Evolution of Carbon Ion Radiotherapy at the National Institute of Radiological Sciences in Japan
Abstract
:1. Introduction
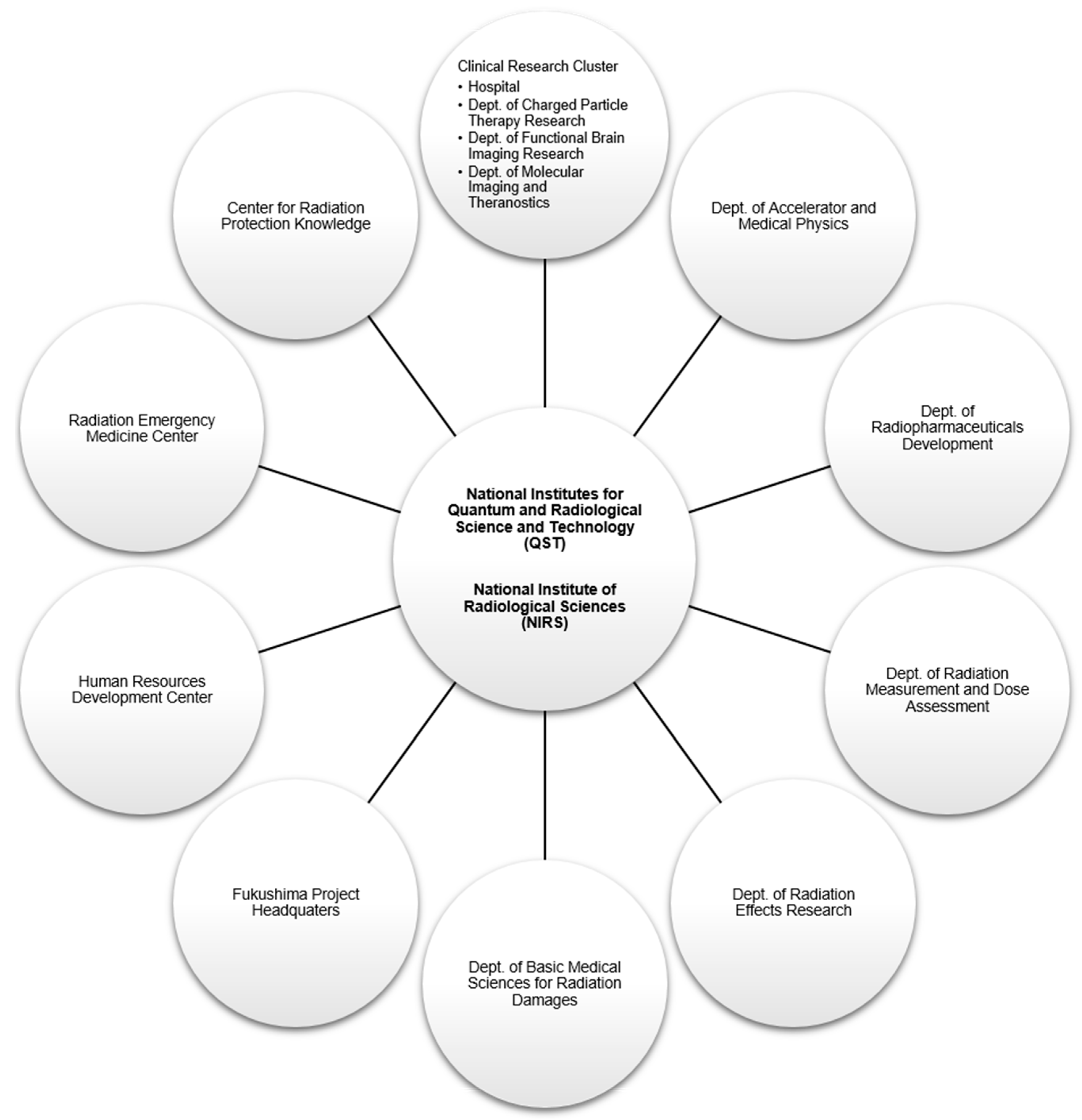
2. A Brief History of the National Institute of Radiological Sciences
- -
- NIRS was founded in 1957.
- -
- Fast neutron therapy started in 1974 and proton therapy in 1979.
- -
- HIMAC planning and construction started in 1984.
- -
- HIMAC construction was completed at the end of 1993.
- -
- HIMAC commissioning was completed and first patient was treated in June 1994. Only passive beam irradiation was available until 2011.
- -
- Hospital for charged particle therapy opened in 1996–1997.
- -
- CIRT was approved as advanced medical technology by the Japanese government Ministry of Health, Welfare and Labor in 2003. As such, the NIRS could receive reimbursement for CIRT. Cost was fixed per treatment independent of the number of fractions.
- -
- Construction of the New Particle Therapy Research Facilities started in 2006.
- -
- NIRS collaborated with Gunma University to construct a compact CIRT center with 1/3 the size and cost of the HIMAC at Gunma University in 2010.
- -
- Active scanning treatment started in May 2011 in the new facility. A benchmark of 6000 patients were treated by 2011.
- -
- Respiratory-gated phase-controlled rescanning irradiation of moving targets started in March 2015.
- -
- A benchmark of 10,000 patients were treated by 2015 (one fourth were patients with prostate cancer).
- -
- Construction of the superconducting rotating gantry was completed in 2015 [10].
- -
- Commissioning of the gantry was completed in 2016 (half the size and weight of the gantry in the Heidelberg ion therapy (HIT) center in Germany).
- -
- The NIRS has established a workflow system allowing the treatment of >800 patients annually.
- -
- In April 2016, the National Institutes for Quantum and Radiological Science and Technology (QST) was established by merging the NIRS with the quantum beam and nuclear fusion departments of the Japan Atomic Energy Agency (JAEA).
