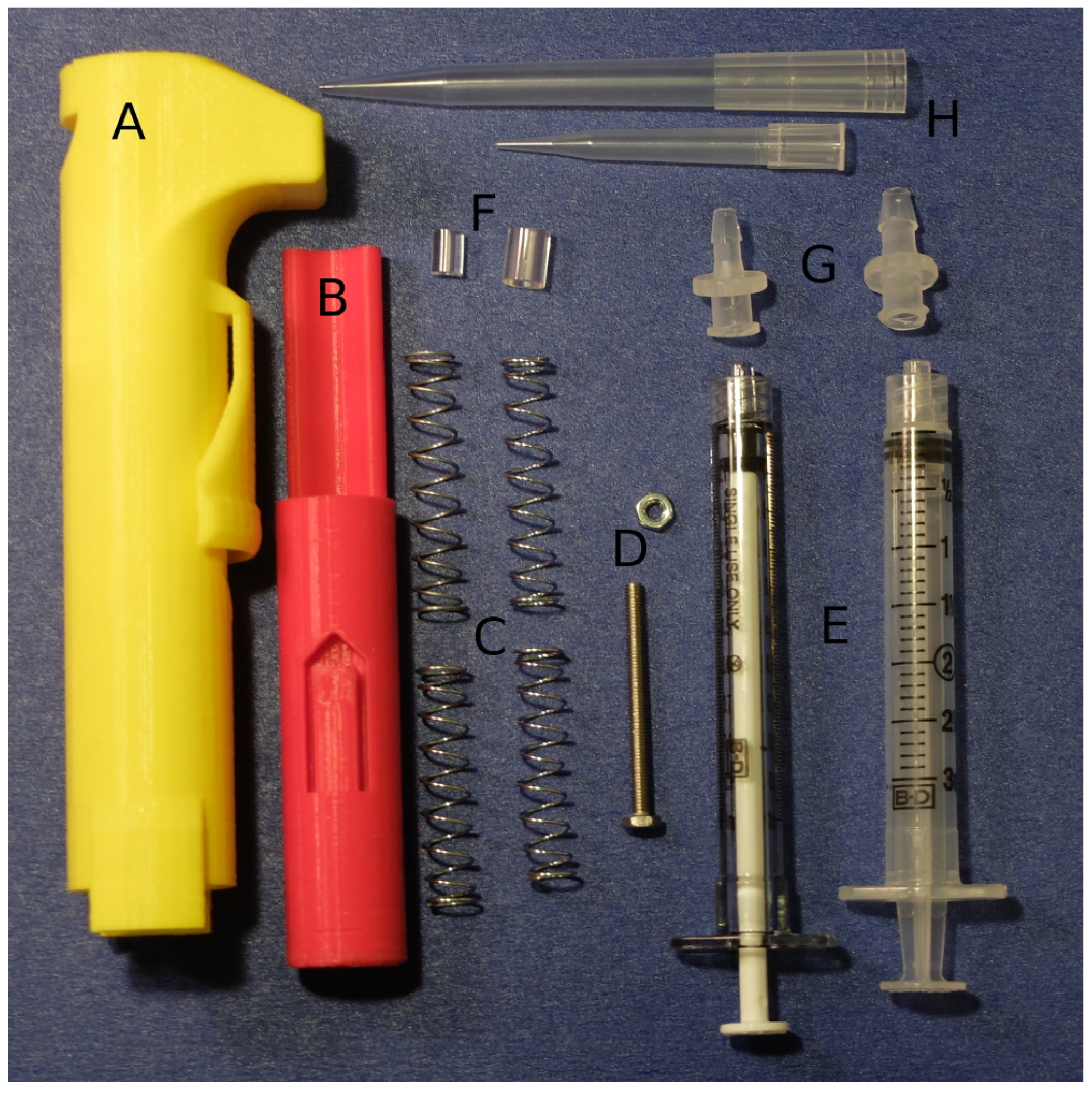
2.1. Fabrication and Assembly
2.2. Validation
3. Results
4. Discussion and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Additional Materials
| 30–300 mL Configuration: | Source | Unit Price | Part Number |
|---|---|---|---|
| Filament | Makerbot | $1.63 | NA |
| 1-mL Syringe | BD Biosciences | $0.15 | 309628 |
| M3 Bolt, 35 mm | McMaster-Carr | $0.12 | 91287A026 |
| M3 Nut | McMaster-Carr | $0.01 | 90591A121 |
| Music Wire Compression Springs * (2) | Jones Spring Co | $1.23 | C10-022-048 |
| Female Luer to 1/8" Hose Barb Adapter | Cole-Parmer | $0.40 | EW-30800-08 |
| Tygon Tubing, 1/16" ID × 3/16" OD | Cole-Parmer | $0.03 | EW-06407-72 |
| 300-L Tips (10) | Fisher Scientific | $0.34 | 02-707-447 |
| Total | $3.91 |
| 100–1000 mL Configuration: | Source | Unit Price | Part Number |
|---|---|---|---|
| Filament | Makerbot | $1.63 | NA |
| 3-mL Syringe | BD Biosciences | $0.73 | 309657 |
| M3 Bolt, 35 mm | McMaster-Carr | $0.12 | 91287A026 |
| M3 Nut | McMaster-Carr | $0.01 | 90591A121 |
