The History and Horizons of Microscale Neural Interfaces
Abstract
:1. Introduction
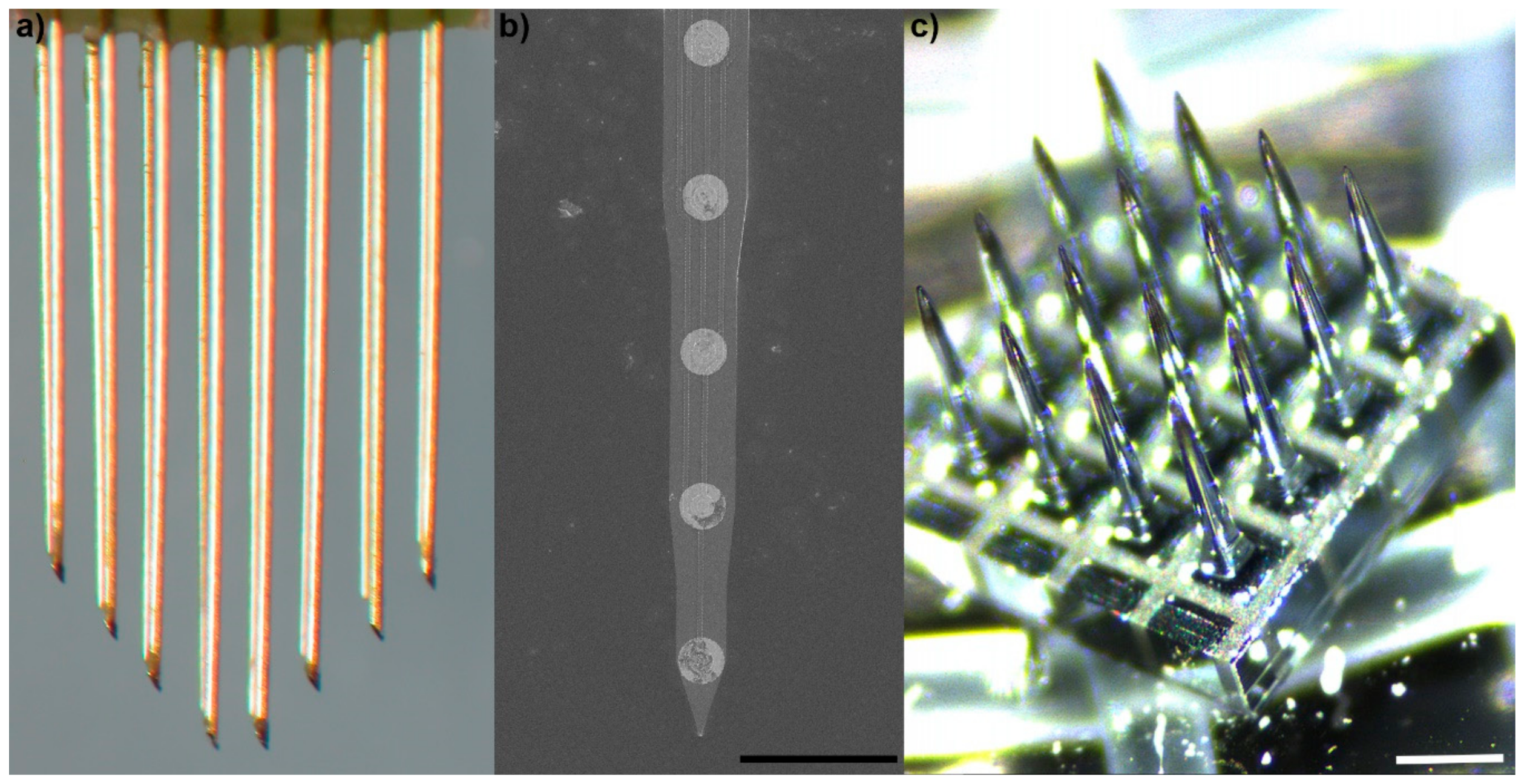
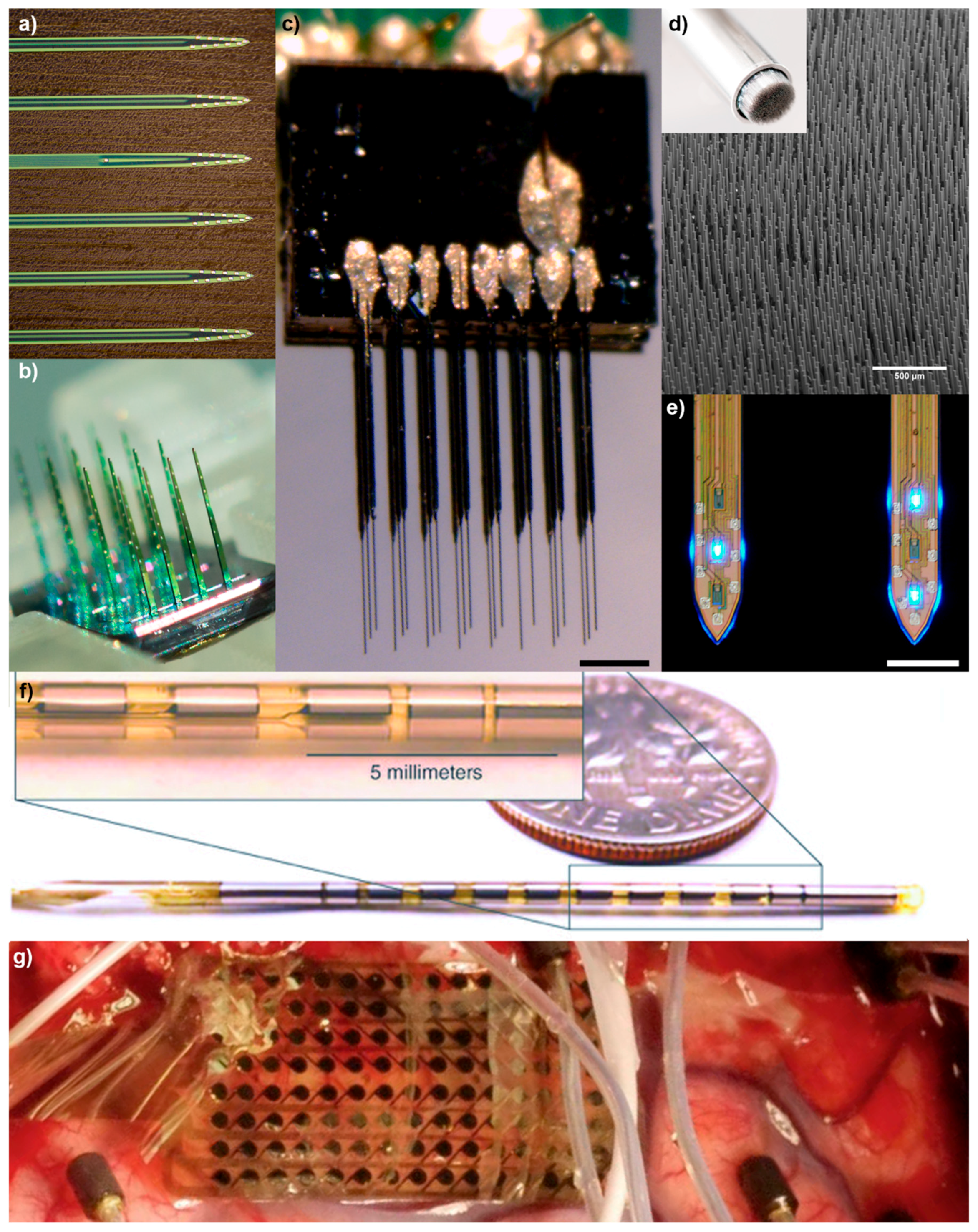
2. Brief History of Microscale Implantable Neural Technologies
3. Challenges on the Horizon
4. Need for the Science of Neural Engineering
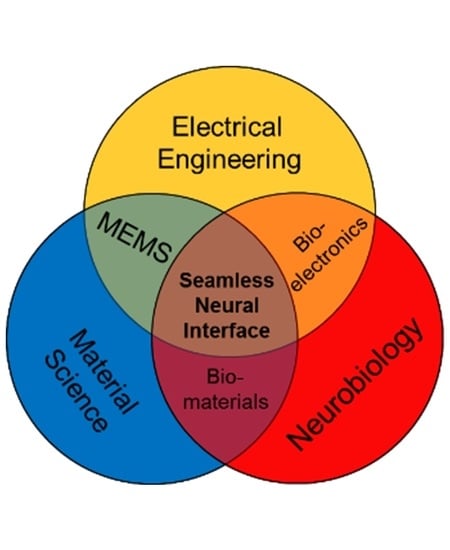
5. Need for Scientific and Engineering Convergence
6. Need for Diversity
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- House, L.R. Cochlear implant: The beginning. Laryngoscope 1987, 97 Pt 1, 996–997. [Google Scholar]
- House, W.F. Cochlear implants. Ann. Otol. Rhinol. Laryngol. 1976, 85 Pt 2 (Suppl. 27), 1–93. [Google Scholar] [CrossRef]
- Dobelle, W.H.; Mladejovsky, M.G.; Girvin, J.P. Artifical vision for the blind: Electrical stimulation of visual cortex offers hope for a functional prosthesis. Science 1974, 183, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Dobelle, W.; Mladejovsky, M. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. J. Physiol. 1974, 243, 553–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wodlinger, B.; Downey, J.E.; Tyler-Kabara, E.C.; Schwartz, A.B.; Boninger, M.L.; Collinger, J.L. Ten-dimensional anthropomorphic arm control in a human brain-machine interface: Difficulties, solutions, and limitations. J. Neural Eng. 2015, 12, 016011. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Collinger, J.L.; Degenhart, A.D.; Tyler-Kabara, E.C.; Schwartz, A.B.; Moran, D.W.; Weber, D.J.; Wodlinger, B.; Vinjamuri, R.K.; Ashmore, R.C.; et al. An electrocorticographic brain interface in an individual with tetraplegia. PLoS ONE 2013, 8, e55344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collinger, J.L.; Wodlinger, B.; Downey, J.E.; Wang, W.; Tyler-Kabara, E.C.; Weber, D.J.; McMorland, A.J.; Velliste, M.; Boninger, M.L.; Schwartz, A.B. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 2013, 381, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, L.R.; Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Simeral, J.D.; Vogel, J.; Haddadin, S.; Liu, J.; Cash, S.S.; van der Smagt, P.; et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012, 485, 372–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H.; Branner, A.; Chen, D.; Penn, R.D.; Donoghue, J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Cushing, H. A note upon the faradic stimulation of the postcentral gyrus in conscious patients. Brain 1909, 32, 44–53. [Google Scholar] [CrossRef]
- Flesher, S.N.; Collinger, J.L.; Foldes, S.T.; Weiss, J.M.; Downey, J.E.; Tyler-Kabara, E.C.; Bensmaia, S.J.; Schwartz, A.B.; Boninger, M.L.; Gaunt, R.A. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 2016, 8, 361ra141. [Google Scholar] [CrossRef] [PubMed]
- Strumwasser, F. Long-term recording’ from single neurons in brain of unrestrained mammals. Science 1958, 127, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Kipke, D.R.; Shain, W.; Buzsaki, G.; Fetz, E.; Henderson, J.M.; Hetke, J.F.; Schalk, G. Advanced neurotechnologies for chronic neural interfaces: New horizons and clinical opportunities. J. Neurosci. 2008, 28, 11830–11838. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.B.; Cui, X.T.; Weber, D.J.; Moran, D.W. Brain-controlled interfaces: Movement restoration with neural prosthetics. Neuron 2006, 52, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Giancoli, D.C. Physics: Principles with Applications; Prentice Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Galvani, L.; Aldini, G. Aloysii Galvani... De Viribus Electricitatis in Motu Musculari Commentarius cum ioannis Aldini Dissertatione et notis. Accesserunt Epistolæ ad Animalis Electricitatis Theoriam Pertinentes; Apud Societatem Typographicam: Paris, France, 1792. [Google Scholar]
- Galvani, L. D Viribus Electricitatis in Motu Musculari: Commentarius; Bologna: Tip; Istituto delle Scienze: Bologna, Italy, 1791; p. 58. [Google Scholar]
- Du Bois-Reymond, E.H. Untersuchungen über Thierische Elektricität. Anal. Phys. 1884, 151, 463–464. [Google Scholar] [CrossRef]
- Fritsch, G.; Hitzig, E. Ueber die elektrische Erregbarkeit des Grosshirns. Arch. Anat. Physiol. Wiss. Medizin. 1870, 37, 300–332. [Google Scholar]
- Volta, A. XVII. On the electricity excited by the mere contact of conducting substances of different kinds. In a letter from Mr. Alexander Volta, FRS Professor of Natural Philosophy in the University of Pavia, to the Rt. Hon. Sir Joseph Banks, Bart. KBPR S. Philos. Trans. R. Soc. Lond. 1800, 90, 403–431. [Google Scholar] [CrossRef]