3. Carbon Ion Radiotherapy in the Context of the Japanese Healthcare System
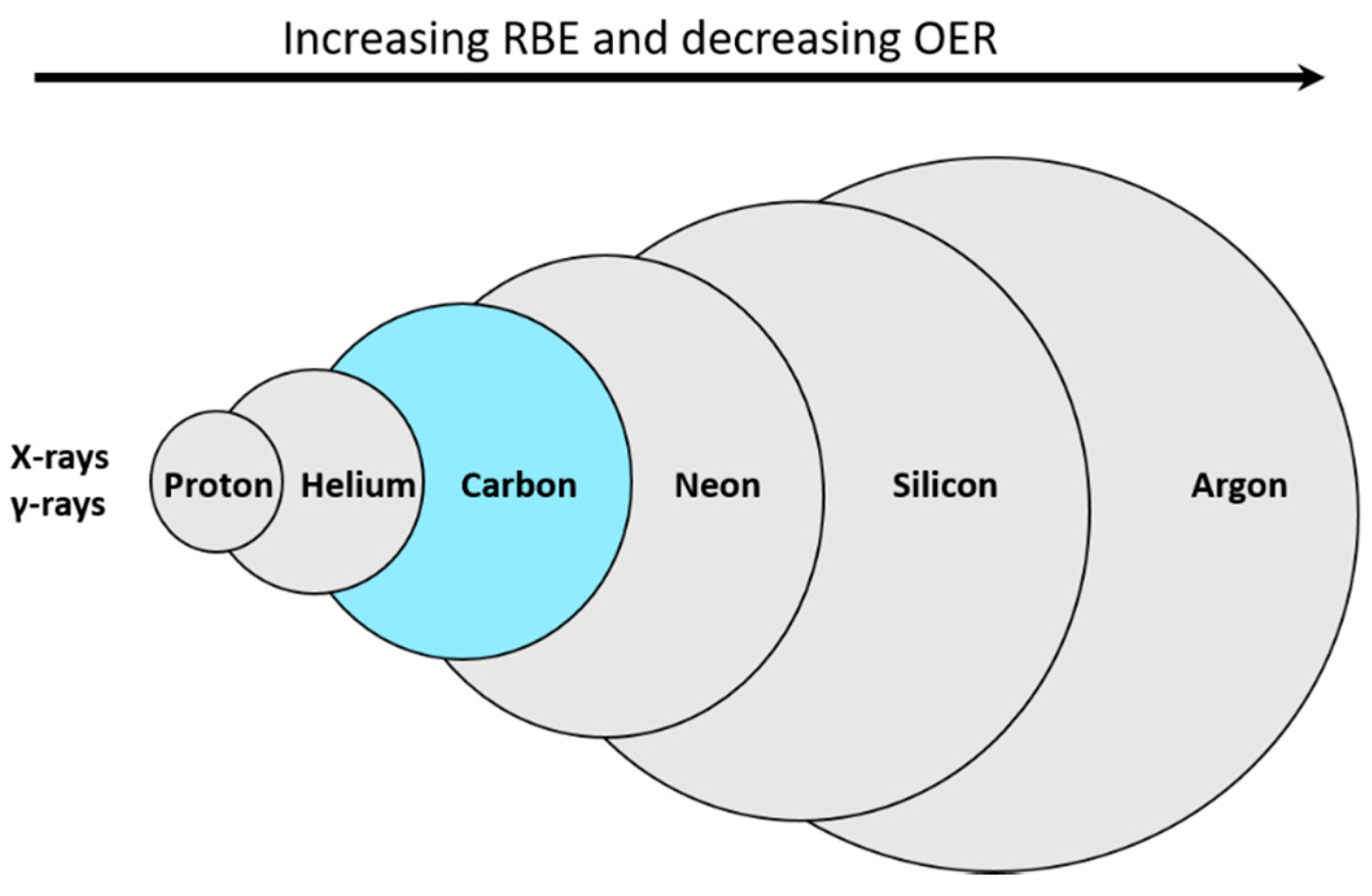
4. Carbon Ions as the Particles of Choice
5. Clinic Design
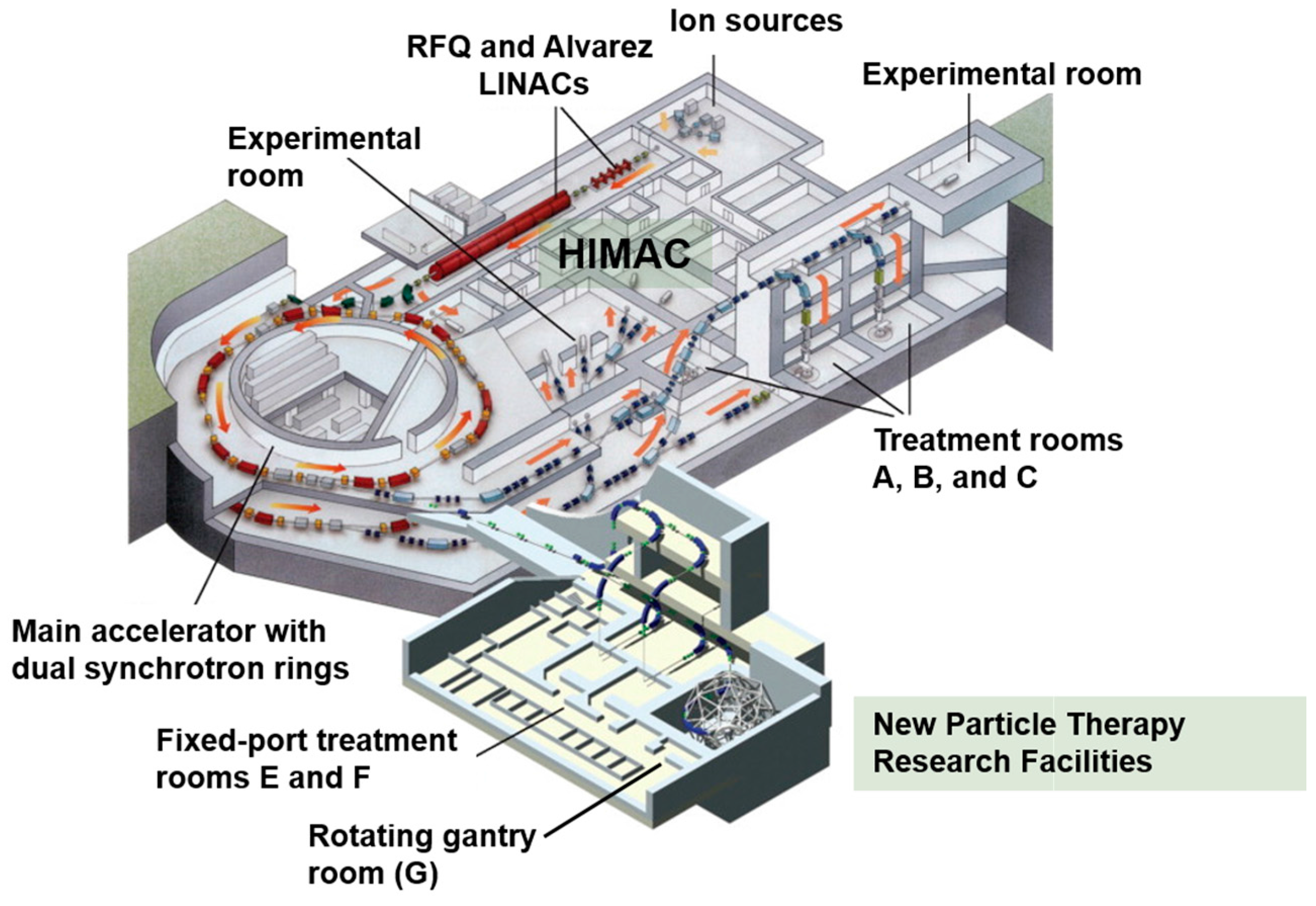
5.1. Heavy Ion Medical Accelerator in Chiba (HIMAC)
5.2. New Particle Therapy Research Facilities
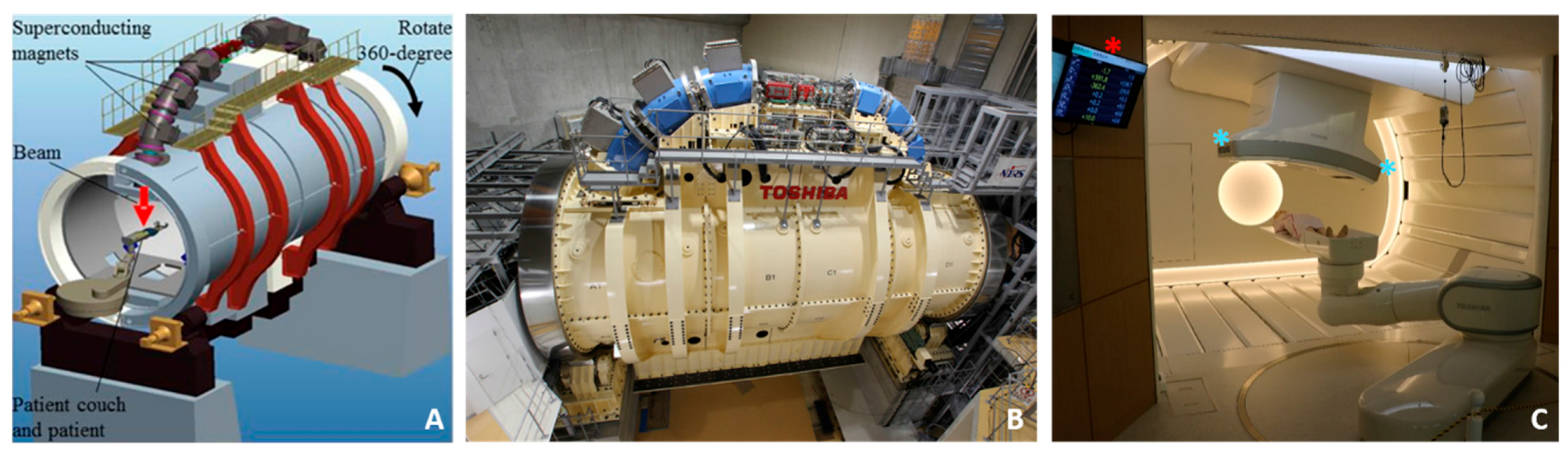
5.3. The World’s First Superconducting Rotating Gantry
6. Treatment Delivery
6.1. Passive Beam Irradiation
6.2. Three-Dimensional Scanning Irradiation
6.3. Motion Management
7. Dose Prescription and Treatment Planning
- -
- Step 1, Determining biological RBE: To create an SOBP with uniform cell kill, the linear-quadratic model variables α and β had to be determined. Cell survival curves were initially created for HSG cells using multiple monoenergetic pristine carbon ion beams and the corresponding α and β values were calculated as a function of depth. An SOBP, however, is characterized by a distribution of LET values. Consequently, α′ and β′ values were determined for a mixed beam of multiple Bragg peaks. Accordingly, survival (10% survival) of HSG cells could be determined at any depth in a given SOBP. This allowed the creation of a ridge filter that could achieve a uniform cell kill effect of HSG cells along the SOBP. Because LET generally increases towards the end of the SOBP, the ridge filter design reduces the weight of the physical dose with depth. This was tested experimentally by irradiating HSG cells using a 6 cm SOBP of 290 MeV/u carbon ion beams, and the results allowed the determination of the biological RBE and RBE-weighted dose distribution [43]. Clearly, this initial model did not account for dose level in RBE calculation.
- -
- Step 2, Determining the neutron equivalent point: The NIRS has a long history using fast neutrons in the clinic since 1974. The NIRS team aimed to find a depth in the carbon beam SOBP at which neutrons would exhibit the same RBE. Using an average of in vitro (again 10% survival of HSG cells) and in vivo (mouse skin dry desquamation) data, it was decided that the depth corresponding to an LET value of 80 keV/µm, 8 mm upstream of the distal fall off of a 6 cm SOBP of carbon ion beams of 290 MeV/u, to be the neutron equivalent point. At this point, it was thought that neutron clinical RBE would be equivalent to carbon ion clinical RBE.
- -