| Music Wire Compression Springs * (2) | Jones Spring Co | $1.23 | C10-022-048 |
| Female luer to 5/32" Hose Barb Adapter | Cole-Parmer | $0.66 | EW-45508-06 |
| Tygon Tubing, 1/8" ID × 1/4" OD | Cole-Parmer | $0.05 | EW-06407-76 |
| 1000-L Tips (10) | Fisher Scientific | $0.41 | 02-707-400 |
| Total | $4.84 |
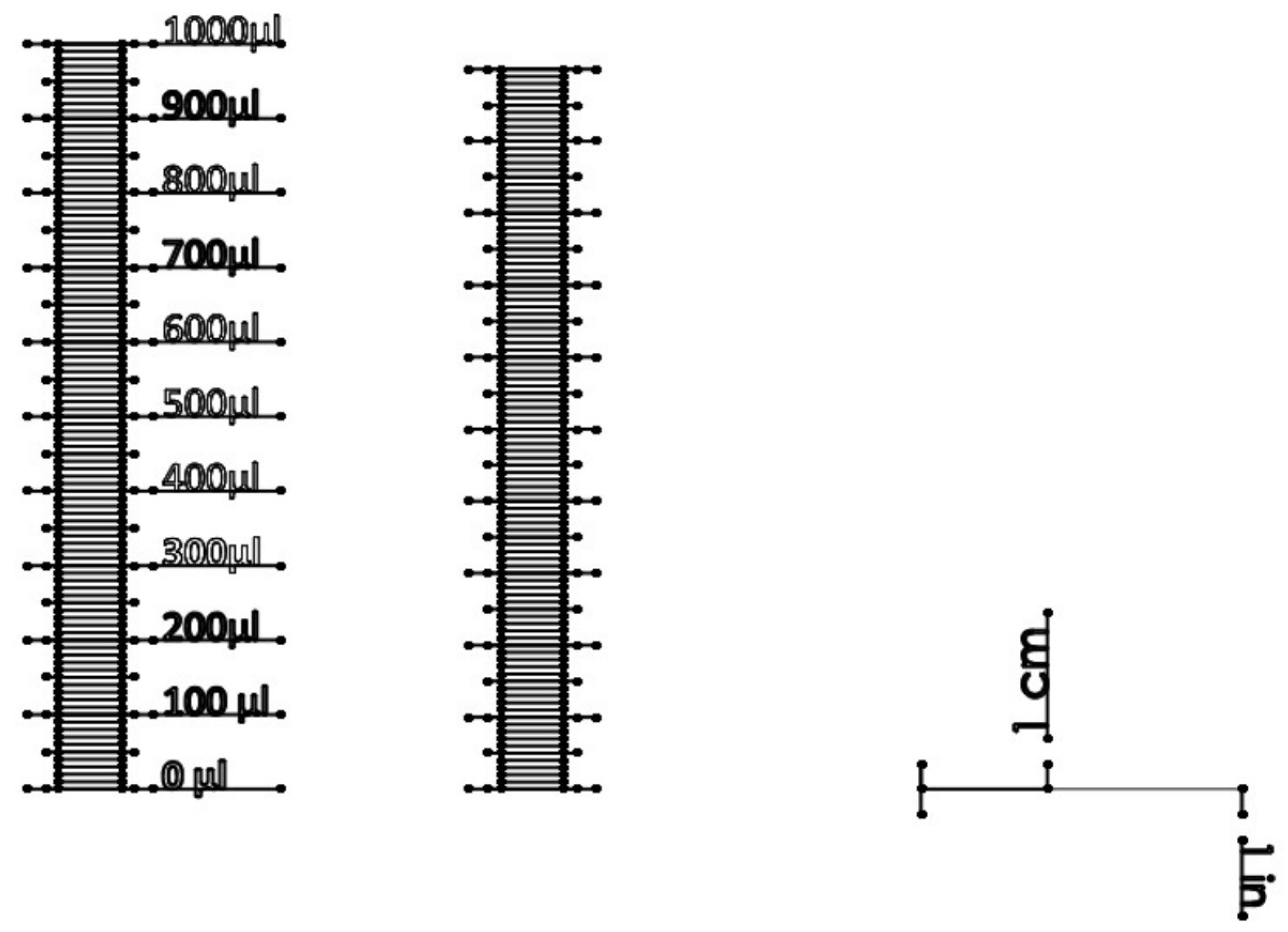
References
- Baden, T.; Chagas, A.M.; Gage, G.; Marzullo, T.; Prieto-Godino, L.L.; Euler, T. Open Labware: 3-D Printing Your Own Lab Equipment. PLoS Biol. 2015, 13, e1002086. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J. M. Open-Source Lab: How to Build Your Own Hardware and Reduce Research Costs; Newnes: New South Wales, Australia, 2013. [Google Scholar]
- Makerbot Industries. Makerbot. 2018. Available online: https://www.makerbot.com/ (accessed on 18 April 2018).
- RepRap Project. RepRap, 2018. Available online: reprap.org (accessed on 18 April 2018).
- Fullerton, J.N.; Frodsham, G.C.M.; Day, R.M. 3D printing for the many, not the few. Nat. Biotechnol. 2014, 32, 1086–1087. [Google Scholar] [CrossRef] [PubMed]
