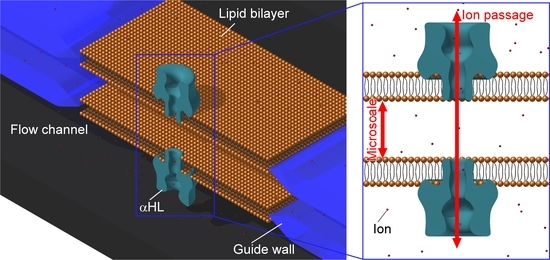
Microfluidic Formation of Double-Stacked Planar Bilayer Lipid Membranes by Controlling the Water-Oil Interface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Regents and Chemicals
2.2. Concept of Double-Stacked pBLM Formation Using Five-Layered Microchannels
2.3. Hydrodynamic Simulation of Five-Layered Microchannels
2.4. Fabrication of Five-Layered Microchannels
2.5. Microfluidic Experiment of Double-Stacked pBLM Formation
2.6. Reconstitution of Membrane Protein to the Double-Stacked pBLMs
3. Results and Discussion
3.1. Hydrodynamic Simulation for Designing the Double-Stacked BLMs
3.2. Formation of Double-Stacked pBLMs Using Five-Layered Microchannels
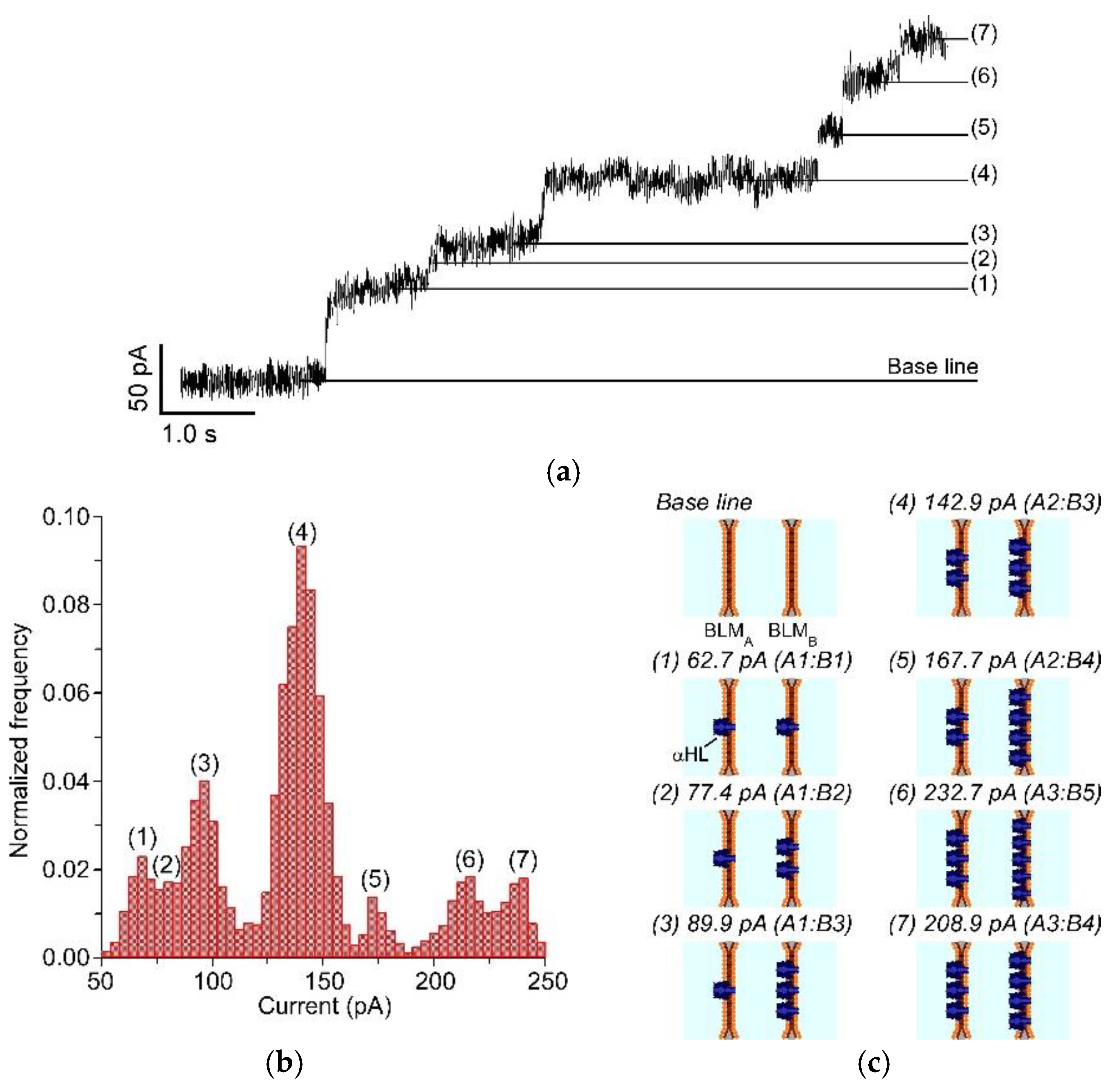
3.3. Channel Current Measurement of Nanopores Reconstituted in the Double-Stacked BLMs
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Latorre, R.; Vergara, C.; Hidalgo, C. Reconstitution in planar lipid bilayers of a Ca2+-dependent K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proc. Natl. Acad. Sci. USA 1982, 79, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Krueger, B.K.; Worley, J.F.; French, R.J. Single sodium channels from rat brain incorporated into planar lipid bilayer membranes. Nature 1983, 303, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.J.; Simon, M.I.; Draper, R.K.; Montal, M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc. Natl. Acad. Sci. USA 1981, 78, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Bayley, H.; Braha, O.; Gu, L.Q. Stochastic sensing with protein pores. Adv. Mater. 2000, 12, 139–142. [Google Scholar] [CrossRef]
- Hladky, S.B.; Haydon, D.A. Discreteness of Conductance Change in Bimolecular Lipid Membranes in the Presence of Certain Antibiotics. Nature 1970, 225, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Kagan, B.L.; Selsted, M.E.; Ganz, T.; Lehrer, R.I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA 1990, 87, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kawano, R. Channel Current Analysis for Pore-forming Properties of an Antimicrobial Peptide, Magainin 1, Using the Droplet Contact Method. Anal. Sci. 2016, 32, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Langecker, M.; Arnaut, V.; Martin, T.G.; List, J.; Renner, S.; Mayer, M.; Dietz, H.; Simmel, F.C. Synthetic lipid membrane channels formed by designed DNA nanostructures. Science 2012, 338, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.R.; Stulz, E.; Howorka, S. Self-assembled DNA nanopores that span lipid bilayers. Nano Lett. 2013, 13, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Wu, H.C.; Jayasinghe, L.; Patel, A.; Reid, S.; Bayley, H. Continuous Base Identification for Single-Molecule Nanopore DNA Sequencing. Nat. Nanotechnol. 2009, 4, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Derrington, I.M.; Butler, T.Z.; Collins, M.D.; Manrao, E.; Pavlenok, M.; Niederweis, M.; Gundlach, J.H. Nanopore DNA Sequencing with MspA. Proc. Natl. Acad. Sci. USA 2010, 107, 16060–16065. [Google Scholar] [CrossRef] [PubMed]