- Dow, B.M.; Vautin, R.G.; Bauer, R. The mapping of visual space onto foveal striate cortex in the macaque monkey. J. Neurosci. 1985, 5, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Iordanova, B.; Vazquez, A.L.; Kozai, T.D.Y.; Fukuda, M.; Kim, S.G. Optogenetic investigation of the variable neurovascular coupling along the interhemispheric circuits. J. Cereb. Blood Flow Metab. 2018, 38, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Stecker, M. Factors Affecting Stimulus Artifact: Solution Factors. EC Neurol. 2017, 5, 52–61. [Google Scholar]
- Ben-Menachem, E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002, 1, 477–482. [Google Scholar] [CrossRef]
- Kahn, A. Motion artifacts and streaming potentials in relation to biological electrodes. In Proceedings of the Dig 6th International Conference Medical Electronics and Biological Engineering, Tokyo, Japan, 22–27 August 1965; Volume 112, pp. 562–563. [Google Scholar]
- Espinosa, J.; Aiello, M.T.; Naritoku, D.K. Revision and removal of stimulating electrodes following long-term therapy with the vagus nerve stimulator. Surg. Neurol. 1999, 51, 659–664. [Google Scholar] [CrossRef]
- Penry, J.K.; Dean, J.C. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: Preliminary results. Epilepsia 1990, 31 (Suppl. 2), S40–S43. [Google Scholar] [CrossRef]
- Rutecki, P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia 1990, 31 (Suppl. 2), S1–S6. [Google Scholar] [CrossRef]
- Brindley, G.S.; Lewin, W.S. The sensations produced by electrical stimulation of the visual cortex. J. Physiol. 1968, 196, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, A.B. Cortical neural prosthetics. Annu. Rev. Neurosci. 2004, 27, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Paralikar, K.; Rao, C.; Clement, R.S. Automated reduction of non-neuronal signals from intra-cortical microwire array recordings by use of correlation technique. In Proceedings of the 30th Annual International Conference of the IEEE EMBS 2008 Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 21–24 August 2008; pp. 46–49. [Google Scholar]
- Agnesi, F.; Muralidharan, A.; Baker, K.B.; Vitek, J.L.; Johnson, M.D. Fidelity of frequency and phase entrainment of circuit-level spike activity during DBS. J. Neurophysiol. 2015, 114, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Collinger, J.L.; Kryger, M.A.; Barbara, R.; Betler, T.; Bowsher, K.; Brown, E.H.; Clanton, S.T.; Degenhart, A.D.; Foldes, S.T.; Gaunt, R.A.; et al. Collaborative approach in the development of high-performance brain-computer interfaces for a neuroprosthetic arm: Translation from animal models to human control. Clin. Transl. Sci. 2014, 7, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; McIntosh, J.; Bak, M. Long-term implants of Parylene-C coated microelectrodes. Med. Biol. Eng. Comput. 1988, 26, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Drake, K.L.; Wise, K.D.; Farraye, J.; Anderson, D.J.; BeMent, S.L. Performance of planar multisite microprobes in recording extracellular single-unit intracortical activity. IEEE Trans. Biomed. Eng. 1988, 35, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Eles, J.R.; Ludwig, K.A.; Seymour, J.P.; Michelson, N.J.; McFadden, W.E.; Vazquez, A.L.; Kozai, T.D. A Materials Roadmap to Functional Neural Interface Design. Adv. Funct. Mater. 2018, 28, 201701269. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.K.; Jones, K.E.; Huber, R.J.; Horch, K.W.; Normann, R.A. A silicon-based, three-dimensional neural interface: Manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 1991, 38, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Zhang, H.; Robbins, M.T.; Nofar, J.B.; Marshall, S.P.; Kobylarek, M.J.; Kozai, T.D.Y.; Kotov, N.A.; Chestek, C.A. Chronic In Vivo Stability Assessment of Carbon Fiber Microelectrode Arrays. J. Neural Eng. 2016, 13, 066002. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Na, K.; Zhang, H.; Kozai, T.D.Y.; Kotov, N.A.; Yoon, E.; Chestek, C.A. Insertion of linear 8.4 mu m diameter 16 channel carbon fiber electrode arrays for single unit recordings. J. Neural Eng. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Mendrela, A.E.; Kim, K.; English, D.; McKenzie, S.; Seymour, J.P.; Buzsáki, G.; Yoon, E. A High-Resolution Opto-Electrophysiology System With a Miniature Integrated Headstage. IEEE Trans. Biomed. Circuits Syst. 2018. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.P.; Wu, F.; Wise, K.D.; Yoon, E. State-of-the-art MEMS and microsystem tools for brain research. Microsyst. Nanoeng. 2017, 3, 16066. [Google Scholar] [CrossRef] [Green Version]
