Perivascular Tumor-Infiltrating Leukocyte Scoring for Prognosis of Resected Hepatocellular Carcinoma Patients
Abstract
1. Introduction
2. Results
2.1. Analysis of Epidemiological and Clinicopathological Data of the Study Patients
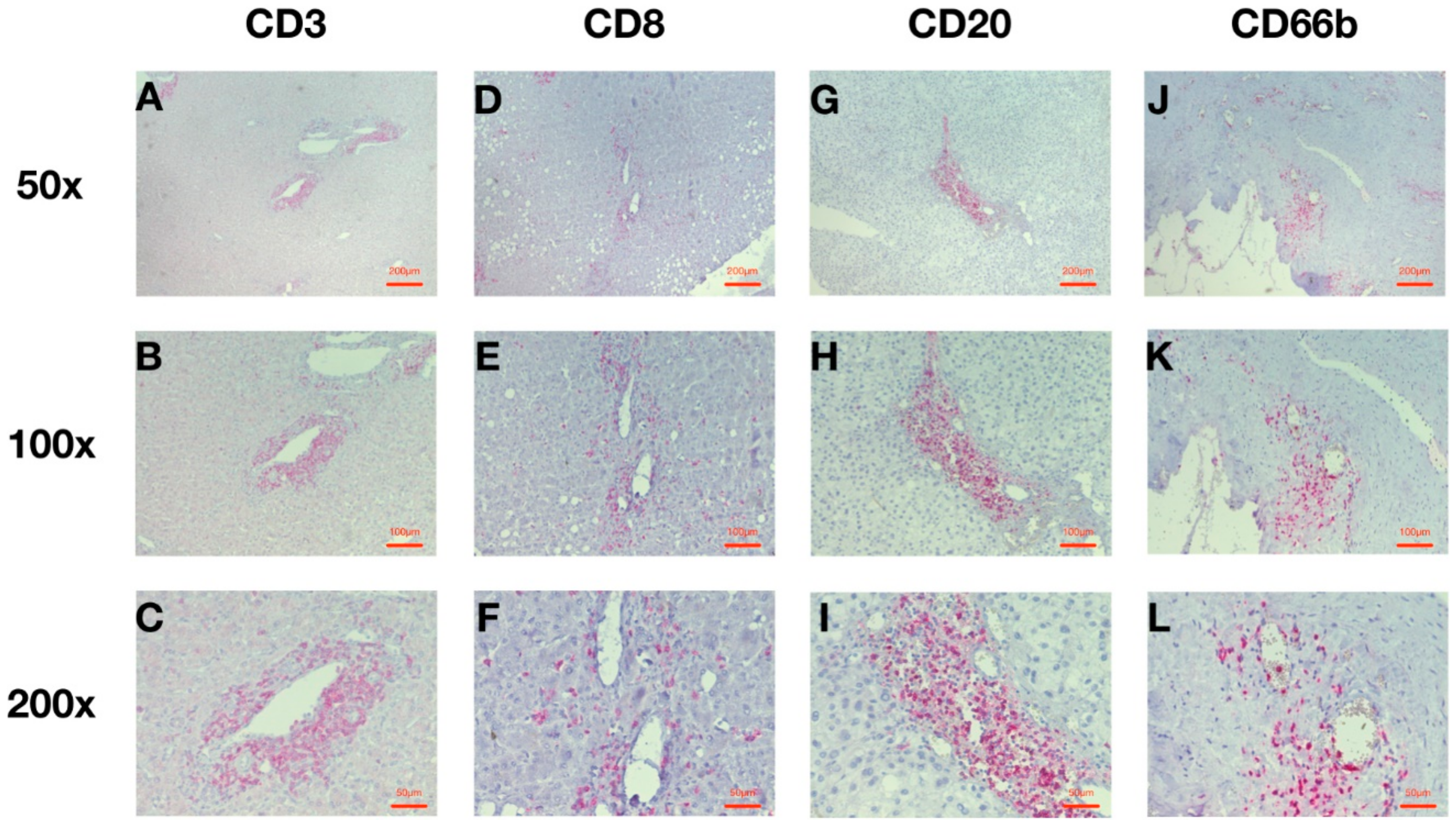
2.2. HCC Infiltration with Leucocytes around the Intratumoral Vessels Is Predominant
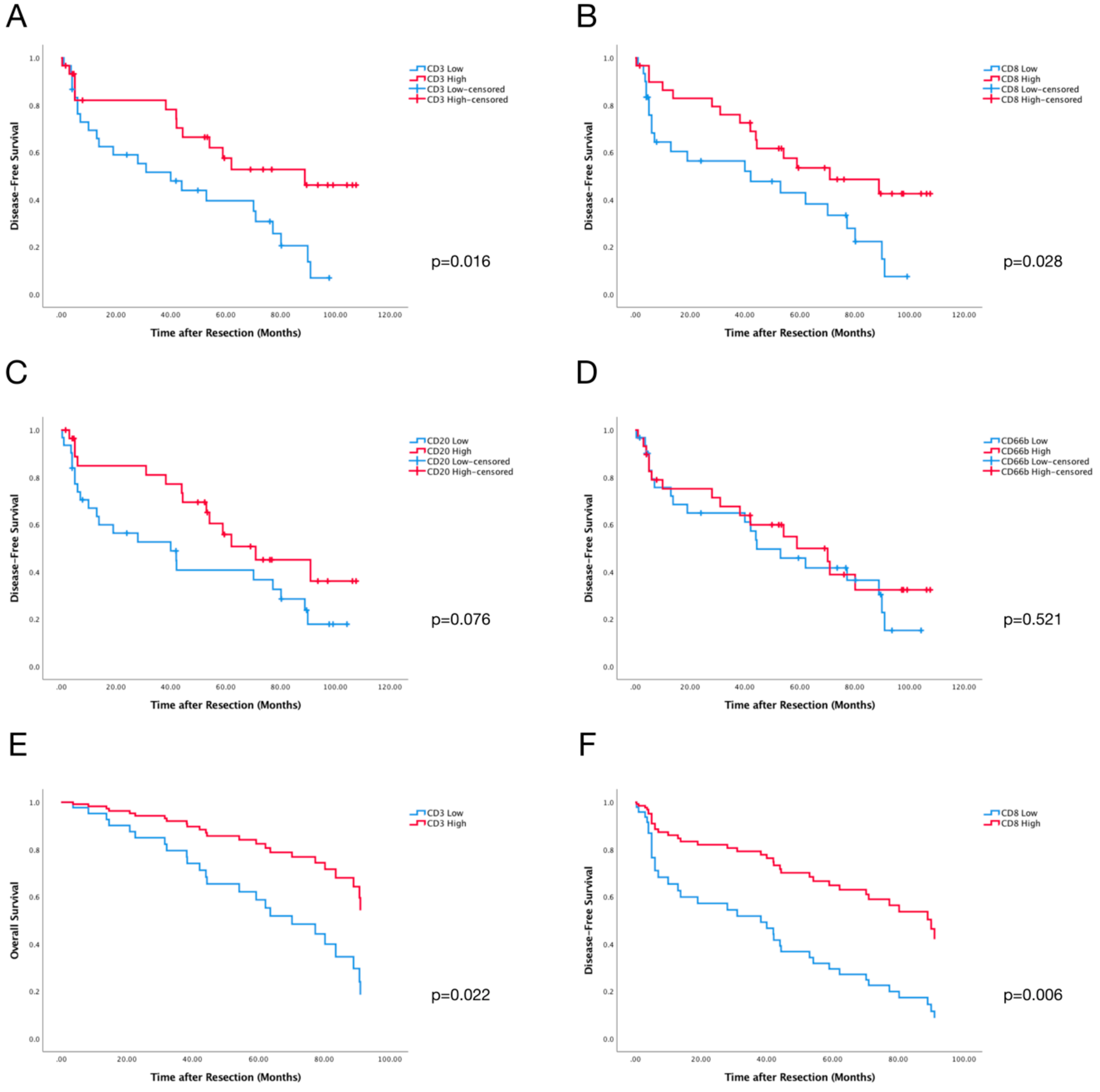
2.3. PVI CD3+ and CD8+ Cells Predict DFS of HCC Patients
2.4. Perivascular Infiltration Scoring (PVIS) Predicts Survival of All HCC Patients
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Patients and Clinical Data
4.3. Immunohistochemical Staining
4.4. Analysis of Immunohistochemical Staining
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer, World Health Organization. Available online: http://www.iarc.fr/ (accessed on 1 April 2018).