- Howorka, S.; Siwy, Z. Nanopore Analytics: Sensing of Single Molecules. Chem. Soc. Rev. 2009, 38, 2360–2384. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Gubbiotti, A.; Chinappi, M.; Takai, N.; Tanaka, K.; Tsumoto, K.; Kawano, R. Analysis of Pore Formation and Protein Translocation Using Large Biological Nanopores. Anal. Chem. 2017, 89, 11269–11277. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, M.; Ohara, M.; Kawano, R. Amplification and quantification of an antisense oligonucleotide from target microRNA using programmable DNA and a biological nanopore. Anal. Chem. 2017, 89, 2312–2317. [Google Scholar] [CrossRef]
- Ohara, M.; Takinoue, M.; Kawano, R. Nanopore logic operation with DNA to RNA transcription in a droplet system. ACS Synth. Biol. 2017, 6, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Maglia, G.; Heron, A.J.; Hwang, W.L.; Holden, M.A.; Mikhailova, E.; Li, Q.; Cheley, S.; Bayley, H. Droplet networks with incorporated protein diodes show collective properties. Nat. Nanotechnol. 2009, 4, 437–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoji, K.; Morishima, K. Stacked biofuel cells separated by artificial lipid bilayers. In Proceedings of the 2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015. [Google Scholar]
- Yasuga, H.; Kawano, R.; Takinoue, M.; Tsuji, Y.; Osaki, T.; Kamiya, K.; Miki, N.; Takeuchi, S. Logic Gate Operation by DNA Translocation through Biological Nanopores. PLoS ONE 2016, 11, e0149667. [Google Scholar] [CrossRef] [PubMed]
- Booth, M.J.; Schild, V.R.; Downs, F.G.; Bayley, H. Functional aqueous droplet networks. Mol. Biosyst. 2017, 13, 1658–1691. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tabata, K.; Kato-Yamada, Y.; Noji, H.; Takeuchi, S. Planar lipid bilayer reconstitution with a micro-fluidic system. Lab Chip 2004, 4, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Malmstadt, N.; Nash, M.A.; Purnell, R.F.; Schmidt, J.J. Automated formation of lipid-bilayer membranes in a microfluidic device. Nano Lett. 2006, 6, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, K.; Suzuki, H.; Takeuchi, S. Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal. Chem. 2006, 78, 8169–8174. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Suzuki, H.; Le Pioufle, B.; Takeuchi, S. Multichannel simultaneous measurements of single-molecule translocation in alpha-hemolysin nanopore array. Anal. Chem. 2009, 81, 9866–9870. [Google Scholar] [CrossRef] [PubMed]
- Zagnoni, M.; Sandison, M.E.; Morgan, H. Microfluidic array platform for simultaneous lipid bilayer membrane formation. Biosens. Bioelectron. 2009, 24, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Suzuki, H.; Takeuchi, S. Microfluidic lipid membrane formation on microchamber arrays. Lab Chip 2011, 11, 2485–2487. [Google Scholar] [CrossRef] [PubMed]
- Zagnoni, M. Miniaturised technologies for the development of artificial lipid bilayer systems. Lab Chip 2012, 12, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Hromada, L.P.; Nablo, B.J.; Kasianowicz, J.J.; Gaitan, M.A.; DeVoe, D.L. Single molecule measurements within individual membrane-bound ion channels using a polymer-based bilayer lipid membrane chip. Lab Chip 2008, 8, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Sun, B.; Colombini, M.; DeVoe, D.L. Rapid Microfluidic Perfusion Enabling Kinetic Studies of Lipid Ion Channels in a Bilayer Lipid Membrane Chip. Ann. Biomed. Eng. 2011, 39, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Kendall, E.L.; Shao, C.; DeVoe, D.L. Visualizing the Growth and Dynamics of Liquid-Ordered Domains during Lipid Bilayer Folding in a Microfluidic Chip. Small 2012, 8, 3613–3619. [Google Scholar] [CrossRef] [PubMed]