- Wittbrodt, B.; Glover, A.G.; Laureto, J.; Anzalone, G.C.; Oppliger, D.; Irwin, J.L.; Pearce, J.M. Life-cycle economic analysis of distributed manufacturing with open-source 3-D printers. Mechatronics 2013, 23, 713–726. [Google Scholar] [CrossRef]
- Kintel, M. OpenSCAD. 2018. Available online: http://www.openscad.org/ (accessed on 18 April 2018).
- Blender Foudation. Blender, 2018. Available online: www.blender.org (accessed on 18 April 2018).
- Trimble Inc. SketchUp. 2016. Available online: www.sketchup.com (accessed on 18 April 2018).
- Autodesk Inc. Autodesk 123D. 2018. Available online: www.123dapp.com (accessed on 18 April 2018).
- Makerbot Industries. Thingiverse. 2018. Available online: https://www.thingiverse.com/ (accessed on 18 April 2018).
- National Institutes of Health. NIH 3D Print Exchange. 2018. Available online: http://3dprint.nih.gov/ (accessed on 18 April 2018).
- Stratasys. GrabCAD. Available online: https://grabcad.com/ (accessed on 18 April 2018).
- GitHub Inc. GitHub. 2018. Available online: https://github.com/ (accessed on 18 April 2018).
