MEMS Inertial Sensors Based Gait Analysis for Rehabilitation Assessment via Multi-Sensor Fusion
Abstract
:1. Introduction
2. Related Works
3. Materials and Methods
3.1. System Setup
3.2. Accelerometer Non-Orthogonal Error Estimation
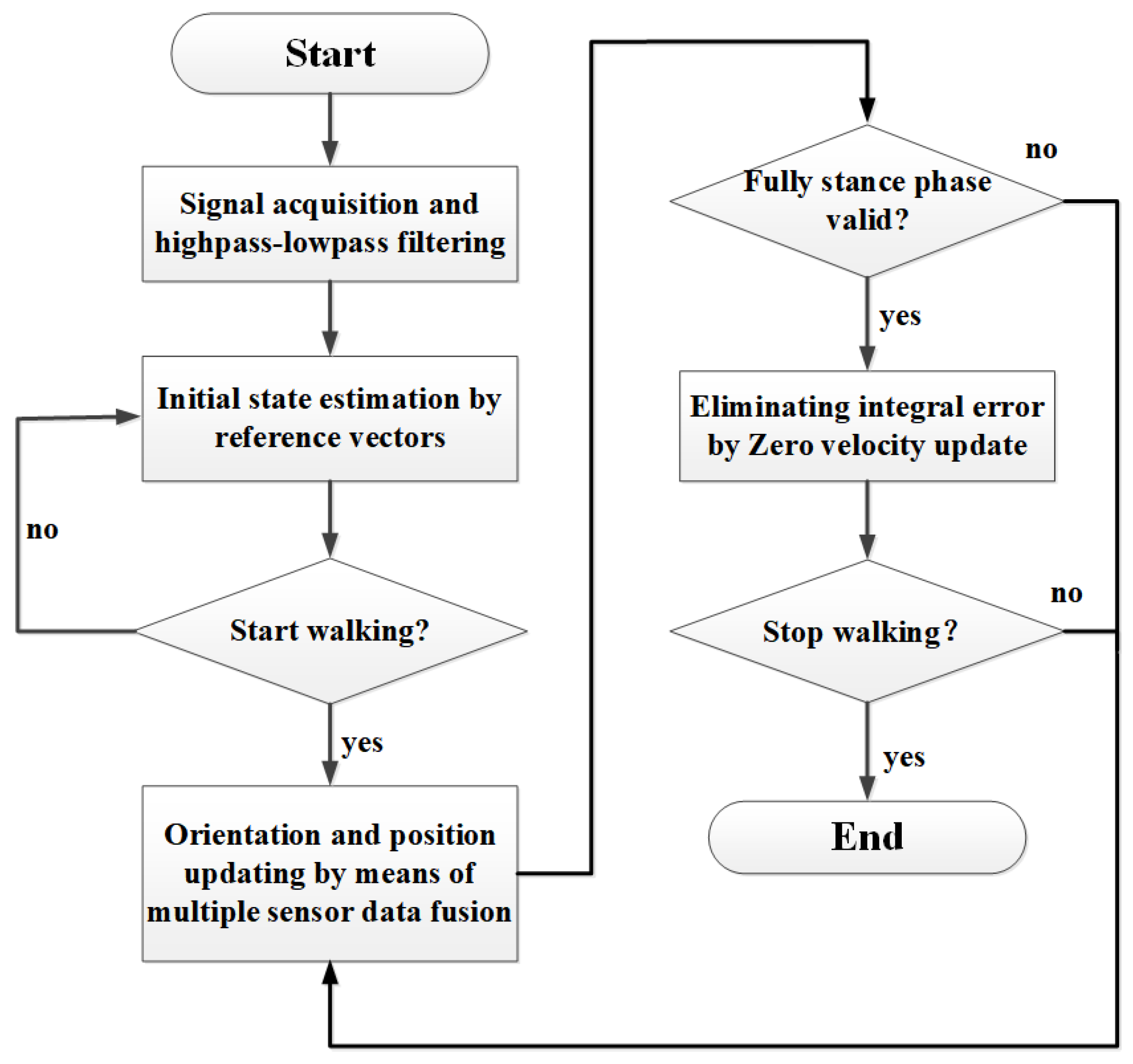
3.3. Stance Phase Detection by Decision Level Data Fusion
3.4. Knee Angle Estimation
3.5. Attitude Estimation and Quaternion Correction in IMUs via Sensor Fusion
4. Experimental Results
4.1. Knee Flexion Monitoring
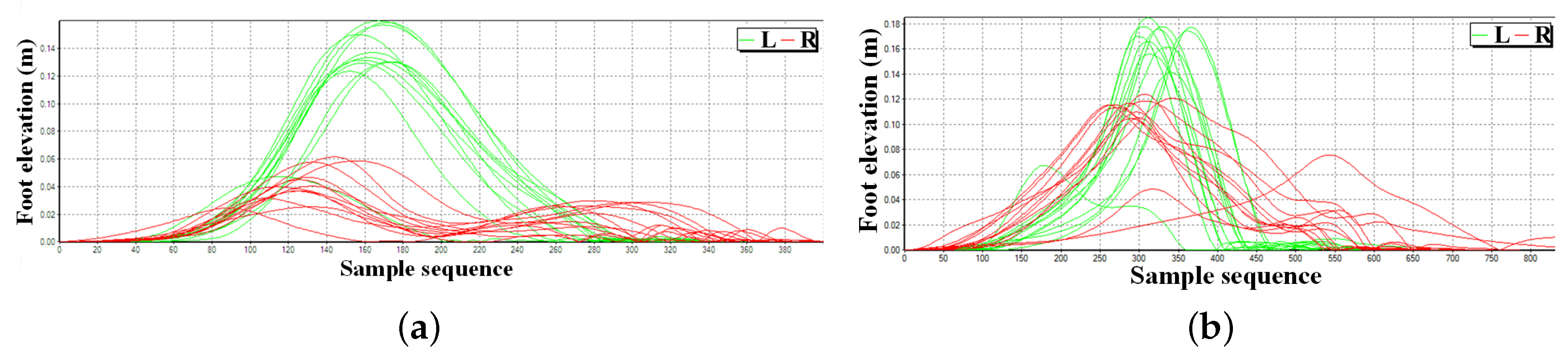
4.2. Feet Clearance Monitoring
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MEMS | Micro-Electro-Mechanical Sensor |
| BSN | Body Sensor Network |
| IMU | Inertial Measurement Unit |
| MARG | Magnetic Angular Rate and Gravity |
| ZVU | Zero Velocity Updating |
References
- Albert, M.V.; Azeze, Y.; Courtois, M.; Jayaraman, A. In-lab versus at-home activity recognition in ambulatory subjects with incomplete spinal cord injury. J. NeuroEng. Rehabil. 2017, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, A.; Cavuoto, L.A.; Crassidis, J.L. Hip and Trunk Kinematics Estimation in Gait through Kalman Filter using IMU Data at the Ankle. IEEE Sens. J. 2018, 18, 4253–4260. [Google Scholar] [CrossRef]
- Farris, R.J.; Quintero, H.A.; Murray, S.A.; Member, S.; Ha, K.H.; Hartigan, C.; Goldfarb, M. A Preliminary Assessment of Legged Mobility Provided by a Lower Limb Exoskeleton for Persons With Paraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Hu, H. Reducing drifts in the inertial measurements of wrist and elbow positions. IEEE Trans. Instrum. Meas. 2010, 59, 575–585. [Google Scholar] [CrossRef]
- Prakash, C.; Gupta, K.; Mittal, A.; Kumar, R.; Laxmi, V. Passive marker based optical system for gait kinematics for lower extremity. Procedia Comput. Sci. 2015, 45, 176–185. [Google Scholar] [CrossRef]
- Tian, Z.; Fang, X.; Zhou, M.; Li, L. Smartphone-based indoor integrated WiFi/MEMS positioning algorithm in a multi-floor environment. Micromachines 2015, 6, 347–363. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.Y.; Kang, H.C.; Kim, S.M. EMG analysis of lower limb muscle activation pattern during pedaling: Experiments and computer simulations. Int. J. Precis. Eng. Manuf. 2012, 13, 601–608. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, Z.; Zhao, H.; Liu, L.; Jiang, Y.; Li, J.; Fortino, G. Body Sensor Network based Robust Gait Analysis: Toward Clinical and at Home Use. IEEE Sens. J. 2018, 18, 1–9. [Google Scholar]