- Kampasi, K.; Stark, E.; Seymour, J.; Na, K.; Winful, H.G.; Buzsáki, G.; Wise, K.D.; Yoon, E. Fiberless multicolor neural optoelectrode for in vivo circuit analysis. Sci. Rep. 2016, 6, 30961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, E.Ç.; Freedman, D.S.; Gökkavas, M.; Özbay, E.; Sahin, M.; Ünlü, M.S. Improved selectivity from a wavelength addressable device for wireless stimulation of neural tissue. Front. Neuroeng. 2014, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurram, A.; Seymour, J.P. Investigation of the photoelectrochemical effect in optoelectrodes and potential uses for implantable electrode characterization. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 3032–3035. [Google Scholar]
- Seymour, J.P.; Langhals, N.B.; Anderson, D.J.; Kipke, D.R. Novel multi-sided, microelectrode arrays for implantable neural applications. Biomed. Microdevices 2011. [Google Scholar] [CrossRef] [PubMed]
- Eles, J.R.; Vazquez, A.L.; Snyder, N.R.; Lagenaur, C.F.; Murphy, M.C.; Kozai, T.D.Y.; Cui, X.T. Neuroadhesive L1 coating attenuates acute microglial attachment to neural electrodes as revealed by live two-photon microscopy. Biomaterials 2017, 113, 279–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.Y.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Khilwani, R.; Gilgunn, P.J.; Kozai, T.D.Y.; Ong, X.C.; Korkmaz, E.; Gunalan, P.K.; Cui, X.T.; Fedder, G.K.; Ozdoganlar, O.B. Ultra-miniature ultra-compliant neural probes with dissolvable delivery needles: Design, fabrication and characterization. Biomed. Microdevices 2016, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Catt, K.; Du, Z.; Na, K.; Srivannavit, O.; Haque, R.-U.M.; Seymour, J.; Wise, K.D.; Yoon, E.; Cui, X.T. Chronic In Vivo Evaluation of PEDOT/CNT for Stable Neural Recordings. IEEE Trans. Bio-Med. Eng. 2016, 63, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolarcik, C.L.; Luebben, S.D.; Sapp, S.A.; Hanner, J.; Snyder, N.; Kozai, T.D.Y.; Chang, E.; Nabity, J.A.; Nabity, S.T.; Lagenaur, C.F.; et al. Elastomeric and soft conducting microwires for implantable neural interfaces. Soft Matter 2015, 11, 4847–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alba, N.A.; Du, Z.J.; Catt, K.A.; Kozai, T.D.Y.; Cui, X.T. In vivo electrochemical analysis of a PEDOT/MWCNT neural electrode coating. Biosensors 2015, 5, 618–646. [Google Scholar] [CrossRef] [PubMed]
- Kolarcik, C.L.; Catt, K.; Rost, E.; Albrecht, I.N.; Bourbeau, D.; Du, Z.; Kozai, T.D.Y.; Luo, X.; Weber, D.J.; Cui, X.T. Evaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglion. J. Neural Eng. 2015, 12, 016008. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Alba, N.A.; Zhang, H.; Kotov, N.A.; Gaunt, R.A.; Cui, X.T. Nanostructured Coatings for Improved Charge Delivery to Neurons. In Nanotechnology and Neuroscience: Nano-electronic, Photonic and Mechanical Neuronal Interfacing; De Vittorio, M., Martiradonna, L., Assad, J., Eds.; Springer: New York, NY, USA, 2014; pp. 71–134. [Google Scholar]
- Gilgunn, P.J.K.R.; Kozai, T.D.Y.; Weber, D.J.; Cui, X.T.; Erdos, G.; Ozdoganlar, O.B.; Fedder, G.K. An ultra-compliant, scalable neural probes with molded biodissolvable delivery vehicle. In Proceedings of the 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), Paris, France, 29 January–2 Febuary 2012; Volume 2012, pp. 56–59. [Google Scholar]
- Kozai, T.D.Y.; Kipke, D.R. Insertion shuttle with carboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain. J. Neurosci. Methods 2009, 184, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escamilla-Mackert, T.; Langhals, N.B.; Kozai, T.D.Y.; Kipke, D.R. Insertion of a three dimensional silicon microelectrode assembly through a thick meningeal membrane. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2009, 1616–1618. [Google Scholar] [PubMed]
- Kozai, T.D.Y.; Jaquins-gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Dexamethasone retrodialysis attenuates microglial response to implanted probes in vivo. Biomaterials 2016, 87, 157–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelson, N.J.; Vazquez, A.L.; Eles, J.R.; Salatino, J.W.; Purcell, E.K.; Williams, J.J.; Cui, X.T.; Kozai, T.D.Y. Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissue-implant neural interface: New Emphasis on the Biological Interface. J. Neural Eng. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Hires, S.A.; Mao, T.; Huber, D.; Chiappe, M.E.; Chalasani, S.H.; Petreanu, L.; Akerboom, J.; McKinney, S.A.; Schreiter, E.R.; et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 2009, 6, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dana, H.; Chen, T.W.; Hu, A.; Shields, B.C.; Guo, C.; Looger, L.L.; Kim, D.S.; Svoboda, K. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS ONE 2014, 9, e108697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cichon, J.; Wang, W.; Qiu, L.; Lee, S.