- Step 3, Determining clinical RBE: The NIRS team decided to initially use a similar fractionation schedule for CIRT as was being used for neutrons. The neutron clinical RBE has been previously determined to be 3.3 for a 20-fraction treatment and thus, an RBE of 3 for an 18-fraction regimen was selected. The flat top of the biological dose is first determined by the treating physician. Using a clinical RBE of 3, the physical carbon ion dose is determined at the neutron equivalent point. The physical dose distribution is then normalized to the neutron equivalent point which allows the determination of the physical dose at the center of SOBP. The RBE values at the center of the SOBP are obtained by dividing the biological dose by the physical dose. A table of clinical RBEs at the center of the SOBP is created for each SOBP width.
- -
- Step 4, Updating the biophysical model: Given the lack of reliable experimental evidence in 1994, some assumptions had to be made to simplify the above biophysical model. LET was assumed to be an accurate predictor of RBE. In reality, LET does not fully explain the distribution of energy deposition around a particle track and RBE is also dependent on other variables such as, but not limited to, tissue type, fractionation and dose level. With the accumulated knowledge, it was necessary to update the biophysical model to account for the improved understanding of the mechanisms of biological effects of carbon beams. Hence, the Microdosimetric Kinetic Model (MKM) [44], which attempts to explain the biological effects of irradiation beams based on the stochastic energy deposition of carbon ions at the micrometer level, was adopted in the updated version of the treatment planning system used for scanning irradiation [45,46]. Comparative experimentation showed that the original model was adequate for clinical use [47] but the updated model better estimated dose distribution especially at the tail beyond the distal fall off where nuclear fragments are in excess [41]. Variable-RBE models are necessary for an utmost utilization of the advantages of CIRT. Given the inadequacy of RBE for the ideal treatment planning system especially for extreme hypofractionation, alternative parameters for plan optimization and evaluation are being considered to decrease the reliance on RBE. The NIRS continues to improve its dose calculation models [48]. A different approach to dose prescription has been proposed, utilized and updated for treatment planning at the Gesellshaft für Schwerionenforschung (GSI) in Germany: namely the Local Effect Model (LEM) [49,50,51,52]. Efforts between the NIRS and the European centers are underway to better understand and minimize the differences between the two models [53,54].