- Yu, L.; Zheng, J.; Wang, Y.; Song, Z.; Zhan, E. Adaptive method for real-time gait phase detection based on ground contact forces. Gait Posture 2015, 41, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wang, Z.; Zhao, H.; Hu, H. Using Distributed Wearable Sensors to Measure and Evaluate Human Lower Limb Motions. IEEE Trans. Instrum. Meas. 2016, 65, 939–950. [Google Scholar] [CrossRef] [Green Version]
- Brzostowski, K. Toward the Unaided Estimation of Human Walking Speed Based on Sparse Modeling. IEEE Trans. Instrum. Meas. 2018, 67, 1389–1398. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, Z.; Zhao, H.; Liu, L.; Jiang, Y. Using Body-Worn Sensors for Preliminary Rehabilitation Assessment in Stroke Victims with Gait Impairment. IEEE Access 2018, 6, 31249–31258. [Google Scholar] [CrossRef]
- Ren, M.; Guo, H.; Shi, J.; Meng, J. Indoor pedestrian navigation based on conditional random field algorithm. Micromachines 2017, 8, 320. [Google Scholar] [CrossRef]
- Barth, J.; Oberndorfer, C.; Pasluosta, C.; Schülein, S.; Gassner, H.; Reinfelder, S.; Kugler, P.; Schuldhaus, D.; Winkler, J.; Klucken, J.; Eskofier, B.M. Stride segmentation during free walk movements using multi-dimensional subsequence dynamic time warping on inertial sensor data. Sensors 2015, 15, 6419–6440. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Destelle, F.; Unzueta, L.; Monaghan, D.S.; Linaza, M.T.; Moran, K.; O’Connor, N.E. 3D human gait reconstruction and monitoring using body-worn inertial sensors and kinematic modeling. IEEE Sens. J. 2016, 16, 8823–8831. [Google Scholar] [CrossRef]
- Anwary, A.R.; Yu, H.; Vassallo, M. Optimal Foot Location for Placing Wearable IMU Sensors and Automatic Feature Extraction for Gait Analysis. IEEE Sens. J. 2018, 18, 2555–2567. [Google Scholar] [CrossRef]
- Chang, H.C.; Hsu, Y.L.; Yang, S.C.; Lin, J.C.; Wu, Z.H. A Wearable Inertial Measurement System With Complementary Filter for Gait Analysis of Patients With Stroke or Parkinson Disease. IEEE Access 2016, 4, 8442–8453. [Google Scholar] [CrossRef]
- Leal-Junior, A.G.; Frizera, A.; Avellar, L.M.; Marques, C.; Pontes, M.J. Polymer Optical Fiber for In-Shoe Monitoring of Ground Reaction Forces during the Gait. IEEE Sens. J. 2018, 18, 2362–2368. [Google Scholar] [CrossRef]