- Marzullo, T.C.; Gage, G.J. The SpikerBox: A Low Cost, Open-Source BioAmplifier for Increasing Public Participation In Neuroscience Inquiry. PLoS ONE 2012, 7, e30837. [Google Scholar] [CrossRef] [PubMed]
- Lang, T. Advancing global health research through digital technology and sharing data. Science 2011, 331, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Fobel, R.; Fobel, C.; Wheeler, A.R. DropBot: An open-source digital microfluidic control system with precise control of electrostatic driving force and instantaneous drop velocity measurement. Appl. Phys. Lett. 2013, 102, 1–5. [Google Scholar] [CrossRef]
- Baker, E. Open source data logger for low-cost environmental monitoring. Biodivers. Data J. 2014, e1059. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, J.S.; Clements, J.; Prakash, M. Foldscope: origami-based paper microscope. PLoS ONE 2014, 9, e98781. [Google Scholar] [CrossRef] [PubMed]
- Bhamla, M.S.; Benson, B.; Chai, C.; Katsikis, G.; Johri, A.; Prakash, M. Hand-powered ultralow-cost paper centrifuge. Nat. Biomed. Eng. 2017, 1, 0009. [Google Scholar] [CrossRef]
- Pearce, J.M. Building Research Equipment with Free, Open-Source Hardware. Science 2012, 337, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Rankin, T.M.; Giovinco, N.A.; Cucher, D.J.; Watts, G.; Hurwitz, B.; Armstrong, D.G. Three-dimensional printing surgical instruments: are we there yet? J. Surg. Res. 2014, 189, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Sulkin, M.S.; Widder, E.; Shao, C.; Holzem, K.M.; Gloschat, C.; Gutbrod, S.R.; Efimov, I.R. Three-dimensional printing physiology laboratory technology. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H1569–H1573. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.D.M.; Wheeler, A.R. DStat: A versatile, open-source potentiostat for electroanalysis and integration. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.T.; Mora, M.F.; Willis, P.A.; Lago, C.L.; Jiao, H.; Garcia, C.D. Getting started with open-hardware: Development and control of microfluidic devices. Electrophoresis 2014, 35, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Tek, P.; Chiganos, T.C.; Mohammed, J.S.; Eddington, D.T.; Fall, C.P.; Ifft, P.; Rousche, P.J. Rapid prototyping for neuroscience and neural engineering. J. Neurosci. Methods 2008, 172, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Chai Biotechnologies Inc. Open PCR. 2017. Available online: http://openpcr.org/ (accessed on 18 April 2018).
- Trachtenberg, J.E.; Mountziaris, P.M.; Miller, J.S.; Wettergreen, M.; Kasper, F.K.; Mikos, A.G. Open-source three-dimensional printing of biodegradable polymer scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4326–4335. [Google Scholar] [CrossRef]
- Rosenegger, D.G.; Tran, C.H.T.; LeDue, J.; Zhou, N.; Gordon, G.R. A High Performance, Cost-Effective, Open-Source Microscope for Scanning Two-Photon Microscopy that Is Modular and Readily Adaptable. PLoS ONE 2014, 9, e110475. [Google Scholar] [CrossRef] [PubMed]
- Garvey, C. DremelFuge. 2009. Available online: https://www.thingiverse.com/thing:1483 (accessed on 18 April 2018).
- Zhang, C.; Anzalone, N.C.; Faria, R.P.; Pearce, J.M. Open-Source 3D-Printable Optics Equipment. PLoS ONE 2013, 8, e59840. [Google Scholar] [CrossRef] [PubMed]
- Baden, T. Raspberry Pi Scope. 2014. Available online: http://3dprint.nih.gov/discover/3dpx-000609 (accessed on 18 April 2018).
- Walus, K. A Fully Printable Microscope. 2014. Available online: http://3dprint.nih.gov/discover/3dpx-000304 (accessed on 18 April 2018).
- Wijnen, B.; Hunt, E.J.; Anzalone, G.C.; Pearce, J.M. Open-Source Syringe Pump Library. PLoS ONE 2014, 9, e107216. [Google Scholar] [CrossRef] [PubMed]
- Patrick, W.G.; Nielsen, A.A.; Keating, S.J.; Levy, T.J.; Wang, C.W.; Rivera, J.J.; Mondragón-Palomino, O.; Carr, P.A.; Voigt, C.A.; Oxman, N.; et al. DNA assembly In 3D printed fluidics. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Symes, M.D.; Kitson, P.J.; Yan, J.; Richmond, C.J.; Cooper, G.J.; Bowman, R.W.; Vilbrandt, T.; Cronin, L. Integrated 3D-printed reactionware for chemical synthesis and analysis. Nat. Chem. 2012, 4, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kitson, P.J.; Rosnes, M.H.; Sans, V.; Dragone, V.; Cronin, L. Configurable 3D-Printed millifluidic and microfluidic ‘lab on a chip’ reactionware devices. Lab Chip 2012, 12, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, J.S.; Rosnes, M.H.; Sans, V.; Kitson, P.J.; Cronin, L. Continuous parallel ESI-MS analysis of reactions carried out In a bespoke 3D printed device. Beilstein J. Nanotechnol. 2013, 4, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Kitson, P.J.; Marshall, R.J.; Long, D.; Forgan, R.S.; Cronin, L. 3D Printed high-throughput hydrothermal reactionware for discovery, optimization, and scale-up. Angew. Chem. Int. Ed. 2014, 53, 12723–12728. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.D.; Rexius-Hall, M.L.; Eddington, D.T. A 3D-Printed Oxygen Control Insert for a 24-Well Plate. PLos ONE 2015, 10, e0137631. [Google Scholar] [CrossRef] [PubMed]
- Dragone, V.; Sans, V.; Rosnes, M.H.; Kitson, P.J.; Cronin, L. 3D-printed devices for continuous-flow organic chemistry. BeilsteIn J. Org. Chem. 2013, 9, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Plos Collections. Open Source Toolkit Hardware. 2017. Available online: http://collections.plos.org/open-source-toolkit-hardware (accessed on 18 April 2018).