8. Hypofractionation in Carbon Ion Radiotherapy
8.1. Physical and Biological Rationale of Hypofractionation in Carbon Ion Radiotherapy
8.2. Economic Rationale of Hypofractionation
9. Ongoing and Future Projects at the NIRS
9.1. Combination with Systemic Therapy
9.2. LET Painting and Mixed Beams Irradiation
9.3. Artificial Intelligence
9.4. Local and International Collaborations
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schulz-Ertner, D.; Jakel, O.; Schlegel, W. Radiation therapy with charged particles. Semin. Radiat. Oncol. 2006, 16, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Pompos, A.; Durante, M.; Choy, H. Heavy ions in cancer therapy. JAMA Oncol. 2016, 2, 1539–1540. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, O.; Sishc, B.J.; Saha, J.; Pompos, A.; Rahimi, A.; Story, M.D.; Davis, A.J.; Kim, D.W.N. Carbon ion radiotherapy: A review of clinical experiences and preclinical research, with an emphasis on DNA damage/repair. Cancers 2017, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-particle therapy in cancer: Clinical uses and future perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Peeters, A.; Grutters, J.P.; Pijls-Johannesma, M.; Reimoser, S.; De Ruysscher, D.; Severens, J.L.; Joore, M.A.; Lambin, P. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010, 95, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jakel, O.; Land, B.; Combs, S.E.; Schulz-Ertner, D.; Debus, J. On the cost-effectiveness of carbon ion radiation therapy for skull base chordoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2007, 83, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, A.; Ohno, T.; Yamada, S.; Sakurai, H.; Nakano, T. Cost-effectiveness of carbon ion radiation therapy for locally recurrent rectal cancer. Cancer Sci. 2010, 101, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Mizoe, J.E.; Aoki, Y.; Morita, S.; Tsunemoto, H. Fast neutron therapy for malignant gliomas—Results from nirs study. National institute of radiological sciences. Strahlenther Onkol. 1993, 169, 222–227. [Google Scholar] [PubMed]
- Morita, S.; Arai, T.; Nakano, T.; Ishikawa, T.; Tsunemoto, H.; Fukuhisa, K.; Kasamatsu, T. Clinical experience of fast neutron therapy for carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 1439–1445. [Google Scholar] [CrossRef]
- Iwata, Y.; Fujimoto, T.; Matsuba, S.; Fujita, T.; Sato, S.; Furukawa, T.; Hara, Y.; Mizushima, K.; Saraya, Y.; Tansho, R.; et al. Beam commissioning of a superconducting rotating-gantry for carbon-ion radiotherapy. Nucl. Instrum. Methods Phys. Res. 2016, 834, 71–80. [Google Scholar] [CrossRef]
- Friedrich, T.; Scholz, U.; Elsasser, T.; Durante, M.; Scholz, M. Systematic analysis of rbe and related quantities using a database of cell survival experiments with ion beam irradiation. J. Radiat. Res. 2013, 54, 494–514. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Fukutsu, K.; Aoki, M.; Itsukaichi, H.; Eguchi-Kasai, K.; Ohara, H.; Yatagai, F.; Kanai, T.; Ando, K. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)he-, (12)c- and (20)ne-ion beams. Radiat. Res. 2000, 154, 485–496. [Google Scholar] [CrossRef]
- Blakely, E.A.; Chang, P.Y. Biology of charged particles. Cancer J. 2009, 15, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T.; et al. Biophysical characteristics of himac clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 201–210. [Google Scholar] [CrossRef]
- Noda, K.; Furukawa, T.; Shibuya, S.; Uesugi, T.; Muramatsu, M.; Kanazawa, M.; Takada, E.; Yamada, S. Advanced rf-ko slow-extraction method for the reduction of spill ripple. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2002, 492, 253–263. [Google Scholar] [CrossRef]
- Noda, K.; Furukawa, T.; Fujimoto, T.; Fukuda, S.; Inaniwa, T.; Himukai, T.; Iwata, Y.; Kanematsu, N.; Katagiri, K.; Kitagawa, A.; et al. Recent progress on new treatment research project at himac. Nucl. Instrum. Methods Phys. Res. Sect. A 2010, 33–38. [Google Scholar] [CrossRef]
- Mori, S.; Shibayama, K.; Tanimoto, K.; Kumagai, M.; Matsuzaki, Y.; Furukawa, T.; Inaniwa, T.; Shirai, T.; Noda, K.; Tsuji, H.; et al. First clinical experience in carbon ion scanning beam therapy: Retrospective analysis of patient positional accuracy. J. Radiat. Res. 2012, 53, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Tessa, C.L.; Berger, T.; Kaderka, R.; Schardt, D.; Burmeister, S.; Labrenz, J.; Reitz, G.; Durante, M. Characterization of the secondary neutron field produced during treatment of an anthropomorphic phantom with X-rays, protons and carbon ions. Phys. Med. Biol. 2014, 59, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
- Yonai, S.; Matsufuji, N.; Kanai, T.; Matsui, Y.; Matsushita, K.; Yamashita, H.; Numano, M.; Sakae, T.; Terunuma, T.; Nishio, T.; et al. Measurement of neutron ambient dose equivalent in passive carbon-ion and proton radiotherapies. Med. Phys. 2008, 35, 4782–4792. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, N.; Endo, M.; Futami, Y.; Kanai, T.; Asakura, H.; Oka, H.; Yusa, K. Treatment planning for the layer-stacking irradiation system for three-dimensional conformal heavy-ion radiotherapy. Med. Phys. 2002, 29, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Inaniwa, T.; Furukawa, T.; Kanematsu, N.; Mori, S.; Mizushima, K.; Sato, S.; Toshito, T.; Shirai, T.; Noda, K. Evaluation of hybrid depth scanning for carbon-ion radiotherapy. Med. Phys. 2012, 39, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Kadowaki, T.; Uchiyama, H.; Fujimoto, T.; Takada, E.; Shirai, T.; Furukawa, T.; Mizushima, K.; Takeshita, E.; Katagiri, K.; et al. Multiple-energy operation with extended flattops at himac. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2010, 624, 33–38. [Google Scholar] [CrossRef]
- Mori, S.; Furukawa, T.; Inaniwa, T.; Zenklusen, S.; Nakao, M.; Shirai, T.; Noda, K. Systematic evaluation of four-dimensional hybrid depth scanning for carbon-ion lung therapy. Med. Phys. 2013, 40, 031720. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Inaniwa, T.; Sato, S.; Tomitani, T.; Minohara, S.; Noda, K.; Kanai, T. Design study of a raster scanning system for moving target irradiation in heavy-ion radiotherapy. Med. Phys. 2007, 34, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Inaniwa, T.; Sato, S.; Shirai, T.; Mori, S.; Takeshita, E.; Mizushima, K.; Himukai, T.; Noda, K. Moving target irradiation with fast rescanning and gating in particle therapy. Med. Phys. 2010, 37, 4874–4879. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Zenklusen, S.; Inaniwa, T.; Furukawa, T.; Imada, H.; Shirai, T.; Noda, K.; Yasuda, S. Conformity and robustness of gated rescanned carbon ion pencil beam scanning of liver tumors at nirs. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 111, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.; Hara, R.; Mori, S.; Yanagi, T.; Asakura, H.; Kishimoto, R.; Kato, H.; Yamada, S.; Kandatsu, S.; Kamada, T. Impact of intrafractional bowel gas movement on carbon ion beam dose distribution in pancreatic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Hara, R.; Yanagi, T.; Sharp, G.C.; Kumagai, M.; Asakura, H.; Kishimoto, R.; Yamada, S.; Kandatsu, S.; Kamada, T. Four-dimensional measurement of intrafractional respiratory motion of pancreatic tumors using a 256 multi-slice ct scanner. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009, 92, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, S.; Sugane, T.; Mori, S.; Asakura, H.; Yamamoto, N.; Kumagai, M.; Kandatsu, S.; Baba, M. Intrafractional respiratory motion for charged particle lung therapy with immobilization assessed by four-dimensional computed tomography. J. Radiat. Res. 2011, 52, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Minohara, S.; Kanai, T.; Endo, M.; Noda, K.; Kanazawa, M. Respiratory gated irradiation system for heavy-ion radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1097–1103. [Google Scholar] [CrossRef]
