Proton Beam Therapy Alone for Intermediate- or High-Risk Prostate Cancer: An Institutional Prospective Cohort Study
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
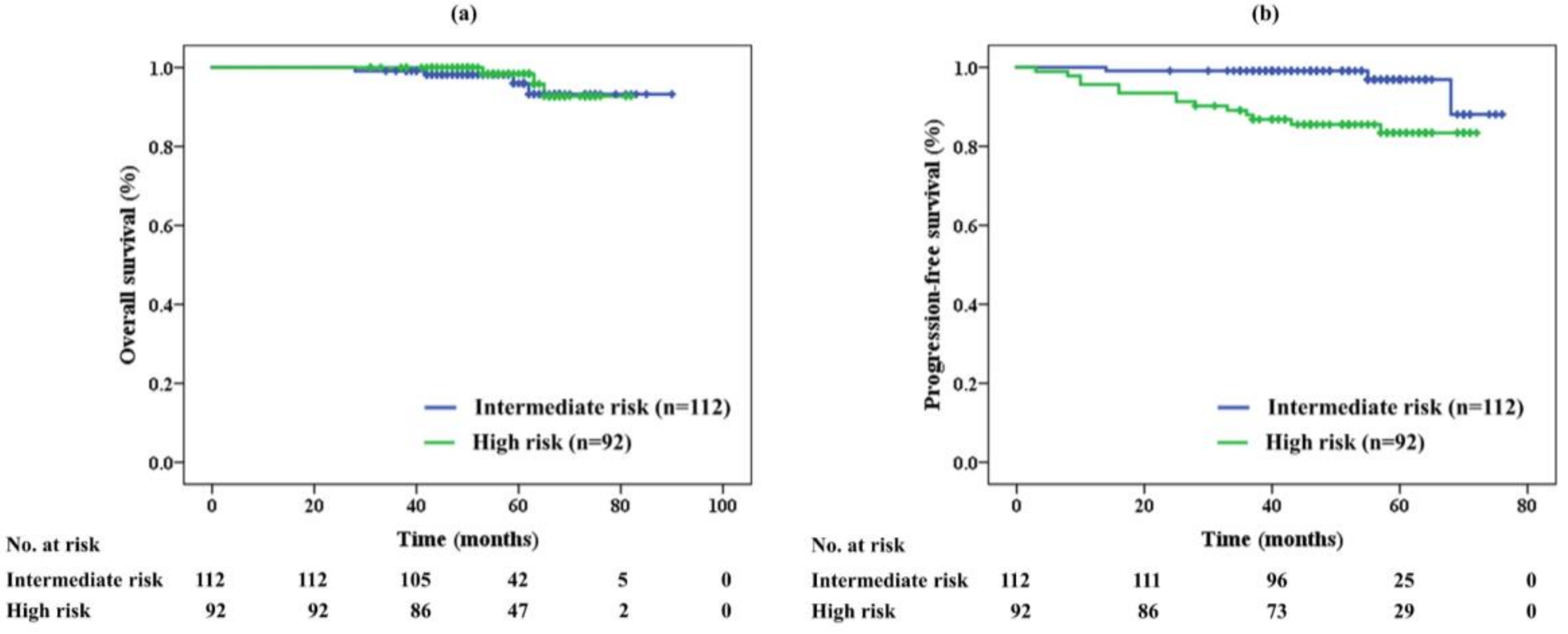
2.2. Efficacy
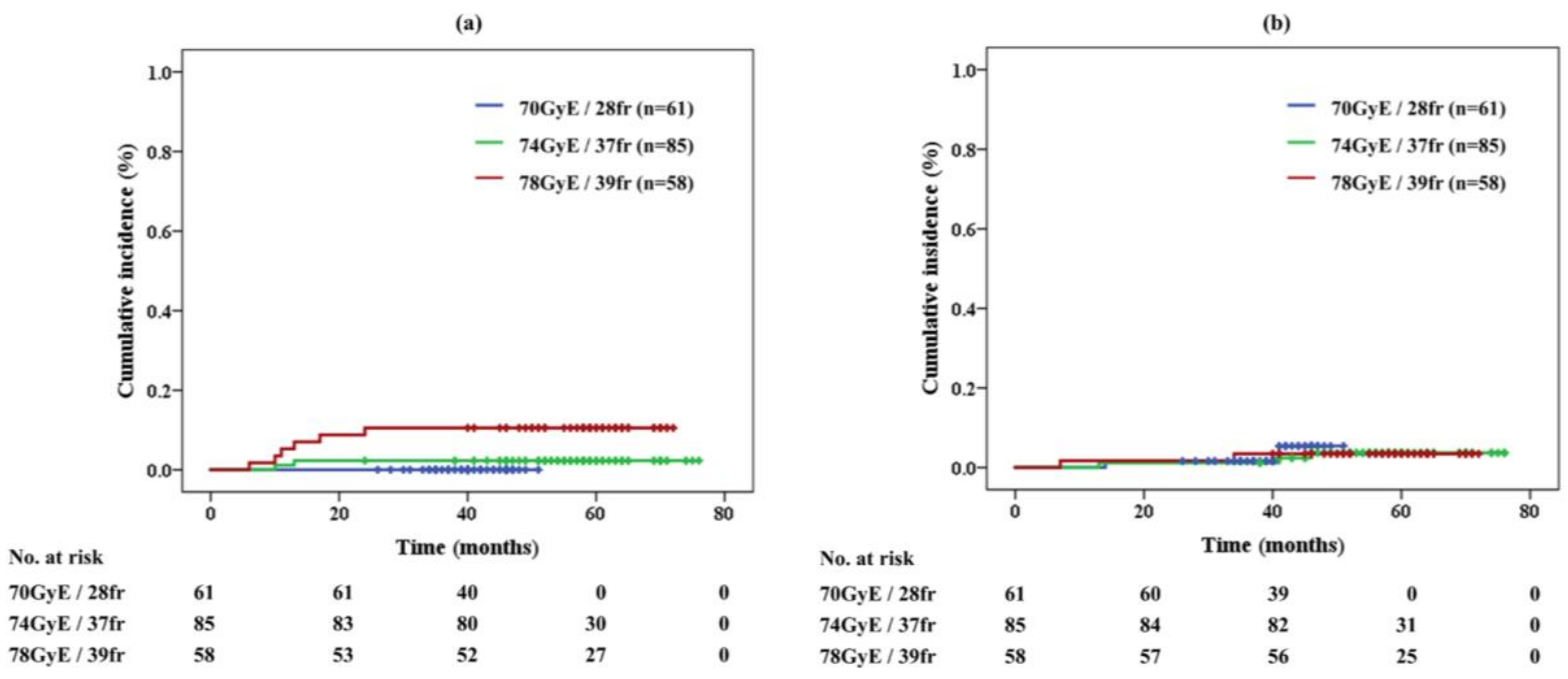
2.3. Adverse Events
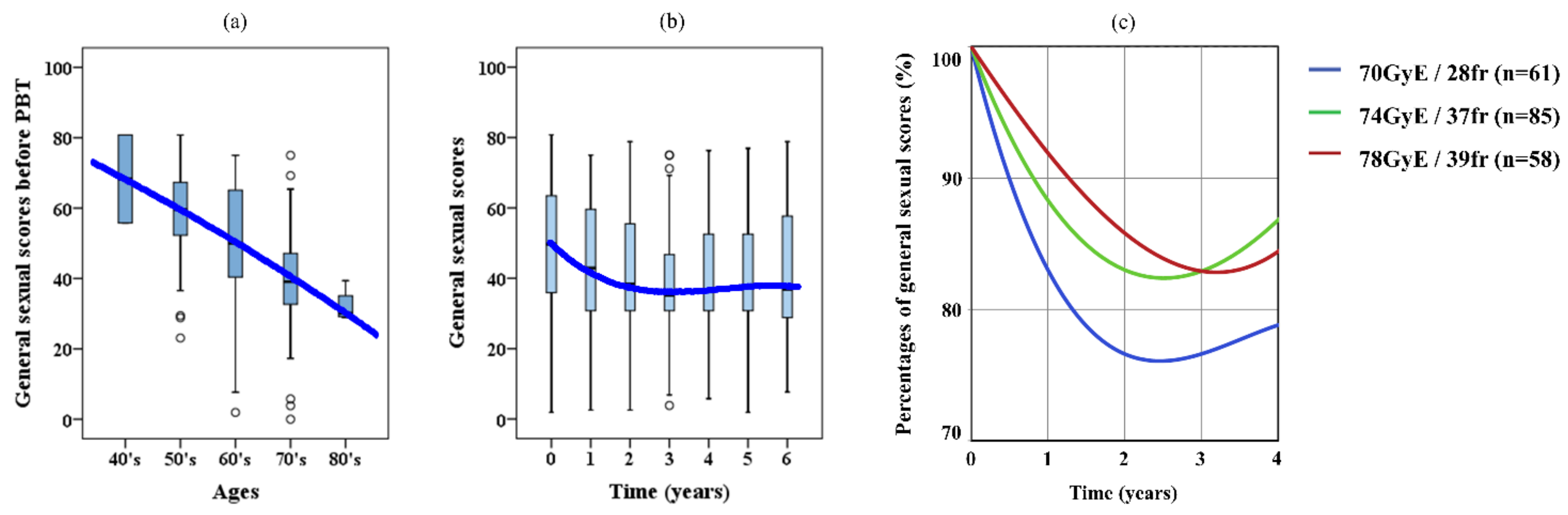
2.4. Sexual QOL
2.5. Costs of PBT
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. PBT
4.3. Follow-Up and Evaluation of Adverse Effects
4.4. Costs
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomarkers Prev. 2015, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Globocan 2012. Available online: http://globocan.iarc.fr/Default.aspx (accessed on 27 December 2017).