-J.; Campbell, N.R.; DeStefino, N.; Goard, M.J.; Fu, Z.; Yasuda, R.; et al. Imaging Neural Activity Using Thy1-GCaMP Transgenic Mice. Neuron 2012, 76, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Chan, A.W.; McGirr, A.; Xue, S.; Xiao, D.; Zeng, H.; Murphy, T.H. Resolution of high-frequency mesoscale intracortical maps using the genetically encoded glutamate sensor iGluSnFR. J. Neurosci. 2016, 36, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Kozai, T.D.Y. Understanding the Inflammatory Tissue Reaction to Brain Implants to Improve Neurochemical Sensing Performance. ACS Chem. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Jaquins-Gerstl, A.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Brain Tissue Responses to Neural Implants Impact Signal Sensitivity and Intervention Strategies. ACS Chem. Neurosci. 2015, 6, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Salatino, J.W.; Ludwig, K.A.; Kozai, T.D.Y.; Purcell, E.K. Glial responses to implanted electrodes in the brain. Nat. BME 2017, 1, 862–877. [Google Scholar] [CrossRef]
- Williams, J.C.; Rennaker, R.L.; Kipke, D.R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Brain Res. Protoc. 1999, 4, 303–313. [Google Scholar] [CrossRef]
- Bedell, H.W.; Hermann, J.K.; Ravikumar, M.; Lin, S.; Rein, A.; Li, X.; Molinich, E.; Smith, P.D.; Selkirk, S.M.; Miller, R.H.; et al. Targeting CD14 on blood derived cells improves intracortical microelectrode performance. Biomaterials 2018, 163, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Rousche, P.J.; Normann, R.A. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J. Neurosci. Methods 1998, 82, 1–15. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Marzullo, T.C.; Hooi, F.; Langhals, N.B.; Majewska, A.K.; Brown, E.B.; Kipke, D.R. Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping. J. Neural Eng. 2010, 7, 046011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozai, T.D.Y.; Catt, K.; Li, X.; Gugel, Z.V.; Olafsson, V.T.; Vazquez, A.L.; Cui, X.T. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials 2015, 37, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.; Xue, Q.S.; Dieme, R.; Sankar, V.; Mayrand, R.C.; Nishida, T.; Streit, W.J.; Sanchez, J.C. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front. Neuroeng. 2014, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013, 10, 066014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.R. Congress’ Joint Committee on Atomic Energy. In Authorizing Legislation for FY 1970; Congress of the United States: Washington, DC, USA, 1969. [Google Scholar]
- Michelson, N.J.; Islam, R.; Vazquez, A.L.; Ludwig, K.A.; Kozai, T.D.Y. Calcium activation of frequency dependent temporally phasic, localized, and dense population of cortical neurons by continuous electrical stimulation. BioRxiv 2018. [Google Scholar] [CrossRef]
- Michelson, N.J.; Kozai, T.D.Y. Isoflurane and Ketamine Differentially Influence Spontaneous and Evoked Laminar Electrophysiology in Mouse V1. J. Neurophysiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Kozai, T.D.Y. In vivo spatiotemporal dynamics of NG2 glia activity caused by neural electrode implantation. Biomaterials 2018, 164, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Eles, J.; Vazquez, A.; Kozai, T.; Cui, X. In vivo imaging of neuronal calcium during electrode implantation: Spatial and temporal mapping of damage and recovery. Biomaterials 2018. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Eles, J.R.; Vazquez, A.L.; Cui, X.T. Two-photon imaging of chronically implanted neural electrodes: Sealing methods and new insights. J. Neurosci. Methods 2016, 256, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Du, Z.; Gugel, Z.V.; Smith, M.A.; Chase, S.M.; Bodily, L.M.; Caparosa, E.M.; Friedlander, R.M.; Cui, X.T. Comprehensive chronic laminar single-unit, multi-unit, and local field potential recording performance with planar single shank electrode arrays. J. Neurosci. Methods 2015, 242, 15–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozai, T.D.Y.; Li, X.; Bodily, L.M.; Caparosa, E.M.; Zenonos, G.A.; Carlisle, D.L.; Friedlander, R.M.; Cui, X.T. Effects of caspase-1 knockout on chronic neural recording quality and longevity: Insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials 2014, 35, 9620–9634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozai, T.D.Y.; Vazquez, A.L.; Weaver, C.L.; Kim, S.-G.; Cui, X.T. In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. J. Neural Eng. 2012, 9, 066001–066001. [Google Scholar] [CrossRef] [PubMed]