- Furukawa, T.; Hara, Y.; Mizushima, K.; Saotome, N.; Tansho, R.; Saraya, Y.; Inaniwa, T.; Mori, S.; Iwata, Y.; Shirai, T.; et al. Development of nirs pencil beam scanning system for carbon ion radiotherapy. Nucl. Instrum. Methods Phys. Res. B 2017, 361–367. [Google Scholar] [CrossRef]
- Mori, S.; Inaniwa, T.; Miki, K.; Shirai, T.; Noda, K. Implementation of a target volume design function for intrafractional range variation in a particle beam treatment planning system. Br. J. Radiol. 2014, 87, 20140233. [Google Scholar] [CrossRef] [PubMed]
- Mori, S. Deep architecture neural network-based real-time image processing for image-guided radiotherapy. Phys. Med. 2017, 40, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Karube, M.; Mori, S.; Tsuji, H.; Yamamoto, N.; Nakajima, M.; Nakagawa, K.; Kamada, T. Carbon-ion pencil beam scanning for thoracic treatment—Initiation report and dose metrics evaluation. J. Radiat. Res. 2016, 57, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Karube, M.; Shirai, T.; Tajiri, M.; Takekoshi, T.; Miki, K.; Shiraishi, Y.; Tanimoto, K.; Shibayama, K.; Yasuda, S.; et al. Carbon-ion pencil beam scanning treatment with gated markerless tumor tracking: An analysis of positional accuracy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Ebner, D.K.; Tsuji, H.; Yasuda, S.; Yamamoto, N.; Mori, S.; Kamada, T. Respiration-gated fast-rescanning carbon-ion radiotherapy. Jpn. J. Clin. Oncol. 2017, 47, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Bert, C.; Durante, M. Motion in radiotherapy: Particle therapy. Phys. Med. Biol. 2011, 56, R113–R144. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Koyama-Ito, H.; Minohara, S.; Miyahara, N.; Tomura, H.; Kanai, T.; Kawachi, K.; Tsujii, H.; Morita, K. Hiplan-a heavy ion treatment planning system at himac. J. Jpn. Soc. Ther. Radiol. Oncol. 1996, 8, 231–238. [Google Scholar]
- Kanai, T.; Matsufuji, N.; Miyamoto, T.; Mizoe, J.; Kamada, T.; Tsuji, H.; Kato, H.; Baba, M.; Tsujii, H. Examination of gye system for himac carbon therapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, N.; Kanai, T.; Kanematsu, N.; Miyamoto, T.; Baba, M.; Kamada, T.; Kato, H.; Yamada, S.; Mizoe, J.E.; Tsujii, H. Specification of carbon ion dose at the national institute of radiological sciences (nirs). J. Radiat. Res. 2007, 48 (Suppl. A), A81–A86. [Google Scholar] [CrossRef] [PubMed]
- Inaniwa, T.; Kanematsu, N.; Matsufuji, N.; Kanai, T.; Shirai, T.; Noda, K.; Tsuji, H.; Kamada, T.; Tsujii, H. Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the national institute of radiological sciences, Japan. Phys. Med. Biol. 2015, 60, 3271–3286. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kase, Y.; Yamaguchi, H.; Kanai, T.; Ando, K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in chiba (himac) carbon-ion beams. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 241–250. [Google Scholar] [CrossRef]
- Kanai, T.; Furusawa, Y.; Fukutsu, K.; Itsukaichi, H.; Eguchi-Kasai, K.; Ohara, H. Irradiation of mixed beam and design of spread-out bragg peak for heavy-ion radiotherapy. Radiat. Res. 1997, 147, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B. A statistical theory of cell killing by radiation of varying linear energy transfer. Radiat. Res. 1994, 140, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Inaniwa, T.; Furukawa, T.; Kase, Y.; Matsufuji, N.; Toshito, T.; Matsumoto, Y.; Furusawa, Y.; Noda, K. Treatment planning for a scanned carbon beam with a modified microdosimetric kinetic model. Phys. Med. Biol. 2010, 55, 6721–6737. [Google Scholar] [CrossRef] [PubMed]
- Kase, Y.; Kanai, T.; Matsumoto, Y.; Furusawa, Y.; Okamoto, H.; Asaba, T.; Sakama, M.; Shinoda, H. Microdosimetric measurements and estimation of human cell survival for heavy-ion beams. Radiat. Res. 2006, 166, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Yamamoto, N.; Nishimura, H.; Koto, M.; Tsujii, H.; Mizoe, J.E.; Kamada, T.; Kato, H.; Yamada, S.; Morita, S.; et al. Carbon ion radiotherapy for stage i non-small cell lung cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2003, 66, 127–140. [Google Scholar] [CrossRef]
- Kanematsu, N.; Inaniwa, T. Biological dose representation for carbon-ion radiotherapy of unconventional fractionation. Phys. Med. Biol. 2017, 62, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Jakel, O.; Haberer, T.; Kraft, G.; Schardt, D.; Weber, U. Treatment planning for heavy-ion radiotherapy: Physical beam model and dose optimization. Phys. Med. Biol. 2000, 45, 3299–3317. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Scholz, M. Treatment planning for heavy-ion radiotherapy: Calculation and optimization of biologically effective dose. Phys. Med. Biol. 2000, 45, 3319–3330. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Kraft, G. The physical and radiobiological basis of the local effect model: A response to the commentary by r. Katz. Radiat. Res. 2004, 161, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Elsasser, T.; Kramer, M.; Scholz, M. Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Molinelli, S.; Matsufuji, N.; Ciocca, M.; Mirandola, A.; Mairani, A.; Mizoe, J.; Hasegawa, A.; Imai, R.; Kamada, T.; et al. Dose prescription in carbon ion radiotherapy: A planning study to compare nirs and lem approaches with a clinically-oriented strategy. Phys. Med. Biol. 2012, 57, 7543–7554. [Google Scholar] [CrossRef] [PubMed]
- Magro, G.; Dahle, T.J.; Molinelli, S.; Ciocca, M.; Fossati, P.; Ferrari, A.; Inaniwa, T.; Matsufuji, N.; Ytre-Hauge, K.S.; Mairani, A. The fluka Monte Carlo code coupled with the nirs approach for clinical dose calculations in carbon ion therapy. Phys. Med. Biol. 2017, 62, 3814–3827. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Tsujii, H.; Tsuji, H.; Yanagi, T.; Mizoe, J.E.; Miyamoto, T.; Kato, H.; Yamada, S.; Morita, S.; Yoshikawa, K.; et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 4466–4471. [Google Scholar] [CrossRef] [PubMed]
- Matsunobu, A.; Imai, R.; Kamada, T.; Imaizumi, T.; Tsuji, H.; Tsujii, H.; Shioyama, Y.; Honda, H.; Tatezaki, S.; Working Group for Bone and Soft Tissue Sarcomas. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer 2012, 118, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, S.; Kamada, T.; Imai, R.; Tsuji, H.; Kameda, N.; Okada, T.; Tsujii, H.; Tatezaki, S.; Working Group for Bone and Soft Tissue Sarcomas. Carbon ion radiotherapy for localized primary sarcoma of the extremities: Results of a phase i/ii trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012, 105, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Imai, R.; Kamada, T.; Maruyama, K.; Tsuji, H.; Tsujii, H.; Shioyama, Y.; Honda, H.; Isu, K.; Working Group for Bone and Soft Tissue Sarcomas. Impact of carbon ion radiotherapy for primary spinal sarcoma. Cancer 2013, 119, 3496–3503. [Google Scholar] [CrossRef] [PubMed]