- Jiménez Ruiz, A.R.; Seco Granja, F.; Prieto Honorato, J.C.; Guevara Rosas, J.I. Accurate pedestrian indoor navigation by tightly coupling foot-mounted IMU and RFID measurements. IEEE Trans. Instrum. Meas. 2012, 61, 178–189. [Google Scholar] [CrossRef] [Green Version]
- Lan, K.C.; Shih, W.Y. Using smart-phones and floor plans for indoor location tracking. IEEE Trans. Hum. Mach. Syst. 2014, 44, 211–221. [Google Scholar]
- Fourati, H. Heterogeneous Data Fusion Algorithm for Pedestrian Navigation via Foot-Mounted Inertial Measurement Unit and Complementary Filter. IEEE Trans. Instru. Meas. 2015, 64, 221–229. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Qiu, S.; Shen, Y.; Zhang, L.; Tang, K. Heading Drift Reduction for Foot-Mounted Inertial Navigation System via Multi-Sensor Fusion and Dual-Gait Analysis. IEEE Sens. J. 2018, 18, 1–8. [Google Scholar]
- Wang, Z.L.; Zhao, H.Y.; Qiu, S.; Gao, Q. Stance phase detection for ZUPT-aided foot-mounted pedestrian navigation system. IEEE/ASME Trans. Mechatron. 2015, 20, 3170–3181. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Z.; Chen, Y.; Zhao, H. Mixed-kernel based weighted extreme learning machine for inertial sensor based human activity recognition with imbalanced dataset. Neurocomputing 2016, 190, 35–49. [Google Scholar] [CrossRef]
- Fortino, G.; Di Fatta, G.; Pathan, M.; Vasilakos, A.V. Cloud-assisted body area networks: State-of-the-art and future challenges. Wirel. Netw. 2014, 20, 1925–1938. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, C.; Qiu, S. A system of human vital signs monitoring and activity recognition based on body sensor network. Sens. Rev. 2014, 34, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Yang, Y.; Hou, J.; Ji, R. Ambulatory estimation of 3D walking trajectory and knee joint angle using MARG Sensors. In Proceedings of the Fourth International Conference on Innovative Computing Technology (INTECH), London, UK, 13–15 Augsut 2014; pp. 191–196. [Google Scholar]
- Gravina, R.; Alinia, P.; Ghasemzadeh, H.; Fortino, G. Multi-Sensor Fusion in Body Sensor Networks: State-of-the-art and research challenges. Inf. Fusion 2016, 35, 68–80. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, S.; Cao, Z.; Jiang, M. Quantitative assessment of dual gait analysis based on inertial sensors with body sensor network. Sens. Rev. 2013, 33, 48–56. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, Z.; Zhao, H. Heterogeneous data fusion for three-dimensional gait analysis using wearable MARG sensors. Int. J. Comput. Sci. Eng. 2017, 14, 222–233. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, Z.; Zhao, H.; Qin, K.; Li, Z.; Hu, H. Inertial/magnetic sensors based pedestrian dead reckoning by means of multi-sensor fusion. Inf. Fusion 2018, 39, 108–119. [Google Scholar] [CrossRef]