- Belghiti, J.; Kianmanesh, R. Surgical Treatment of Hepatocellular Carcinoma. HPB 2005, 7, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Burroughs, A.; Bruix, J. Hepatocellular carcinoma. Lancet 2003, 362, 1907–1917. [Google Scholar] [CrossRef]
- Duffy, J.P.; Vardanian, A.; Benjamin, E.; Watson, M.; Farmer, D.G.; Ghobrial, R.M.; Lipshutz, G.; Yersiz, H.; Lu, D.S.K.; Lassman, C.; et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: A 22-year experience with 467 patients at UCLA. Ann. Surg. 2007, 246, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, M.; Bucher, J.N.; Vater, A.; Bazhin, A.V.; Hao, J.; Guba, M.O.; Angele, M.K.; Werner, J.; Rentsch, M. Resection or transplant in early hepatocellular carcinoma—A systematic review and meta-analysis. Deutsch. Ärztebl. Int. 2017, 114, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, A.; Wu, Y.; Wang, H.; Jiang, J.; Kallakury, B.; Gatalica, Z.; Reddy, S.; Kleiner, D.; Fishbein, T.; Johnson, L.; et al. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer Immunol. Res. 2016, 4, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Chawla, Y.K.; Arora, S.K. Immunology of hepatocellular carcinoma. World J. Hepatol. 2015, 7, e2080. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.-Y.; Gao, Q.; Qiu, S.-J.; Ye, S.-L.; Wu, Z.-Q.; Fan, J.; Tang, Z.-Y. Dendritic cell infiltration and prognosis of human hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2006, 132, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Unitt, E.; Marshall, A.; Gelson, W.; Rushbrook, S.M.; Davies, S.; Vowler, S.L.; Morris, L.S.; Coleman, N.; Alexander, G.J.M. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J. Hepatol. 2006, 45, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Qiu, S.-J.; Fan, J.; Zhou, J.; Gao, Q.; Xiao, Y.-S.; Xu, Y.-F. Intratumoral neutrophils: A poor prognostic factor for hepatocellular carcinoma following resection. J. Hepatol. 2011, 54, 497–505. [Google Scholar] [CrossRef]
- Chew, V.; Chen, J.; Lee, D.; Loh, E.; Lee, J.; Lim, K.H.; Weber, A.; Slankamenac, K.; Poon, R.T.P.; Yang, H.; et al. Chemokine-driven lymphocyte infiltration: An early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012, 61, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xu, J.; Song, J.; Liu, C.Q.; Wang, J.; Weng, C.; Sun, H.; Wei, H.; Xiao, W.; Sun, R.; et al. The predictive value of centre tumour CD8(+) T cells in patients with hepatocellular carcinoma: Comparison with Immunoscore. Oncotarget 2015, 6, 35602–35615. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.M.; Rubner, C.; Kessalring, R.; Martin, M.; Griesshammer, E.; Ruemmele, P.; Stempfl, T.; Teufel, A.; Schlitt, H.J.; Fichtner-Feigl, S. Tumor-infiltrating, interleukin-33-producing effector-memory CD8(+) T cells in resected hepatocellular carcinoma prolong patient survival. Hepatology 2015, 61, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Ma, L.; Liu, L.; Zhao, G.; Zhang, S.; Dong, L.; Xue, R.; Chen, S. CD86+/CD206+, Diametrically Polarized Tumor-Associated Macrophages, Predict Hepatocellular Carcinoma Patient Prognosis. Int. J. Mol. Sci. 2016, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Jiang, N.; Tang, Z.-W.; Li, D.-W.; Huang, P.; Luo, S.-Q.; Gong, J.-P.; Du, C.-Y. Systemic and intratumoral balances between monocytes/macrophages and lymphocytes predict prognosis in hepatocellular carcinoma patients after surgery. Oncotarget 2016, 7, 30951–30961. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhou, J.; Wang, X.-Y.; Qiu, S.-J.; Song, K.; Huang, X.-W.; Sun, J.; Shi, Y.-H.; Li, B.-Z.; Xiao, Y.