- International Organization for Standardization. Piston-Operated Volumetric Apparatus—Part 1: Terminology, General Requirements and User Recommendations; ISO 8655-1:2002(en); ISO: Geneva, Switzerland, 2002. [Google Scholar]
- Takagishi, K.; Umezu, S. Development of the Improving Process for the 3D Printed Structure. Sci. Rep. 2017, 7, 39852. [Google Scholar] [CrossRef] [PubMed]
- Baden, T. Biropette: Customisable, High Precision Pipette. 2014. Available online: http://www.thingiverse.com/thing:255519 (accessed on 18 April 2018).
- Eddington Lab. 3D Printable Micropipette. 2017. Available online: https://github.com/Biological-Microsystems-Laboratory/micropipette (accessed on 18 April 2018).


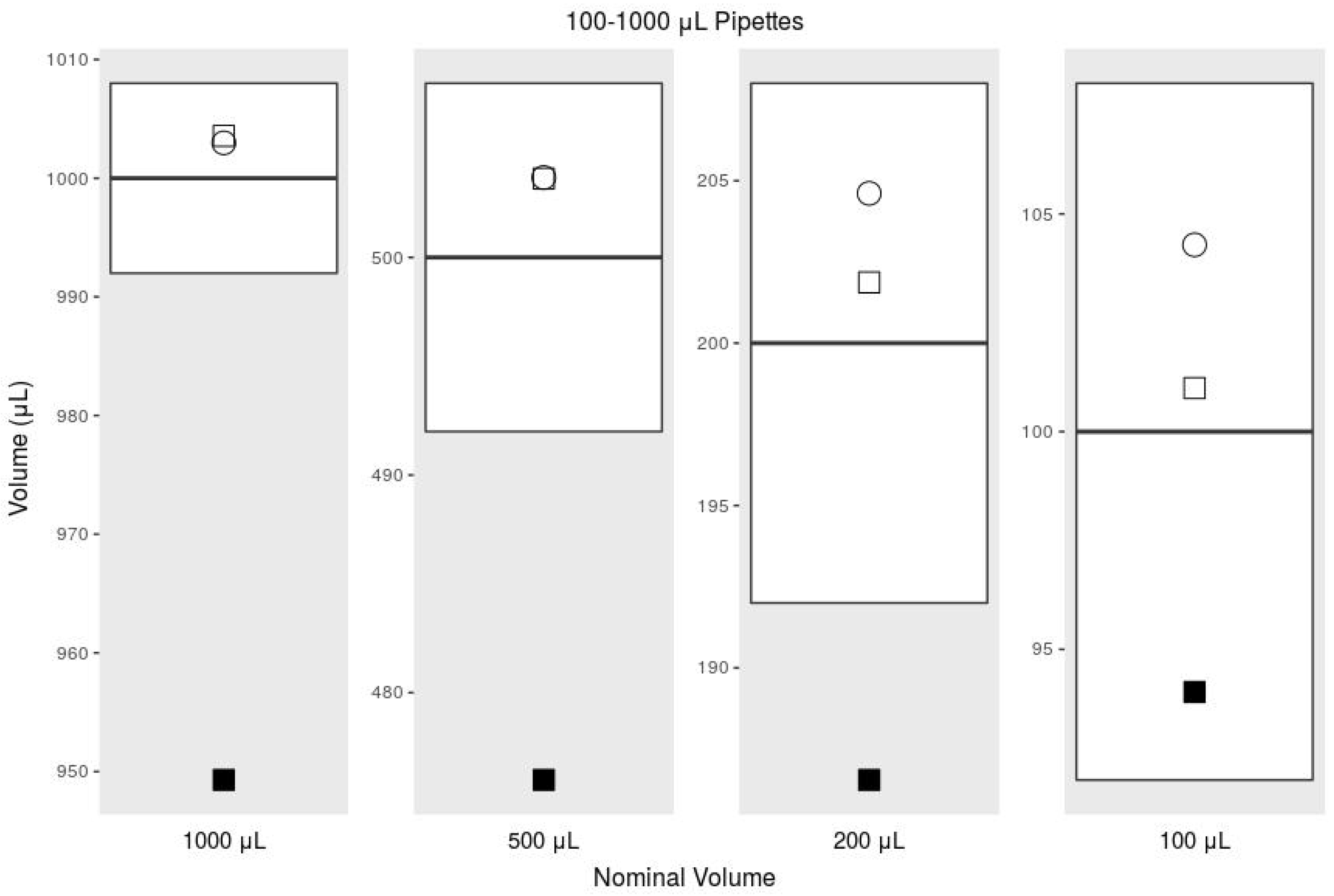
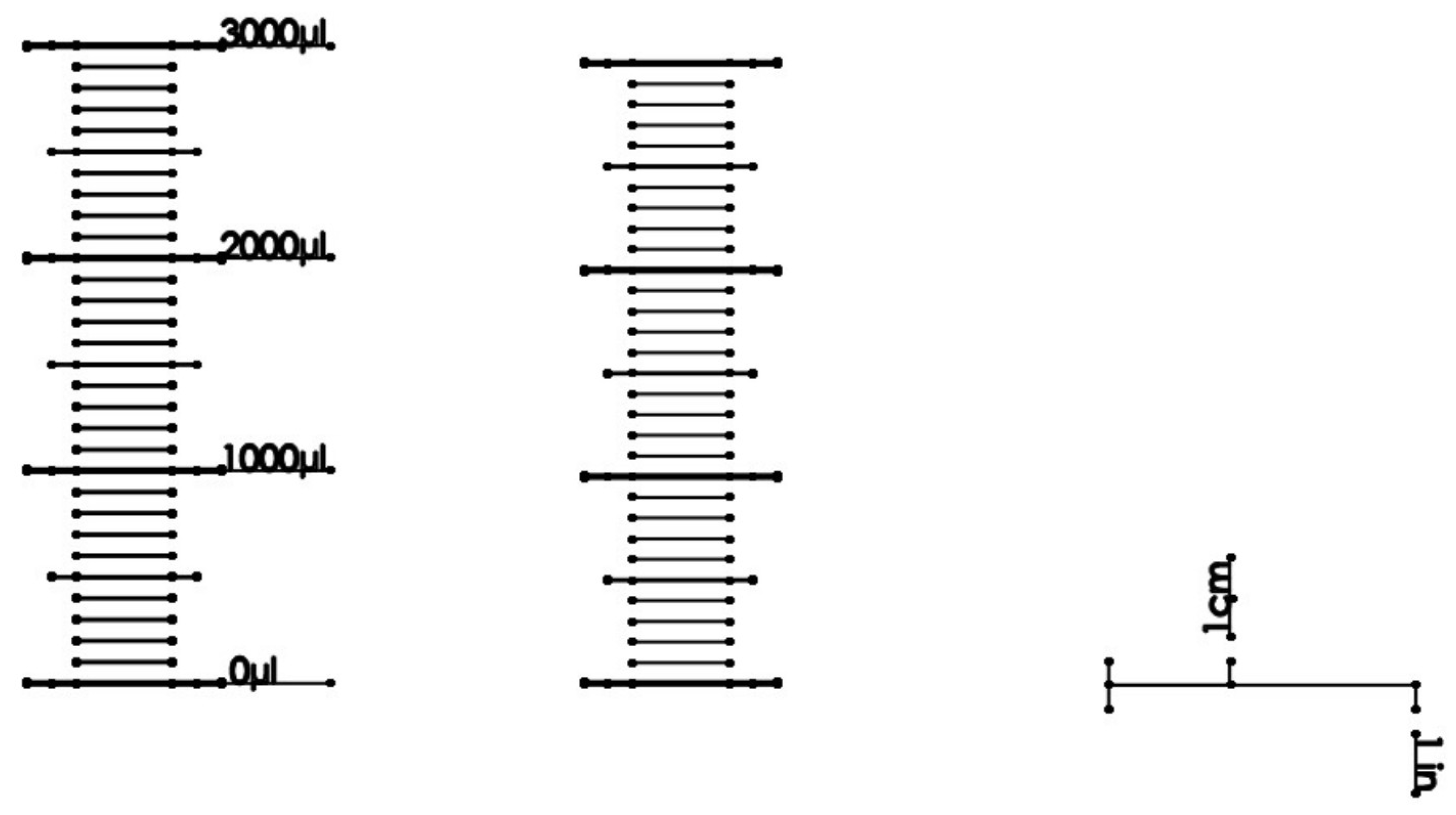
| Mean | Systematic Error | % Sys. Err. | Random Error | % Rand. Err. | ||
|---|---|---|---|---|---|---|
| 1000 L | ISO 8655, 100–1000 L | 1000 | 8.00 | 0.80 | 3.00 | 0.30 |