- Bao, S.D.; Meng, X.L.; Xiao, W.; Zhang, Z.Q. Fusion of inertial/magnetic sensor measurements and map information for pedestrian tracking. Sensors 2017, 17, 340. [Google Scholar] [CrossRef] [PubMed]
- Luinge, H.J.; Veltink, P.H.; Baten, C.T.M. Ambulatory measurement of arm orientation. J. Biomech. 2007, 40, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Dejnabadi, H.; Jolles, B.M.; Aminian, K. A new approach to accurate measurement of uniaxial joint angles based on a combination of accelerometers and gyroscopes. IEEE Trans. Biomed. Eng. 2005, 52, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Favre, J.; Aissaoui, R.; Jolles, B.M.; de Guise, J.A.; Aminian, K. Functional calibration procedure for 3D knee joint angle description using inertial sensors. J. Biomech. 2009, 42, 2330–2335. [Google Scholar] [CrossRef] [PubMed]
- Roetenberg, D.; Luinge, H.; Slycke, P. Xsens MVN: Full 6DOF Human Motion Tracking Using Miniature Inertial Sensors; Xsens Technologies: Enschede, The Netherlands, 2013; pp. 1–9. [Google Scholar]
- Wang, Z.; Li, J.; Wang, J.; Zhao, H.; Qiu, S.; Yang, N.; Shi, X. Inertial Sensor-Based Analysis of Equestrian Sports between Beginner and Professional Riders under. IEEE Trans. Instrum. Meas. 2018, 14, 1–13. [Google Scholar]
- Gheorghe, M.V.; Member, S.; Bodea, M.C.; Member, L.S. Calibration Optimization Study for Tilt-Compensated Compasses. IEEE Trans. Instrum. Meas. 2018, 67, 1486–1494. [Google Scholar] [CrossRef]
- Skog, I.; Händel, P.; Nilsson, J.O.; Rantakokko, J. Zero-velocity detection—An algorithm evaluation. IEEE Trans. Bio-Med. Eng. 2010, 57, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Bebek, O.; Suster, M.a.; Rajgopal, S.; Fu, M.J.; Huang, X.; Cavusoglu, M.C.; Young, D.J.; Mehregany, M.; Van Den Bogert, A.J.; Mastrangelo, C.H. Personal navigation via shoe mounted inertial measurement units. IEEE Trans. Instrum. Meas. 2010, 59, 3018–3027. [Google Scholar] [CrossRef]
- Martinez-Hernandez, U.; Mahmood, I.; Dehghani-Sanij, A.A. Simultaneous Bayesian Recognition of Locomotion and Gait Phases with Wearable Sensors. IEEE Sens. J. 2018, 18, 1282–1290. [Google Scholar] [CrossRef]