-S.; et al. Infiltrating memory/senescent T cell ratio predicts extrahepatic metastasis of hepatocellular carcinoma. Ann. Surg. Oncol. 2012, 19, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Liu, Y.; Liu, C.; Cui, A.; Liang, Z.; Wang, G.; Peng, H.; Cui, L.; Li, C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: Systematic review and meta-analysis. Br. J. Cancer 2014, 110, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, Q.; Liu, G.; Dang, L.; Chu, D.; Tao, K.; Wang, W. Presence of FOXP3(+) Treg cells is correlated with colorectal cancer progression. Int. J. Clin. Exp. Med. 2014, 7, 1781–1785. [Google Scholar] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.C.; Maurer, M.J.; Oberg, A.L.; Visscher, D.W.; Kalli, K.R.; Hartmann, L.C.; Goode, E.L.; Knutson, K.L. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3-T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS ONE 2013, 8, e80063. [Google Scholar] [CrossRef] [PubMed]
- Tewari, N.; Zaitoun, A.M.; Arora, A.; Madhusudan, S.; Ilyas, M.; Lobo, D.N. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: An immunohistochemical study of tissue microarrays. BMC Cancer 2013, 13, e436. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.M.; Al-Foheidi, M.E.; Al-Mansour, M.M.; Kazkaz, G.A. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2014, 148, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Mella, M.; Kauppila, J.H.; Karihtala, P.; Lehenkari, P.; Jukkola-Vuorinen, A.; Soini, Y.; Auvinen, P.; Vaarala, M.H.; Ronkainen, H.; Kauppila, S.; et al. Tumor infiltrating CD8+ T lymphocyte count is independent of tumor TLR9 status in treatment naive triple negative breast cancer and renal cell carcinoma. Oncoimmunology 2015, 4, e1002726. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Anitei, M.G.; Zeitoun, G.; Mlecnik, B.; Marliot, F.; Haicheur, N.; Todosi, A.M.; Kirilovsky, A.; Lagorce, C.; Bindea, G.; Ferariu, D.; et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin. Cancer Res. 2014, 20, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, F.M.; Wang, T.; Wang, Y.J.; Zhu, Z.Y.; Gao, Y.T.; Du, Z. Tumor-infiltrating FoxP3+ Tregs and CD8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion 2012, 86, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Kojiro, M. Histological Growth Patterns of Hepatocellular Carcinoma. In Hepatocellular Carcinoma: An Atlas of Its Pathology; Springer: Tokyo, Japan, 1987; pp. 81–104. [Google Scholar]
- Wu, T.H.; Yu, M.C.; Chen, T.C.; Lee, C.F.; Chan, K.M.; Wu, T.J.; Chou, H.S.; Lee, W.C.; Chen, M.F. Encapsulation is a significant prognostic factor for better outcome in large hepatocellular carcinoma. J. Surg. Oncol. 2012, 105, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Muto, J.; Shirabe, K.; Sugimachi, K.; Maehara, Y. Review of angiogenesis in hepatocellular carcinoma. Hepatol. Res. 2015, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O. Imaging of multistep human hepatocarcinogenesis by CT during intra-arterial contrast injection. Intervirology 2004, 47, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Duda, D.G.; Sahani, D.V.; Jain, R.K. HCC and angiogenesis: Possible targets and future directions. Nat. Rev. Clin. Oncol. 2011, 8, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Martinet, L.; Garrido, I.; Filleron, T.; Le Guellec, S.; Bellard, E.; Fournie, J.J.; Rochaix, P.; Girard, J.P. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011, 71, 5678–5687. [Google Scholar] [CrossRef] [PubMed]
- Bellone, M.; Calcinotto, A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front. Oncol. 2013, 3, e231. [Google Scholar] [CrossRef] [PubMed]