| Commercial Pipette | 1002.98 | 2.98 | 0.30 | 1.72 | 0.17 | |
| Printed Pipette | 949.29 | −50.71 | −5.07 | 0.60 | 0.06 | |
| Printed Pipette Scale | 1003.57 | 3.57 | 0.36 | 0.89 | 0.09 | |
| 500 L | ISO 8655, 100–1000 L | 500 | 8.00 | 1.60 | 3.00 | 0.60 |
| Commercial Pipette | 503.67 | 3.67 | 0.73 | 0.49 | 0.10 | |
| Printed Pipette | 475.99 | −24.01 | −4.80 | 4.75 | 1.00 | |
| Printed Pipette Scale | 503.62 | 3.62 | 0.72 | 1.64 | 0.33 | |
| 200 L | ISO 8655, 100–1000 L | 200 | 8.00 | 4.00 | 3.00 | 1.50 |
| Commercial Pipette | 204.61 | 4.61 | 2.30 | 0.15 | 0.07 | |
| Printed Pipette | 186.55 | −13.45 | −6.72 | 1.31 | 0.70 | |
| Printed Pipette Scale | 201.87 | 1.87 | 0.94 | 1.47 | 0.73 | |
| 100 L | ISO 8655, 100–1000 L | 100 | 8.00 | 8.00 | 3.00 | 3.00 |
| Commercial Pipette | 104.29 | 4.29 | 4.29 | 1.65 | 1.58 | |
| Printed Pipette | 94.02 | −5.98 | −5.98 | 4.81 | 5.12 | |