- Gouwanda, D.; Gopalai, A.A.; Khoo, B.H. A Low Cost Alternative to Monitor Human Gait Temporal Parameters-Wearable Wireless Gyroscope. IEEE Sens. J. 2016, 16, 9029–9035. [Google Scholar] [CrossRef]
- Wang, C.; Qu, X.; Zhang, X.; Zhu, W.; Fang, G. A fast calibration method for magnetometer array and the application of ferromagnetic target localization. IEEE Trans. Instrum. Meas. 2017, 66, 1743–1750. [Google Scholar] [CrossRef]
- Vargas-Valencia, L.; Elias, A.; Rocon, E.; Bastos-Filho, T.; Frizera, A. An IMU-to-Body Alignment Method Applied to Human Gait Analysis. Sensors 2016, 16, 2090. [Google Scholar] [CrossRef] [PubMed]
- Choe, N.; Zhao, H.; Qiu, S.; So, Y. A Sensor-to-Segment Calibration Method for Motion Capture system based on low cost mimu. Measurement 2018. [Google Scholar] [CrossRef]







| Gait Parameter | Description |
|---|---|
| Stride length (m) | Distance between two consecutive footprint of the same foot. |
| Stride speed (m/s) | Stride length divided by walking cycle. |
| Stride frequency | Number of steps taken per minute during walking. |
| Walking cycle (s) | Duration of a single stride, inversely proportional to cadence. |
| Stance time (s) | Duration of stance phase when feet contact with the ground, starting with initial-contact (IC) and ending with foot-off (FO) of the same foot. |
| Swing time (s) | Duration of swing phase when feet swing above the ground, starting with FO and ending with IC. |
| Clearance (m) | Foot elevation in swing phase, which reflects the muscular strength of lower limbs and can be diversified as maximum and minimum foot elevation. |
| Plantar & dorsiflex (degrees) | The angle between the dorsum of the foot and the back of the leg. |
| Knee ROM (degrees) | Range of knee flexion during a single stride. |
| Items | Observation Method | Optical System | Inertial Body Sensor Network (BSN) |
|---|---|---|---|
| Objectivity | subjective | objective | objective |
| Robustness | poor | sensitive to occlusion | very stable |
| Repeatability | poor | high | high |
| Efficiency | medium | low | high |
| Set-up time | several minutes | half-hour | several minutes |
| Usability | high | low | high |
| Visual text | no | partial | fully |
| Unit | Accelerometer | Gyroscope | Magnetometer |
|---|---|---|---|
| Dimensions | 3 axes | 3 axes | 3 axes |
| Dynamic Range | m/s | deg/s | mGauss |
| Bandwidth (Hz) | 30 | 40 | 10 |