- Cariani, E.; Pilli, M.; Zerbini, A.; Rota, C.; Olivani, A.; Pelosi, G.; Schianchi, C.; Soliani, P.; Campanini, N.; Silini, E.M.; et al. Immunological and molecular correlates of disease recurrence after liver resection for hepatocellular carcinoma. PLoS ONE 2012, 7, e32493. [Google Scholar] [CrossRef] [PubMed]
- Reuschenbach, M.; von Knebel, D.M.; Wentzensen, N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol. Immunother. 2009, 58, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.H. CD20+ B cells: The other tumor-infiltrating lymphocytes. J. Immunol. 2010, 185, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef] [PubMed]
- Al-Shibli, K.I.; Donnem, T.; Al-Saad, S.; Persson, M.; Bremnes, R.M.; Busund, L.T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, B.S.; Ladekarl, M.; Nyengaard, J.R.; Nielsen, K. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol. Oncol. 2008, 108, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Farid, S.; Malik, H.Z.; Young, A.L.; Toogood, G.J.; Lodge, J.P.; Prasad, K.R. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J. Surg. 2008, 32, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Hardy, M.A.; Rana, A.A.; Woodland, D.C.; Luyten, E.J.; Mahadev, S.; Witkowski, P.; Siegel, A.B.; Brown, R.S., Jr.; Emond, J.C. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann. Surg. 2009, 250, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Huang, H.; Zhang, Z.; Wang, F.S. The role of neutrophils in the development of liver diseases. Cell Mol. Immunol. 2014, 11, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Ngai, P.; Ho, D.W.; Yu, W.C.; Ng, M.N.; Lau, C.K.; Li, M.L.; Tam, K.H.; Lam, C.T.; Poon, R.T.; et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology 2008, 47, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.R.; Schneck, F.X.; Gagnon, M.L.; Corless, C.; Soker, S.; Niknejad, K.; Peoples, G.E.; Klagsbrun, M. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: A potential role for T cells in angiogenesis. Cancer Res. 1995, 55, 4140–4145. [Google Scholar] [PubMed]
- Huang, H.; Langenkamp, E.; Georganaki, M.; Loskog, A.; Fuchs, P.F.; Dieterich, L.C.; Kreuger, J.; Dimberg, A. VEGF suppresses T-lymphocyte infiltration in the tumor microenvironment through inhibition of NF-kappaB-induced endothelial activation. FASEB J. 2015, 29, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Xu, Y.; Youn, J.; Cabrera, R.; Zhang, X.; Gabrilovich, D.; Nelson, D.R.; Liu, C. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab Investig. 2011, 91, 598–608. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 48, 908–943. [Google Scholar]
- Thasler, W.E.; Weiss, T.S.; Schillhorn, K.; Stoll, P.T.; Irrgang, B.; Jauch, K.W. Charitable State-Controlled Foundation Human Tissue and Cell Research: Ethic and Legal Aspects in the Supply of Surgically Removed Human Tissue for Research in the Academic and Commercial Sector in Germany. Cell Tissue Bank 2003, 4, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bazhin, A.V.; Wiedemann, N.; Schnölzer, M.; Schadendorf, D.; Eichmüller, S.B. Expression of GAGE family proteins in malignant melanoma. Cancer Lett. 2007, 251, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Miksch, R.C.; Hao, J.; Schoenberg, M.B.; Dötzer, K.; Schlüter, F.; Weniger, M.; Yin, S.; Ormanns, S.; D’Haese, J.G.; Guba, M.O.; et al. Development of a reliable and accurate algorithm to quantify the tumor immune stroma (QTiS) across tumor types. Oncotarget 2017, 8, 114935–114944. [Google Scholar] [CrossRef] [PubMed]