| Printed Pipette Scale | 101.00 | 1.00 | 1.00 | 1.05 | 1.04 |
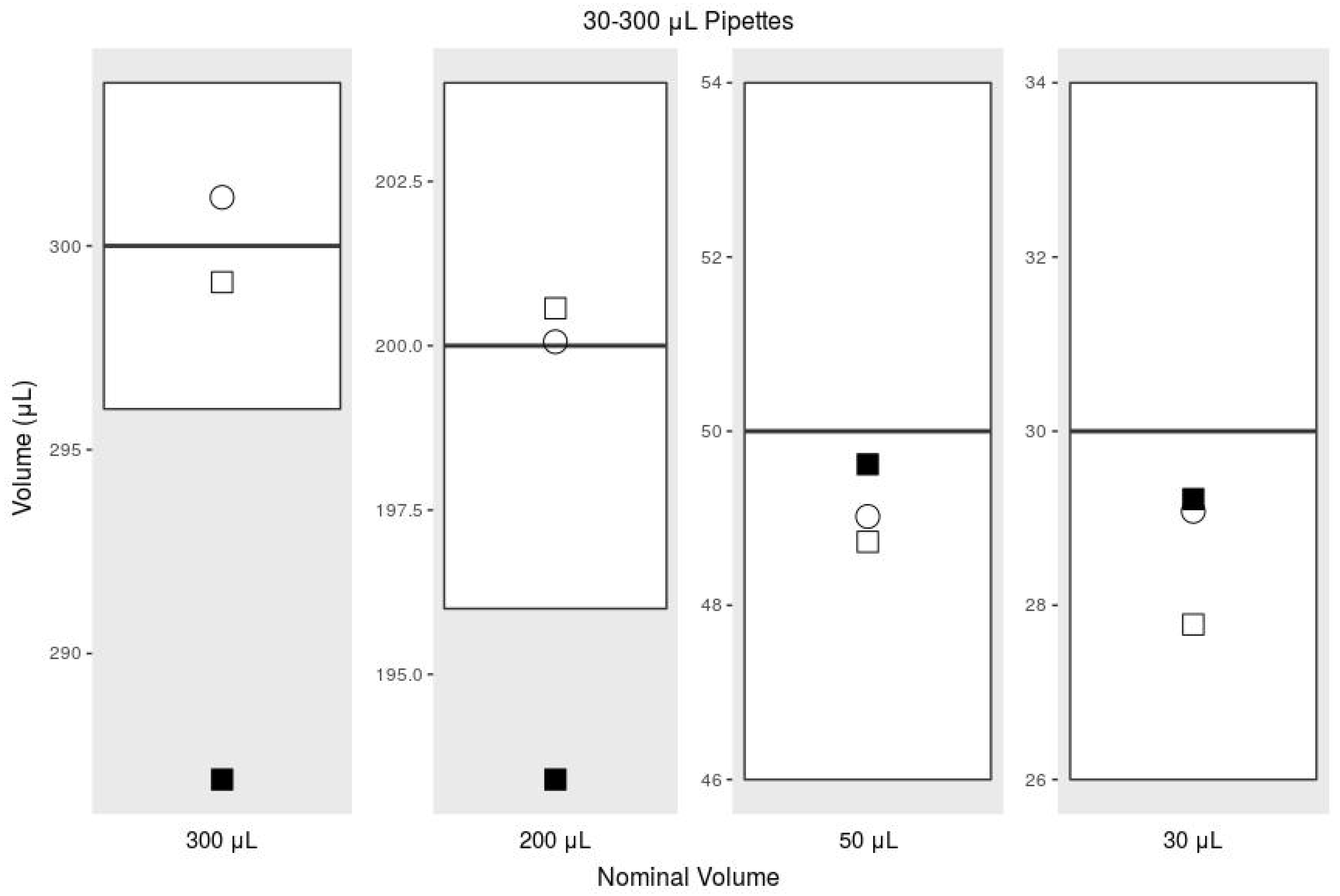
| Mean | Systematic Error | % Sys. Err. | Random Error | % Rand. Err. | ||
|---|---|---|---|---|---|---|
| 300 L | ISO 8655, 30–300 L | 300 | 4.00 | 1.33 | 1.50 | 0.50 |
| Commercial Pipette | 301.19 | 1.19 | 0.40 | 0.53 | 0.18 | |
| Printed Pipette | 286.91 | −13.09 | −4.36 | 0.42 | 0.15 | |
| Printed Pipette Scale | 299.11 | −0.89 | −0.30 | 0.48 | 0.16 | |
| 200 L | ISO 8655, 30–300 L | 200 | 4 | 2 | 1.5 | 0.75 |
| Commercial Pipette | 200.06 | 0.06 | 0.03 | 0.46 | 0.23 | |
| Printed Pipette | 193.40 | −6.60 | −3.30 | 2.86 | 1.48 | |
| Printed Pipette Scale | 200.57 | 0.57 | 0.28 | 0.86 | 0.43 | |
| 50 L | ISO 8655, 30–300 L | 50 | 4 | 8 | 1.5 | 3 |
| Commercial Pipette | 49.02 | −0.98 | −1.96 | 0.10 | 0.20 | |
| Printed Pipette | 49.62 | −0.38 | −0.76 | 1.26 | 2.53 | |
| Printed Pipette Scale | 48.73 | −1.27 | −2.54 | 1.11 | 2.27 | |
| 30 L | ISO 8655, 30–300 L | 30 | 4 | 13.3 | 1.5 | 5 |
| Commercial Pipette | 29.08 | −0.92 | −3.06 | 0.09 | 0.31 | |
| Printed Pipette | 29.22 | −0.78 | −2.59 | 0.31 | 1.07 | |
| Printed Pipette Scale | 27.78 | −2.22 | −7.41 | 1.37 | 4.93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brennan, M.D.; Bokhari, F.F.; Eddington, D.T. Open Design 3D-Printable Adjustable Micropipette that Meets the ISO Standard for Accuracy. Micromachines 2018, 9, 191. https://doi.org/10.3390/mi9040191
Brennan MD, Bokhari FF, Eddington DT. Open Design 3D-Printable Adjustable Micropipette that Meets the ISO Standard for Accuracy. Micromachines. 2018; 9(4):191. https://doi.org/10.3390/mi9040191
Chicago/Turabian StyleBrennan, Martin D., Fahad F. Bokhari, and David T. Eddington. 2018. "Open Design 3D-Printable Adjustable Micropipette that Meets the ISO Standard for Accuracy" Micromachines 9, no. 4: 191. https://doi.org/10.3390/mi9040191