- Golabchi, A.; Wu, B.; Li, X.; Carlisle, D.L.; Kozai, T.D.Y.; Friedlander, R.M.; Cui, X.T. Melatonin improves quality and longevity of chronic neural recording. Biomaterials 2018, 180, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Xue, Q.-S.; Sankar, V.; Nishida, T.; Shaw, G.; Streit, W.J.; Sanchez, J.C. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J. Neural Eng. 2012, 9, 056015. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.K.; Ravikumar, M.; Shoffstall, A.; Ereifej, E.S.; Kovach, K.; Chang, J.; Soffer, A.; Wong, C.T.; Srivastava, V.; Smith, P.; et al. Inhibition of the cluster of differentiation 14 innate immunity pathway with IAXO-101 improves chronic microelectrode performance. J. Neural Eng. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, M.; Sunil, S.; Black, J.; Barkauskas, D.S.; Haung, A.Y.; Miller, R.H.; Selkirk, S.M.; Capadona, J.R. The roles of blood-derived macrophages and resident microglia in the neuroinflammatory response to implanted intracortical microelectrodes. Biomaterials 2014, 35, 8049–8064. [Google Scholar] [CrossRef] [PubMed]
- Nolta, N.F.; Christensen, M.B.; Crane, P.D.; Skousen, J.L.; Tresco, P.A. BBB leakage, astrogliosis, and tissue loss correlate with silicon microelectrode array recording performance. Biomaterials 2015, 53, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Martin, D.C.; Tresco, P.A. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J. Biomed. Mater. Res. A 2007, 82, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Saxena, T.; Karumbaiah, L.; Gaupp, E.A.; Patkar, R.; Patil, K.; Betancur, M.; Stanley, G.B.; Bellamkonda, R.V. The impact of chronic blood-brain barrier breach on intracortical electrode function. Biomaterials 2013. [Google Scholar] [CrossRef] [PubMed]
- Karumbaiah, L.; Saxena, T.; Carlson, D.; Patil, K.; Patkar, R.; Gaupp, E.A.; Betancur, M.; Stanley, G.B.; Carin, L.; Bellamkonda, R.V. Relationship between intracortical electrode design and chronic recording function. Biomaterials 2013, 34, 8061–8074. [Google Scholar] [CrossRef] [PubMed]
- Karumbaiah, L.; Norman, S.E.; Rajan, N.B.; Anand, S.; Saxena, T.; Betancur, M.; Patkar, R.; Bellamkonda, R.V. The upregulation of specific interleukin (IL) receptor antagonists and paradoxical enhancement of neuronal apoptosis due to electrode induced strain and brain micromotion. Biomaterials 2012, 33, 5983–5996. [Google Scholar] [CrossRef] [PubMed]
- McConnell, G.C.; Rees, H.D.; Levey, A.I.; Gutekunst, C.A.; Gross, R.E.; Bellamkonda, R.V. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 2009, 6, 56003. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.K.; Seymour, J.P.; Yandamuri, S.; Kipke, D.R. In vivo evaluation of a neural stem cell-seeded prosthesis. J. Neural Eng. 2009, 6, 026005. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 2007, 28, 3594–3607. [Google Scholar] [CrossRef] [PubMed]
- Cody, P.A.; Eles, J.R.; Lagenaur, C.F.; Kozai, T.D.; Cui, X.T. Unique electrophysiological and impedance signatures between encapsulation types: An analysis of biological Utah array failure and benefit of a biomimetic coating in a rat model. Biomaterials 2018, 161, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Henze, D.A.; Borhegyi, Z.; Csicsvari, J.; Mamiya, A.; Harris, K.D.; Buzsaki, G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J. Neurophysiol. 2000, 84, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Kipke, D.R. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 2012, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozai, T.D.Y.; Gugel, Z.; Li, X.; Gilgunn, P.J.; Khilwani, R.; Ozdoganlar, O.B.; Fedder, G.K.; Weber, D.J.; Cui, X.T. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes. Biomaterials 2014, 35, 9255–9268. [Google Scholar] [CrossRef] [PubMed]
- Potter, K.A.; Buck, A.C.; Self, W.K.; Capadona, J.R. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses. J. Neural Eng. 2012, 9, 046020. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Nikolakopoulou, A.M.; Zhao, Z.; Sagare, A.P.; Si, G.; Lazic, D.; Barnes, S.R.; Daianu, M.; Ramanathan, A.; Go, A. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat. Med. 2018, 24, 326. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Y.-J.; Yang, N.; Chen, X.-J.; Huang, N.-X.; Zhang, J.; Wu, Y.; Liu, Z.; Gao, X.; Li, T. Enhancing Oligodendrocyte Myelination Rescues Synaptic Loss and Improves Functional Recovery after Chronic Hypoxia. Neuron 2018. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Cambi, F.; Kozai, T.D.Y. The role of oligodendrocytes and their progenitors on neural interface technology: A novel perspective on tissue regeneration and repair. Biomaterials 2018. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.P.; Rajdev, P.; Ellison, C.; Irazoqui, P.P. Toward a comparison of microelectrodes for acute and chronic recordings. Brain Res. 2009, 1282, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Tresco, P.A.; Winslow, B.D. The challenge of integrating devices into the central nervous system. Crit. Rev. Biomed. Eng. 2011, 39, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; McCreery, D.B.; Bullara, L.A.; Agnew, W.F. Evaluation of the stability of intracortical microelectrode arrays. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; McCreery, D.B.; Carter, R.R.; Bullara, L.A.; Yuen, T.G.; Agnew, W.F. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans. Rehabil. Eng. 1999, 7, 315–326. [Google Scholar] [PubMed]
- Córdova, F.A. Dear Colleague Letter: NSF INCLUDES (Inclusion across the Nation of Communities of Learners of Underrepresented Discoverers in Engineering and Science); National Science Foundation: Alexandria, VA, USA, 2016. [Google Scholar]
- Orfield, G. Diversity Challenged: Evidence on the Impact of Affirmative Action; ERIC: New York, NY, USA, 2001. [Google Scholar]
- Ellison, S.F.; Mullin, W.P. Diversity, social goods provision, and performance in the firm. J. Econ. Manag. Strategy 2014, 23, 465–481. [Google Scholar] [CrossRef]
- Hunt, V.; Layton, D.; Prince, S. Diversity Matters; McKinsey & Company: New York, NY, USA, 2015. [Google Scholar]
- Cox, T.H.; Lobel, S.A.; McLeod, P.L. Effects of ethnic group cultural differences on cooperative and competitive behavior on a group task. Acad. Manag. J. 1991, 34, 827–847. [Google Scholar]
- Miller, T.; del Carmen Triana, M. Demographic diversity in the boardroom: Mediators of the board diversity–firm performance relationship. J. Manag. Stud. 2009, 46, 755–786. [Google Scholar] [CrossRef]
- Richard, O.C.; Murthi, B.S.; Ismail, K. The impact of racial diversity on intermediate and long-term performance: The moderating role of environmental context. Strateg. Manag. J. 2007, 28, 1213–1233. [Google Scholar] [CrossRef]
- Buttner, E.H.; Lowe, K.B.; Billings-Harris, L. The challenge of increasing minority-group professional representation in the United States: Intriguing findings. Int. J. Hum. Resour. Manag. 2009, 20, 771–789. [Google Scholar] [CrossRef]
- Athey, S.; Avery, C.; Zemsky, P. Mentoring and diversity. Am. Econ. Rev. 2000, 90, 765–786. [Google Scholar] [CrossRef]
- Hong, L.; Page, S.E. Groups of diverse problem solvers can outperform groups of high-ability problem solvers. Proc. Natl. Acad. Sci. USA 2004, 101, 16385–16389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, L.R.; Maier, N.R. Quality and acceptance of problem solutions by members of homogeneous and heterogeneous groups. J. Abnorm. Soc. Psychol. 1961, 62, 401. [Google Scholar] [CrossRef] [PubMed]
- McLeod, P.L.; Lobel, S.A.; Cox, T.H., Jr. Ethnic diversity and creativity in small groups. Small Group Res. 1996, 27, 248–264. [Google Scholar] [CrossRef]
- Herring, C. Does diversity pay: Race, gender, and the business case for diversity. Am. Sociol. Rev. 2009, 74, 208–224. [Google Scholar] [CrossRef]
- Grossman, G.M.; Maggi, G. Diversity and trade. Am. Econ. Rev. 2000, 90, 1255–1275. [Google Scholar] [CrossRef]
- Jones, J.R.; Wilson, D.C.; Jones, P. Toward Achieving the “Beloved Community” in the Workplace: Lessons for Applied Business Research and Practice From the Teachings of Martin Luther King Jr. Bus. Soc. 2008, 47, 457–483. [Google Scholar] [CrossRef]
- Watson, W.E.; Kumar, K.; Michaelsen, L.K. Cultural diversity’s impact on interaction process and performance: Comparing homogeneous and diverse task groups. Acad. Manag. J. 1993, 36, 590–602. [Google Scholar]
- Ostrom, E. The Difference: How the Power of Diversity Creates Better Groups, Firms, Schools, and Societies. By Page Scott E. Princeton: Princeton University Press, 2007. 448p. $27.95 cloth, $19.95 paper. Perspect. Politics 2008, 6, 828–829. [Google Scholar] [CrossRef]
- Bristow, L.R.; Butler, A.S.; Smedley, B.D. In the Nation’s Compelling Interest: Ensuring Diversity in the Health-Care Workforce; National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Antonio, A.L.; Chang, M.J.; Hakuta, K.; Kenny, D.A.; Levin, S.; Milem, J.F. Effects of racial diversity on complex thinking in college students. Psychol. Sci. 2004, 15, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, J.; Eatman, J. Effects of racial composition on small work groups. Small Group Behav. 1977, 8, 479–486. [Google Scholar] [CrossRef]
- Watson, W.E.; Kumar, K. Differences in decision making regarding risk taking: A comparison of culturally diverse and culturally homogeneous task groups. Int. J. Int. Relat. 1992, 16, 53–65. [Google Scholar] [CrossRef]