| Linearity (% of FS) | 0.2 | 0.1 | 0.2 |
| Bias stability (unit ) | 0.02 | 1 | 0.1 |
| Alignment Error (deg) | 0.1 | 0.1 | 0.1 |
| Parameter | Healthy | Neurological | Arthropathy |
|---|---|---|---|
| Stride length (m) | 1.21 | 0.68 | |
| Stride speed (m/s) | 0.94 | ||
| Stride frequency | 92 | 64 | 72 |
| Walking cycle (s) | 1.32 | 1.68 | 1.48 |
| Stance time (s) | 0.86 | 1.14 | 0.99 |
| Swing time (s) | 0.46 | 0.54 | 0.49 |
| Clearance (m) | 0.22 | 0.08 | 0.14 |
| Knee ROM (degrees) |
| Joint Angle ° | Heel Strike | Foot Flat | Heel Off | Swing |
|---|---|---|---|---|
| Knee joint (Healthy subject) | ||||
| Knee joint (Stroke Patient) | ||||
| Ankle joint (Healthy subject) | ||||
| Ankle joint (Stroke Patient) |
| Item | Source | SS | df | MS | F | p-Value |
|---|---|---|---|---|---|---|
| Prior treatment | Columns | 285.1 | 19 | 15.0053 | 0.39 | 0.9787 |
| Error | 778 | 20 | 38.9 | |||
| Total | 1063.1 | 39 | ||||
| Post treatment 2 weeks | Columns | 260.275 | 19 | 13.6987 | 1.26 | 0.3061 |
| Error | 217.5 | 20 | 10.875 | |||
| Total | 477.775 | 39 | ||||
| Post treatment 6 weeks | Columns | 227.275 | 19 | 11.9618 | 3.39 | 0.0046 |
| Error | 70.5 | 20 | 3.525 | |||
| Total | 297.775 | 39 |
| Item | Source | SS | df | MS | F | p-Value |
|---|---|---|---|---|---|---|
| Prior treatment | Columns | 304.28 | 19 | 16.0145 | 0.23 | 0.9988 |
| Error | 1385.5 | 20 | 69.275 | |||
| Total | 1689.78 | 39 | ||||
| Post treatment 2 weeks | Columns | 203.9 | 19 | 10.7316 | 0.21 | 0.9993 |
| Error | 1006 | 20 | 50.3 | |||
| Total | 1209.9 | 39 | ||||
| Post treatment 6 weeks | Columns | 165.6 | 19 | 8.7158 | 0.6 | 0.8637 |
| Error | 290 | 20 | 14.5 | |||
| Total | 455.6 | 39 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Liu, L.; Zhao, H.; Wang, Z.; Jiang, Y. MEMS Inertial Sensors Based Gait Analysis for Rehabilitation Assessment via Multi-Sensor Fusion. Micromachines 2018, 9, 442. https://doi.org/10.3390/mi9090442
Qiu S, Liu L, Zhao H, Wang Z, Jiang Y. MEMS Inertial Sensors Based Gait Analysis for Rehabilitation Assessment via Multi-Sensor Fusion. Micromachines. 2018; 9(9):442. https://doi.org/10.3390/mi9090442
Chicago/Turabian StyleQiu, Sen, Long Liu, Hongyu Zhao, Zhelong Wang, and Yongmei Jiang. 2018. "MEMS Inertial Sensors Based Gait Analysis for Rehabilitation Assessment via Multi-Sensor Fusion" Micromachines 9, no. 9: 442. https://doi.org/10.3390/mi9090442