- Imai, R.; Kamada, T.; Araki, N.; Working Group for Bone and Soft Tissue Sarcomas. Carbon ion radiation therapy for unresectable sacral chordoma: An analysis of 188 cases. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Imai, R.; Kamada, T.; Tsuji, H.; Tsujii, H. Carbon ion radiation therapy for chondrosarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84. [Google Scholar] [CrossRef]
- Mizoe, J.E.; Hasegawa, A.; Jingu, K.; Takagi, R.; Bessyo, H.; Morikawa, T.; Tonoki, M.; Tsuji, H.; Kamada, T.; Tsujii, H.; et al. Results of carbon ion radiotherapy for head and neck cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Fujii, O.; Terashima, K.; Mima, M.; Hashimoto, N.; Niwa, Y.; Akagi, T.; Daimon, T.; Murakami, M.; Fuwa, N. Particle therapy for mucosal melanoma of the head and neck. A single-institution retrospective comparison of proton and carbon ion therapy. Strahlenther. Onkol. 2014, 190, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, T.; Mizoe, J.E.; Hasegawa, A.; Takagi, R.; Bessho, H.; Onda, T.; Kamada, T.; Okamoto, Y.; Tsujii, H. Mucosal malignant melanoma of the head and neck treated by carbon ion radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Hasegawa, A.; Takagi, R.; Sasahara, G.; Ikawa, H.; Mizoe, J.E.; Jingu, K.; Tsujii, H.; Kamada, T.; Okamoto, Y.; et al. Feasibility of carbon ion radiotherapy for locally advanced sinonasal adenocarcinoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 113, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, N.; Tsuji, H.; Toyama, S.; Kamada, T.; Tsujii, H.; Nakayama, Y.; Mizota, A.; Ohnishi, Y.; Working Group for Ophthalmologic Tumors. Carbon-ion radiotherapy for locally advanced primary or postoperative recurrent epithelial carcinoma of the lacrimal gland. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2015, 114, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Koyama-Ito, H.; Kanai, T.; Minohara, S.; Tsuji, H.; Tsujii, H. Carbon ion therapy for ocular melanoma: Planning orthogonal two-port treatment. Phys. Med. Biol. 2007, 52, 5341–5352. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Ishikawa, H.; Yanagi, T.; Hirasawa, N.; Kamada, T.; Mizoe, J.E.; Kanai, T.; Tsujii, H.; Ohnishi, Y.; Working Group for Ophthalmologic Tumors. Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: A phase i/ii dose-escalation study. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Debus, J.; Haberer, T.; Schulz-Ertner, D.; Jakel, O.; Wenz, F.; Enghardt, W.; Schlegel, W.; Kraft, G.; Wannenmacher, M. Carbon ion irradiation of skull base tumors at gsi. First clinical results and future perspectives. Strahlentherapie und Onkologie 2000, 176, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Haberer, T.; Jakel, O.; Thilmann, C.; Kramer, M.; Enghardt, W.; Kraft, G.; Wannenmacher, M.; Debus, J. Radiotherapy for chordomas and low-grade chondrosarcomas of the skull base with carbon ions. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 36–42. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Haberer, T.; Scholz, M.; Thilmann, C.; Wenz, F.; Jakel, O.; Kraft, G.; Wannenmacher, M.; Debus, J. Acute radiation-induced toxicity of heavy ion radiotherapy delivered with intensity modulated pencil beam scanning in patients with base of skull tumors. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2002, 64, 189–195. [Google Scholar] [CrossRef]
- Jingu, K.; Tsujii, H.; Mizoe, J.E.; Hasegawa, A.; Bessho, H.; Takagi, R.; Morikawa, T.; Tonogi, M.; Tsuji, H.; Kamada, T.; et al. Carbon ion radiation therapy improves the prognosis of unresectable adult bone and soft-tissue sarcoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Miyamoto, T.; Nakajima, M.; Karube, M.; Hayashi, K.; Tsuji, H.; Tsujii, H.; Kamada, T.; Fujisawa, T. A dose escalation clinical trial of single-fraction carbon ion radiotherapy for peripheral stage i non-small cell lung cancer. J. Thorac. Oncol. 2017, 12, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Tsujii, H.; Miyamoto, T.; Mizoe, J.E.; Kamada, T.; Tsuji, H.; Yamada, S.; Kandatsu, S.; Yoshikawa, K.; Obata, T.; et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Imada, H.; Kato, H.; Yasuda, S.; Yamada, S.; Yanagi, T.; Kishimoto, R.; Kandatsu, S.; Mizoe, J.E.; Kamada, T.; Yokosuka, O.; et al. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010, 96, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S. Carbon-Ion Radiotherapy: Principles, Practices and Treatment Planning; Tsujii, H., Kamada, T., Shirai, T., Noda, K., Tsuji, H., Karasawa, K., Eds.; Springer: Tokyo, Japan, 2014; pp. 213–218. [Google Scholar]
- Kato, H.; Yamada, S.; Yasuda, S.; Yamaguchi, K.; Kitabayashi, H.; Kamada, T.; Mizoe, J.E.; Ohto, M.; Tsujii, H. Two-fraction carbon ion radiotherapy for hepatocellular carcinoma: Preliminary results of a phase i/ii clinical trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, S4124. [Google Scholar] [CrossRef]
- Kasuya, G.; Kato, H.; Yasuda, S.; Tsuji, H.; Yamada, S.; Haruyama, Y.; Kobashi, G.; Ebner, D.K.; Okada, N.N.; Makishima, H.; et al. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: Combined analyses of 2 prospective trials. Cancer 2017, 123, 3955–3965. [Google Scholar] [CrossRef] [PubMed]
- Shinoto, M.; Yamada, S.; Terashima, K.; Yasuda, S.; Shioyama, Y.; Honda, H.; Kamada, T.; Tsujii, H.; Saisho, H.; Working Group for Pancreas Cancer. Carbon ion radiation therapy with concurrent gemcitabine for patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Kawashiro, S.; Mori, S.; Yamada, S.; Miki, K.; Nemoto, K.; Tsuji, H.; Kamada, T. Dose escalation study with respiratory-gated carbon-ion scanning radiotherapy using a simultaneous integrated boost for pancreatic cancer: Simulation with four-dimensional computed tomography. Br. J. Radiol. 2017, 90, 20160790. [Google Scholar] [CrossRef] [PubMed]
- Shinoto, M.; Yamada, S.; Yasuda, S.; Imada, H.; Shioyama, Y.; Honda, H.; Kamada, T.; Tsujii, H.; Saisho, H.; Working Group for Pancreas Cancer. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer 2013, 119, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, Y.; Yasuda, S.; Nagata, M.; Izumi, Y.; Okazumi, S.; Shimada, H.; Nakatani, Y.; Tsujii, H.; Kamada, T.; Yamada, S.; et al. A phase i/ii clinical trial of preoperative short-course carbon-ion radiotherapy for patients with squamous cell carcinoma of the esophagus. J. Surg. Oncol. 2012, 105, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Kamada, T.; Ebner, D.K.; Shinoto, M.; Terashima, K.; Isozaki, Y.; Yasuda, S.; Makishima, H.; Tsuji, H.; Tsujii, H.; et al. Carbon-ion radiation therapy for pelvic recurrence of rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, T.; Tsuji, H.; Hirasawa, N.; Kato, H.; Kamada, T.; Mizoe, J.; Kishi, H.; Kamura, K.; Wada, H.; Nemoto, K.; et al. Carbon ion radiation therapy for primary renal cell carcinoma: Initial clinical experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Akakura, K.; Tsujii, H.; Morita, S.; Tsuji, H.; Yagishita, T.; Isaka, S.; Ito, H.; Akaza, H.; Hata, M.; Fujime, M.; et al. Phase i/ii clinical trials of carbon ion therapy for prostate cancer. The Prostate 2004, 58, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, T.; Tsuji, H.; Maruyama, K.; Toyama, S.; Suzuki, H.; Akakura, K.; Shimazaki, J.; Nemoto, K.; Kamada, T.; Tsujii, H.; et al. Phase i/ii trial of definitive carbon ion radiotherapy for prostate cancer: Evaluation of shortening of treatment period to 3 weeks. Br. J. Cancer 2014, 110, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Tsuji, H.; Kamada, T.; Akakura, K.; Suzuki, H.; Shimazaki, J.; Tsujii, H.; Working Group for Genitourinary Tumors. Carbon ion radiotherapy in advanced hypofractionated regimens for prostate cancer: From 20 to 16 fractions. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Ohno, T.; Tsujii, H.; Nakano, T.; Mizoe, J.E.; Kamada, T.; Miyamoto, T.; Tsuji, H.; Kato, H.; Yamada, S.; et al. Dose escalation study of carbon ion radiotherapy for locally advanced carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, M.; Kato, S.; Ohno, T.; Karasawa, K.; Ando, K.; Kiyohara, H.; Tsujii, H.; Nakano, T.; Kamada, T.; Shozu, M. Dose-escalation study of carbon ion radiotherapy for locally advanced squamous cell carcinoma of the uterine cervix (9902). Gynecol. Oncol. 2014, 132, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, M.; Kato, S.; Ohno, T.; Karasawa, K.; Kiyohara, H.; Tamaki, T.; Ando, K.; Tsujii, H.; Nakano, T.; Kamada, T.; et al. Clinical outcomes of carbon ion radiotherapy for locally advanced adenocarcinoma of the uterine cervix in phase 1/2 clinical trial (protocol 9704). Cancer 2014, 120, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Paganetti, H. Nuclear physics in particle therapy: A review. Rep Prog Phys 2016, 79, 096702. [Google Scholar] [CrossRef] [PubMed]
- Tinganelli, W.; Ma, N.Y.; Von Neubeck, C.; Maier, A.; Schicker, C.; Kraft-Weyrather, W.; Durante, M. Influence of acute hypoxia and radiation quality on cell survival. J. Radiat. Res. 2013, 54 (Suppl. 1), i23–i30. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Zhang, P.; Zhang, S.; Naidu, M.; Wang, H.; Wang, Y. S-phase cells are more sensitive to high-linear energy transfer radiation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Tamulevicius, P.; Wang, M.; Iliakis, G. Homology-directed repair is required for the development of radioresistance during s phase: Interplay between double-strand break repair and checkpoint response. Radiat. Res. 2007, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Scholz, U.; Durante, M.; Scholz, M. Rbe of ion beams in hypofractionated radiotherapy (sbrt). Phys. Med. 2014, 30, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Koike, S.; Uzawa, A.; Takai, N.; Fukawa, T.; Furusawa, Y.; Aoki, M.; Miyato, Y. Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J. Radiat. Res. 2005, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Grutters, J.P.; Kessels, A.G.; Pijls-Johannesma, M.; De Ruysscher, D.; Joore, M.A.; Lambin, P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: A meta-analysis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Grutters, J.P.; Pijls-Johannesma, M.; Ruysscher, D.D.; Peeters, A.; Reimoser, S.; Severens, J.L.; Lambin, P.; Joore, M.A. The cost-effectiveness of particle therapy in non-small cell lung cancer: Exploring decision uncertainty and areas for future research. Cancer Treat Rev. 2010, 36, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Vanderstraeten, B.; Verstraete, J.; De Croock, R.; De Neve, W.; Lievens, Y. In search of the economic sustainability of hadron therapy: The real cost of setting up and operating a hadron facility. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Demizu, Y.; Saitoh, J.I.; Suefuji, H.; Tsuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Takagi, R.; Nemoto, K.; et al. Multicenter study of carbon-ion radiation therapy for mucosal melanoma of the head and neck: Subanalysis of the japan carbon-ion radiation oncology study group (j-cros) study (1402 hn). Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Wakatsuki, M.; Kato, S.; Ohno, T.; Okonogi, N.; Karasawa, K.; Kiyohara, H.; Tsujii, H.; Nakano, T.; Kamada, T.; et al. Carbon-ion radiotherapy for locally advanced cervical cancer with bladder invasion. J. Radiat. Res. 2016, 57, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, J.; Hu, J.; Hu, W.; Guan, X.; Lu, R.; Lu, J.J. Phase i/ii trial evaluating concurrent carbon-ion radiotherapy plus chemotherapy for salvage treatment of locally recurrent nasopharyngeal carcinoma. Chin. J. Cancer 2016, 35, 101. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, F.; Brons, S.; Haberer, T.; Debus, J.; Combs, S.E.; Weber, K.J. Comparison of the effects of photon versus carbon ion irradiation when combined with chemotherapy in vitro. Radiat. Oncol. 2013, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- El Shafie, R.A.; Habermehl, D.; Rieken, S.; Mairani, A.; Orschiedt, L.; Brons, S.; Haberer, T.; Weber, K.J.; Debus, J.; Combs, S.E. In vitro evaluation of photon and raster-scanned carbon ion radiotherapy in combination with gemcitabine in pancreatic cancer cell lines. J. Radiat. Res. 2013, 54 (Suppl. 1), i113–i119. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Zipp, L.; Rieken, S.; Habermehl, D.; Brons, S.; Winter, M.; Haberer, T.; Debus, J.; Weber, K.J. In vitro evaluation of photon and carbon ion radiotherapy in combination with chemotherapy in glioblastoma cells. Radiat. Oncol. 2012, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Bohl, J.; Elsasser, T.; Weber, K.J.; Schulz-Ertner, D.; Debus, J.; Weyrather, W.K. Radiobiological evaluation and correlation with the local effect model (lem) of carbon ion radiation therapy and temozolomide in glioblastoma cell lines. Int. J. Radiat. Biol. 2009, 85, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Baris, D.; Habermehl, D.; Rieken, S.; Brons, S.; Weber, K.J.; Roth, W.; Debus, J.; Combs, S.E. Evaluation of chemoradiotherapy with carbon ions and the influence of p53 mutational status in the colorectal carcinoma cell line hct 116. Tumori 2014, 100, 675–684. [Google Scholar] [PubMed]
- Harrabi, S.; Combs, S.E.; Brons, S.; Haberer, T.; Debus, J.; Weber, K.J. Temozolomide in combination with carbon ion or photon irradiation in glioblastoma multiforme cell lines—Does scheduling matter? Int. J. Radiat. Biol. 2013, 89, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, H.; Shimada, H.; Yamada, S.; Yasuda, S.; Kamata, T.; Ando, K.; Tsujii, H.; Ochiai, T. Synergistic growth suppression induced in esophageal squamous cell carcinoma cells by combined treatment with docetaxel and heavy carbon-ion beam irradiation. Oncol. Rep. 2006, 15, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Harrabi, S.B.; Adeberg, S.; Winter, M.; Haberer, T.; Debus, J.; Weber, K.J. S-phase-specific radiosensitization by gemcitabine for therapeutic carbon ion exposure in vitro. J. Radiat. Res. 2016, 57, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Fujita, H.; Hosoi, A.; Nakawatari, M.; Nakamura, E.; Kakimi, K.; Nakano, T.; Imai, T.; Shimokawa, T. Effective suppression of pulmonary metastasis in combined carbon ion radiation therapy with dendritic-cell immunotherapy in murine tumor models. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, S642. [Google Scholar] [CrossRef]