- Stimberg, V.C.; Bomer, J.G.; Uitert, I.V.; van den Berg, A.; Gac, S.L. High Yield, Reproducible and Quasi-Automated Bilayer Formation in a Microfluidic Format. Small 2013, 9, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.; Kieffer, R.; Padmos, R.; Aubin-Tam, M.E. Stable Free-Standing Lipid Bilayer Membranes in Norland Optical Adhesive 81 Microchannels. Anal. Chem. 2016, 88, 7466–7470. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.A.; Needham, D.; Bayley, H. Functional bionetworks from nanoliter water droplets. J. Am. Chem. Soc. 2007, 129, 8650–8655. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Holden, M.A.; White, S.; Bayley, H. Electrical behavior of droplet interface bilayer networks: Experimental analysis and modeling. J. Am. Chem. Soc. 2007, 129, 11854–11864. [Google Scholar] [CrossRef] [PubMed]
- Bayley, H.; Cronin, B.; Heron, A.; Holden, M.A.; Hwang, W.L.; Syeda, R.; Thompson, J.; Wallace, M. Droplet interface bilayers. Mol. Biosyst. 2008, 4, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Tsuji, Y.; Sato, K.; Osaki, T.; Kamiya, K.; Hirano, M.; Ide, T.; Miki, N.; Takeuchi, S. Automated parallel recordings of topologically identified single ion channels. Sci. Rep. 2013, 3, 1995. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Tsuji, Y.; Kamiya, K.; Kodama, T.; Osaki, T.; Miki, N.; Takeuchi, S. A portable lipid bilayer system for environmental sensing with a transmembrane protein. PLoS ONE 2014, 9, e102427. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Srijanto, B.; Collier, C.P.; Retterer, S.T.; Sarles, S.A. Hydrodynamic trapping for rapid assembly and in situ electrical characterization of droplet interface bilayer arrays. Lab Chip 2016, 16, 3576–3588. [Google Scholar] [CrossRef] [PubMed]
- Czekalska, M.A.; Kaminski, T.S.; Horka, M.; Jakiela, S.; Garstecki, P. An automated microfluidic system for the generation of droplet interface bilayer networks. Micromachines 2017, 8, 93. [Google Scholar] [CrossRef]
- Venkatesan, G.A.; Lee, J.; Farimani, A.B.; Heiranian, M.; Collier, C.P.; Aluru, N.R.; Sarles, S.A. Adsorption Kinetics Dictate Monolayer Self-Assembly for Both Lipid-In and Lipid-Out Approaches to Droplet Interface Bilayer Formation. Langmuir 2015, 31, 12883–12893. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Jensen, K.F. Multiphase microfluidics: From flow characteristics to chemical and materials synthesis. Lab Chip 2006, 6, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Surmeian, M.; Slyadnev, M.N.; Hisamoto, H.; Hibara, A.; Uchiyama, K.; Kitamori, T. Three-layer flow membrane system on a microchip for investigation of molecular transport. Anal. Chem. 2002, 74, 2014–2020. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoji, K.; Kawano, R. Microfluidic Formation of Double-Stacked Planar Bilayer Lipid Membranes by Controlling the Water-Oil Interface. Micromachines 2018, 9, 253. https://doi.org/10.3390/mi9050253
Shoji K, Kawano R. Microfluidic Formation of Double-Stacked Planar Bilayer Lipid Membranes by Controlling the Water-Oil Interface. Micromachines. 2018; 9(5):253. https://doi.org/10.3390/mi9050253
Chicago/Turabian StyleShoji, Kan, and Ryuji Kawano. 2018. "Microfluidic Formation of Double-Stacked Planar Bilayer Lipid Membranes by Controlling the Water-Oil Interface" Micromachines 9, no. 5: 253. https://doi.org/10.3390/mi9050253