- Collett, D. Modelling Survival Data in Medical Research, 3rd ed.; CRC Press: New York, NY, USA, 2015; p. 538. [Google Scholar]


| Variables | Results |
|---|---|
| Gender (Male/Female) | 49 (81.7%)/11 (18.3%) |
| Age (Years) (Median (IQR)) | 66.00 (16.00) |
| Hepatitis (HBV/HCV) | 7 (11.7%)/0 (0.0%) |
| Cirrhosis | 15 (25.0%) |
| AFP (ng/mL) (Median (IQR)) | 12.60 (153.00) |
| Tumor multiplicity | 9 (15.0%) |
| Microvascular invasion | 18 (30.0%) |
| Macrovascular invasion | 6 (10.0%) |
| Beyond Milan Criteria | 47 (78.3%) |
| Bilirubin (mg/dL) (Median (IQR)) | 0.70 (0.30) |
| Albumin (mg/dL) (Median (IQR)) | 43.00 (5.00) |
| ALT (U/L) (Median (IQR)) | 42.00 (33.00) |
| AST (U/L) (Median (IQR)) | 45.00 (37.00) |
| APTT (s) (Median (IQR)) | 28.50 (5.25) |
| Creatinine (mg/dL) (Median (IQR)) | 1.00 (0.20) |
| CRP (mg/L) (Median (IQR)) | 6.00 (16.00) |
| Leukocytes (103/µL) (Median (IQR)) | 7246.67 (2094.03) |
| Platelets (103/µL) (Median (IQR)) | 222.00 (120.50) |
| Variables | Overall Survival | Disease-free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Gender | 1.243 | 0.781 | 1.978 | 0.355 | 2.312 | 1.112 | 4.808 | 0.021 |
| Age (≥60 years) | 0.440 | 0.150 | 1.291 | 0.124 | 1.496 | 0.754 | 2.971 | 0.246 |
| Hepatitis | 0.237 | 0.077 | 0.732 | 0.007 | 2.451 | 0.932 | 6.447 | 0.059 |
| Cirrhosis | 0.525 | 0.219 | 1.256 | 0.141 | 0.843 | 0.399 | 1.779 | 0.652 |
| AFP (≥20 ng/mL) | 0.913 | 0.389 | 2.145 | 0.835 | 0.638 | 0.322 | 1.266 | 0.195 |
| AFP (≥400 ng/mL) | 1.798 | 0.420 | 7.706 | 0.423 | 1.364 | 0.478 | 3.889 | 0.560 |
| Tumor Multiplicity | 1.521 | 0.355 | 6.517 | 0.570 | 0.818 | 0.339 | 1.973 | 0.653 |
| Microvascular Invasion | 0.677 | 0.272 | 1.690 | 0.401 | 0.708 | 0.346 | 1.447 | 0.338 |
| Macrovascular Invasion | 0.702 | 0.201 | 2.457 | 0.578 | 0.618 | 0.232 | 1.644 | 0.328 |
| Beyond Milan | 0.553 | 0.073 | 4.187 | 0.560 | 0.272 | 0.037 | 2.006 | 0.169 |
| Low CD3+ Cells | 2.196 | 0.956 | 5.046 | 0.057 | 2.277 | 1.145 | 4.527 | 0.016 |
| Low CD8+ Cells | 1.531 | 0.684 | 3.425 | 0.297 | 2.074 | 1.064 | 4.043 | 0.028 |
| Low CD20+ Cells | 1.291 | 0.574 | 2.904 | 0.535 | 0.549 | 0.279 | 1.079 | 0.076 |
| Low CD66b+ Cells | 1.228 | 0.550 | 2.744 | 0.616 | 1.239 | 0.641 | 2.394 | 0.521 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoenberg, M.B.; Hao, J.; Bucher, J.N.; Miksch, R.C.; Anger, H.J.W.; Mayer, B.; Mayerle, J.; Neumann, J.; Guba, M.O.; Werner, J.; et al. Perivascular Tumor-Infiltrating Leukocyte Scoring for Prognosis of Resected Hepatocellular Carcinoma Patients. Cancers 2018, 10, 389. https://doi.org/10.3390/cancers10100389
Schoenberg MB, Hao J, Bucher JN, Miksch RC, Anger HJW, Mayer B, Mayerle J, Neumann J, Guba MO, Werner J, et al. Perivascular Tumor-Infiltrating Leukocyte Scoring for Prognosis of Resected Hepatocellular Carcinoma Patients. Cancers. 2018; 10(10):389. https://doi.org/10.3390/cancers10100389
Chicago/Turabian StyleSchoenberg, Markus Bo, Jingcheng Hao, Julian Nikolaus Bucher, Rainer Christoph Miksch, Hubertus Johann Wolfgang Anger, Barbara Mayer, Julia Mayerle, Jens Neumann, Markus Otto Guba, Jens Werner, and et al. 2018. "Perivascular Tumor-Infiltrating Leukocyte Scoring for Prognosis of Resected Hepatocellular Carcinoma Patients" Cancers 10, no. 10: 389. https://doi.org/10.3390/cancers10100389
APA StyleSchoenberg, M. B., Hao, J., Bucher, J. N., Miksch, R. C., Anger, H. J. W., Mayer, B., Mayerle, J., Neumann, J., Guba, M. O., Werner, J., & Bazhin, A. V. (2018). Perivascular Tumor-Infiltrating Leukocyte Scoring for Prognosis of Resected Hepatocellular Carcinoma Patients. Cancers, 10(10), 389. https://doi.org/10.3390/cancers10100389