- Matsunaga, A.; Ueda, Y.; Yamada, S.; Harada, Y.; Shimada, H.; Hasegawa, M.; Tsujii, H.; Ochiai, T.; Yonemitsu, Y. Carbon-ion beam treatment induces systemic antitumor immunity against murine squamous cell carcinoma. Cancer 2010, 116, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Bassler, N.; Toftegaard, J.; Luhr, A.; Sorensen, B.S.; Scifoni, E.; Kramer, M.; Jakel, O.; Mortensen, L.S.; Overgaard, J.; Petersen, J.B. Let-painting increases tumour control probability in hypoxic tumours. Acta Oncol. 2014, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tinganelli, W.; Durante, M.; Hirayama, R.; Kramer, M.; Maier, A.; Kraft-Weyrather, W.; Furusawa, Y.; Friedrich, T.; Scifoni, E. Kill-painting of hypoxic tumours in charged particle therapy. Sci. Rep. 2015, 5, 17016. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Schwartz, R.; Flickinger, J.; Beriwal, S. Machine learning approaches for predicting radiation therapy outcomes: A clinician’s perspective. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Men, K.; Chen, X.; Zhang, Y.; Dai, J.; Yi, J.; Li, Y. Deep deconvolutional neural network for target segmentation of nasopharyngeal cancer in planning ct images. Front. Oncol. 2017, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Furukawa, T.; Fujisawa, T.; Iwata, Y.; Kanai, T.; Kanazawa, M.; Kitagawa, A.; Komori, M.; Minohara, S.; Murakami, T.; et al. New accelerator facility for carbon-ion cancer-therapy. J. Radiat. Res. 2007, 48 (Suppl. A), A43–A54. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Tsujii, H.; Blakely, E.A.; Debus, J.; De Neve, W.; Durante, M.; Jakel, O.; Mayer, R.; Orecchia, R.; Potter, R.; et al. Carbon ion radiotherapy in Japan: An assessment of 20 years of clinical experience. Lancet Oncol. 2015, 16, e93–e100. [Google Scholar] [CrossRef]
- Maruyama, K.; Tsuji, H.; Nomiya, T.; Katoh, H.; Ishikawa, H.; Kamada, T.; Wakatsuki, M.; Akakura, K.; Shimazaki, J.; Aoyama, H.; et al. Five-year quality of life assessment after carbon ion radiotherapy for prostate cancer. J. Radiat. Res. 2017, 58, 260–266. [Google Scholar] [CrossRef] [PubMed]
| Specification | Value |
|---|---|
| Treatment rooms | Rooms E and F: H and V beams in each |
| Room G: Rotating gantry (26 possible angles) | |
| Accelerated energies | 140–430 MeV/u |
| Range | Up to 30 cm |
| Field size | 22 × 22 cm in rooms E and F |
| 20 × 20 cm in room G (compared to 15 × 15 cm in the HIMAC) | |
| Dose rate | Up to 5 GyE/min |
| Irradiation method | 3D fast rescanning with gating for moving targets |
| Scanning technology | Multiple-energy operation with extended flattops with >200 energy steps |
| Site | Number (%) |
|---|---|
| Prostate | 2863 (24.7%) |
| Bone & soft tissue | 1336 (11.5%) |
| Head & neck | 1107 (9.6%) |
| Lung | 1062 (9.2%) |
| Pancreas | 624 (5.4%) |
| Liver | 613 (5.3%) |
| Rectum (post-operative relapse) | 572 (4.9%) |
| Uterus (cervix & body) | 289 (2.5%) |
| Uveal melanoma | 206 (1.8%) |
| Abdominal lymph nodes | 143 (1.2%) |
| CNS | 106 (0.9%) |
| Skull base | 104 (0.9%) |
| Gastrointestinal tract | 97 (0.8%) |
| Lacrimal Gland | 37 (0.3%) |
| Scanning beams (clinical trial) | 21 (0.2%) |
| Breast | 9 (0.1%) |
| Kidney | 8 (0.1%) |
| Rotating gantry (clinical trial) | 8 (0.1%) |
| Re-irradiation | 1065 (9.2%) |
| Others | 1310 (11.3%) |
| Total | 11,580 (100%) |
| Cancer Type | Number of Fractions in Initial Protocols | Current Clinical Practice (Shortest Regimen) |
|---|---|---|
| Osteosarcoma and Soft Tissue Sarcoma [55,56,57,58,59,60] | 16 | 16 |
| Head and neck cancer a [61,62,63,64] | 18 | 16 |
| Lacrimal gland tumor [65] | 12 | 12 |
| Ocular melanoma [66,67] | 5 | 4 |
| Skull base cancer [68,69,70,71] | 16 | 16 |
| Early stage lung cancer [47,72] | 18 | 1 |
| Hepatocellular carcinoma [73,74,75,76,77] | 15 | 2 |
| Liver metastasis from colorectal cancer b | 1 | 1 |
| Pancreas cancer [78,79,80] | ||
| -Preoperative | 16 | 8 |
| -Definitive c | 12 | 12 |
| Esophageal cancer [81] | ||
| -Preoperative d | 8 | 8 |
| -Definitive | 12 | 12 |
| Recurrent rectal cancer [82] | 16 | 16 |
| Renal cell carcinoma [83] | 16 | 4 (on protocol) |
| Prostate cancer e [84,85,86] | 20 | 12 |
| Locally advanced uterine (cervical) cancer f [87,88,89] | 24 | 20 |
| Recommendation | Progress |
|---|---|
| Clinical | |
| “Continued research in ultra-short fractionation” | Significant milestones achieved (single fraction NSCLC) and multiple clinical trials in progress |
| “Continued research in combined modalities” | Ongoing work in pancreas, melanoma, uterine, and esophageal cancers |
| “Reduction in size and cost of technology” | Significant milestones achieved (Section 9.4) and more work in progress |
| “Improving patient throughput including use of gantry, immobilization devices” | Patients, including those with moving tumors, are currently being treated in the gantry room |
| “Analyzing incidence of SMN after CIRT” | Ongoing work |
| “Publish studies in peer reviewed journals and provide detailed reporting of methods in studies” | Tens of papers have been published with updated reporting of results and methodology |
| “Increase use of QOL assessment” | QOL studies are in progress [119] |
| “Announce and register clinical trials internationally” | Clinical trials are announced online at www.umin.ac.jp/ctr |
| “Analysis of relations between dose and local recurrence to estimate potential of dose-painting” | Ongoing work |
| “Start scanning beams CIRT for moving targets” | Patients with moving tumors are treated regularly with scanning beams |
| “Use MRI for adaptive therapy for cervical cancer” | MRI is now standard practice for adaptive planning in cervical cancer |
| “Start randomized phase III trials” | CIPHER trial will start accruing soon (Section 9.4) |
| “Start trials on GBM” | No ongoing trials at the NIRS for GBM |
| Radiobiology | |
| “Intensify international collaborations and harmonize reporting of data/methods” | Ongoing work |
| “Achieve international standard for biophysical modeling in treatment planning” | Ongoing work with European teams [54] |
| “Establish dose-dependent RBE especially for hypofractionation” | Ongoing work [48] |
| “Continue work on combinations of CIRT and immunotherapy” | Ongoing preclinical studies |
| Medical physics | |
| “Continue commissioning of moving target irradiation, PCR and tumor tracking gating system” and “continue research on the interplay effect of scanning beams” | Patients with moving tumors are treated regularly using PCR and respiratory gating |
| “Continue work on optimization of multiple energy operations of synchrotrons” | Significant progress achieved [22] |
| “Continue work on gantry commissioning” | Patients, including those with moving tumors, are currently being treated in the gantry room |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad, O.; Makishima, H.; Kamada, T. Evolution of Carbon Ion Radiotherapy at the National Institute of Radiological Sciences in Japan. Cancers 2018, 10, 66. https://doi.org/10.3390/cancers10030066
Mohamad O, Makishima H, Kamada T. Evolution of Carbon Ion Radiotherapy at the National Institute of Radiological Sciences in Japan. Cancers. 2018; 10(3):66. https://doi.org/10.3390/cancers10030066
Chicago/Turabian StyleMohamad, Osama, Hirokazu Makishima, and Tadashi Kamada. 2018. "Evolution of Carbon Ion Radiotherapy at the National Institute of Radiological Sciences in Japan" Cancers 10, no. 3: 66. https://doi.org/10.3390/cancers10030066