- Copeland, L. Valuing Diversity, Part 1: Making the Most of Cultural Differences at the Workplace. Personnel 1988, 65, 52–54. [Google Scholar]
- Cox, T., Jr. Creating the Multicultural Organization: A Strategy for Capturing the Power of Diversity; Jossey-Bass: San Francisco, CA, USA, 2001. [Google Scholar]
- Cox, T., Jr. The multicultural organization. Acad. Manag. Perspect. 1991, 5, 34–47. [Google Scholar] [CrossRef]
- Cox, T.H.; Blake, S. Managing cultural diversity: Implications for organizational competitiveness. Acad. Manag. Perspect. 1991, 5, 45–56. [Google Scholar] [CrossRef]
- Maznevski, M.L. Understanding our differences: Performance in decision-making groups with diverse members. Hum. Relat. 1994, 47, 531–552. [Google Scholar] [CrossRef]
- Mohammed, S.; Angell, L.C. Surface-and deep-level diversity in workgroups: Examining the moderating effects of team orientation and team process on relationship conflict. J. Organ. Behav. 2004, 25, 1015–1039. [Google Scholar] [CrossRef]
- Richard, O.; McMillan, A.; Chadwick, K.; Dwyer, S. Employing an innovation strategy in racially diverse workforces: Effects on firm performance. Group Organ. Manag. 2003, 28, 107–126. [Google Scholar] [CrossRef]
- Thomas, D.A. Diversity as strategy. Harv. Bus. Rev. 2004, 82, 98–98. [Google Scholar] [PubMed]
- Johnson, N.L. Science of CI: Resources for change. In Collective Intelligence: Creating a Prosperous World at Peace; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2008; pp. 265–274. [Google Scholar]
- Johnson, N.L.; Watkins, J.H. The Where-How of Leadership Emergence (WHOLE) Landscape: Charting Emergent Collective Leadership. 2009. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=1516618 (accessed on 26 Aug 2018).[Green Version]
- Whitla, D.K.; Orfield, G.; Silen, W.; Teperow, C.; Howard, C.; Reede, J. Educational benefits of diversity in medical school: A survey of students. Acad. Med. 2003, 78, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E. Diversification of US medical schools via affirmative action implementation. BMC Med. Educ. 2003, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, W.M. A Success Story: Recruiting & Retaining Underrepresented Minority Doctoral Students in Biomedical Engineering. Lib. Educ. 2006, 92, 52–55. [Google Scholar]
- Rabinowitz, H.K.; Diamond, J.J.; Veloski, J.J.; Gayle, J.A. The impact of multiple predictors on generalist physicians’ care of underserved populations. Am. J. Public Health 2000, 90, 1225. [Google Scholar] [PubMed]
- Saha, S.; Taggart, S.H.; Komaromy, M.; Bindman, A.B. Do patients choose physicians of their own race? Health Aff. 2000, 19, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Traylor, A.H.; Schmittdiel, J.A.; Uratsu, C.S.; Mangione, C.M.; Subramanian, U. Adherence to cardiovascular disease medications: Does patient-provider race/ethnicity and language concordance matter? J. Gen. Intern. Med. 2010, 25, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Noah, B.A. The participation of underrepresented minorities in clinical research. Am. J. Law Med. 2003, 29, 221. [Google Scholar] [PubMed]
- Saha, S.; Shipman, S.A. Race-neutral versus race-conscious workforce policy to improve access to care. Health Aff. 2008, 27, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Holm, K.; Gerard, P.; McElmurry, B.; Foreman, M.; Poslusny, S.; Dallas, C. Bridges to the doctorate: Mentored transition to successful completion of doctoral study for underrepresented minorities in nursing science. Nurs. Outlook 2009, 57, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Magnus, S.A.; Mick, S.S. Medical schools, affirmative action, and the neglected role of social class. Am. J. Public Health 2000, 90, 1197. [Google Scholar] [PubMed]
- Shaya, F.T.; Gbarayor, C.M. The case for cultural competence in health professions education. Am. J. Pharm. Educ. 2006, 70, 124. [Google Scholar] [CrossRef] [PubMed]
- Komaromy, M.; Grumbach, K.; Drake, M.; Vranizan, K.; Lurie, N.; Keane, D.; Bindman, A.B. The role of black and Hispanic physicians in providing health care for underserved populations. N. Engl. J. Med. 1996, 334, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Guiton, G.; Chang, M.J.; Wilkerson, L. Student body diversity: Relationship to medical students’ experiences and attitudes. Acad. Med. 2007, 82, S85–S88. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Lassiter, S.L. Addressing health care disparities and increasing workforce diversity: The next step for the dental, medical, and public health professions. Am. J. Public Health 2006, 96, 2093–2097. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, J.J.; Back, M.R.; Brotherton, S.E. The respective racial and ethnic diversity of US pediatricians and American children. Pediatrics 2000, 105, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Okunseri, C.; Bajorunaite, R.; Abena, A.; Self, K.; Iacopino, A.M.; Flores, G. Racial/ethnic disparities in the acceptance of Medicaid patients in dental practices. J. Public Health Dent. 2008, 68, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Friedemann, M.-L.; Pagan-Coss, H.; Mayorga, C. The workings of a multicultural research team. J. Transcult. Nurs. 2008, 19, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Ginther, D.K.; Schaffer, W.T.; Schnell, J.; Masimore, B.; Liu, F.; Haak, L.L.; Kington, R.S. Diversity in Academic Biomedicine: An Evaluation of Education and Career Outcomes with Implications for Policy. 2009. Available online: https://core.ac.uk/download/pdf/6268557.pdf (accessed on 26 Aug 2018).
- Brown, T.T.; Scheffler, R.M.; Tom, S.E.; Schulman, K.A. Does the Market Value Racial and Ethnic Concordance in Physician–Patient Relationships? Health Serv. Res. 2007, 42, 706–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teal, C.R.; Street, R.L. Critical elements of culturally competent communication in the medical encounter: A review and model. Soc. Sci. Med. 2009, 68, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Grumbach, K.; Coffman, J.M.; Young, J.Q.; Vranizan, K.; Blick, N. Physician supply and medical education in California. A comparison with national trends. West. J. Med. 1998, 168, 412. [Google Scholar] [PubMed]
- Katz, R.V.; Kegeles, S.S.; Kressin, N.R.; Green, B.L.; James, S.A.; Wang, M.Q.; Russell, S.L.; Claudio, C. Awareness of the Tuskegee Syphilis Study and the US presidential apology and their influence on minority participation in biomedical research. Am. J. Public Health 2008, 98, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.; Mahan, J.; Perry, R. Minority recruitment to the health professions: A matched comparison six-year follow-up. J. Med. Educ. 1981, 56, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.A.; Ferry, P.G.; King, J.E.; Martinez-Wedig, C.; Michael, L.H. Increasing access to medical education for students from medically underserved communities: One program’s success. Acad. Med. 2003, 78, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Guiton, G.; Wimmers, P.F.; Wilkerson, L. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA 2008, 300, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Daley, S.; Wingard, D.L.; Reznik, V. Improving the retention of underrepresented minority faculty in academic medicine. J. Natl. Med. Assoc. 2006, 98, 1435. [Google Scholar] [PubMed]
- Thom, D.H.; Tirado, M.D.; Woon, T.L.; McBride, M.R. Development and evaluation of a cultural competency training curriculum. BMC Med. Educ. 2006, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Pohlhaus, J.R.; Jiang, H.; Wagner, R.M.; Schaffer, W.T.; Pinn, V.W. Sex differences in application, success, and funding rates for NIH extramural programs. Acad. Med. 2011, 86, 759. [Google Scholar] [CrossRef] [PubMed]
- Tabak, L.A.; Collins, F.S. Weaving a richer tapestry in biomedical science. Science 2011, 333, 940–941. [Google Scholar] [CrossRef] [PubMed]
- Heggeness, M.L.; Evans, L.; Pohlhaus, J.R.; Mills, S.L. Measuring diversity of the National Institutes of Health-funded workforce. Acad. Med. 2016, 91, 1164. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Fode, K.L. The effect of experimenter bias on the performance of the albino rat. Syst. Res. Behav. Sci. 1963, 8, 183–189. [Google Scholar] [CrossRef]
- Sowell, T. Affirmative Action around the World: An Empirical Study; Yale University Press: New Haven, CT, USA, 2004. [Google Scholar]
- Massey, D.S.; Mooney, M. The effects of America’s three affirmative action programs on academic performance. Soc. Prob. 2007, 54, 99–117. [Google Scholar] [CrossRef]
- Sander, R.H. A systemic analysis of affirmative action in American law schools. Stan. Law Rev. 2004, 57, 367. [Google Scholar]
- Holzer, H.; Neumark, D. Assessing affirmative action. J. Econ. Lit. 2000, 38, 483–568. [Google Scholar] [CrossRef]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozai, T.D.Y. The History and Horizons of Microscale Neural Interfaces. Micromachines 2018, 9, 445. https://doi.org/10.3390/mi9090445
Kozai TDY. The History and Horizons of Microscale Neural Interfaces. Micromachines. 2018; 9(9):445. https://doi.org/10.3390/mi9090445
Chicago/Turabian StyleKozai, Takashi D. Y. 2018. "The History and Horizons of Microscale Neural Interfaces" Micromachines 9, no. 9: 445. https://doi.org/10.3390/mi9090445