- Hori, M.; Matsuda, T.; Shibata, A.; Katanoda, K.; Sobue, T.; Nishimoto, H. Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn. J. Clin. Oncol. 2015, 45, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Broering, J.M.; Litwin, M.S.; Lubeck, D.P.; Mehta, S.S.; Henning, J.M.; Carroll, P.R. CaPSURE Investigators. The contemporary management of prostate cancer in the United States: Lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J. Urol. 2004, 171, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network: NCCN Clinical Guidelines in Oncology for Prostate Cancer. Available online: http://www.nccn.org/professionals/physician_gls/pdf/prostate_blocks.pdf (accessed on 27 December 2017).
- Lu-Yao, G.L.; Albertsen, P.C.; Moore, D.F.; Shih, W.; Lin, Y.; DiPaola, R.S.; Yao, S.L. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA 2008, 300, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Studer, U.E.; Collette, L.; Whelan, P.; Albrecht, W.; Casselman, J.; de Reijke, T.; Knönagel, H.; Loidl, W.; Isorna, S.; Sundaram, S.K.; et al. Using PSA to guide timing of androgen deprivation in patients with T0-4 N0-2 M0 prostate cancer not suitable for local curative treatment (EORTC 30891). Eur. Urol. 2008, 53, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Lee, W.C.; Brandman, J.; Wang, Q.; Botteman, M.; Pashos, C.L. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. Clin. Oncol. 2005, 23, 7897–7903. [Google Scholar] [CrossRef] [PubMed]
- Braga-Basaria, M.; Dobs, A.S.; Muller, D.C.; Carducci, M.A.; John, M.; Egan, J.; Basaria, S. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J. Clin. Oncol. 2006, 24, 3979–3983. [Google Scholar] [CrossRef] [PubMed]
- Keating, N.L.; O’Malley, A.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2006, 24, 4448–4456. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, S.; Garmo, H.; Holmberg, L.; Adolfsson, J.; Stattin, P.; Van Hemelrijck, M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J. Clin. Oncol. 2015, 33, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Dinh, K.T.; Reznor, G.; Muralidhar, V.; Mahal, B.A.; Nezolosky, M.D.; Choueiri, T.K.; Hoffman, K.E.; Hu, J.C.; Sweeney, C.J.; Trinh, Q.D.; et al. Association of androgen deprivation therapy with depression in localized prostate cancer. J. Clin. Oncol. 2016, 34, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Kal, H.B.; Van Gellekom, M.P.R. How low is the α/β ratio for prostate cancer? Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 1116–1121. [Google Scholar] [CrossRef]
- Leborgne, F.; Fowler, J.; Leborgne, J.H.; Mezzera, J. Later outcomes and alpha/beta estimate from hypofractionated conformal three-dimensionalradiotherapy versus standard fractionation for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.U.; Hunt, D.; McGowan, D.G.; Amin, M.B.; Chetner, M.P.; Bruner, D.W.; Leibenhaut, M.H.; Husain, S.M.; Rotman, M.; Souhami, L.; et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med. 2011, 365, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, Z.S.; Spratt, D.E.; Pei, X.; Yamada, Y.; Kalikstein, A.; Kuk, D.; Zhang, Z.; Zelefsky, M.J. Short-term androgen-deprivation therapy improves prostate cancer-specific mortality in intermediate-risk prostate cancer patients undergoing dose-escalated external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 85, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; Gonzalez San Segundo, C.; Cabeza Rodríguez, M.A.; Macias, V.; Pedro Olive, A.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 320–327. [Google Scholar] [CrossRef]
- Bragg, W.H.; Kleeman, R.D. On the α particles of radium, and their loss of range in passing through various atoms and molecules. Philos. Mag. 1905, 10, 318–340. [Google Scholar] [CrossRef]
- Wilson, R.R. Radiological use of fast protons. Radiology 1946, 47, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Jones, K.M.; Barry, M.J.; Gerald, L.; Andriole, G.L.; Culkin, D.; Wheeler, T.; Aronson, W.J.; Brawer, M.K. Follow-up of prostatectomy versus observation for early prostate cancer. N. Engl. J. Med. 2017, 377, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Rhamy, R.K.; Wilson, S.K.; Caldwell, W.L. Biopsy-proved tumor following definitive irradiation for resectable carcinoma of the prostate. J. Urol. 1972, 107, 627–630. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Zelefsky, M.J. Short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer undergoing dose-escalated radiotherapy: The standard of care? Lancet Oncol. 2012, 13, e259–e269. [Google Scholar] [CrossRef]
- Yang, D.D.; Muralidhar, V.; Mahal, B.A.; Nguyen, P.L.; Devlin, P.M.; King, M.T.; Orio, P.F. Lack of Apparent survival benefit with use of androgen deprivation therapy in patients with high-risk prostate cancer receiving combined external beam radiation therapy and brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; Collette, L.; Blank, L.; Warde, P.; Dubois, J.B.; Mirimanoff, R.O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet 2002, 360, 103–106. [Google Scholar] [CrossRef]
- Edelman, S.; Liauw, S.L.; Rossi, P.J.; Cooper, S.; Jani, A.B. High-dose radiotherapy with or without androgen deprivation therapy for intermediate-risk prostate cancer: Cancer control and toxicity outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Reuter, V.E.; Fuks, Z.; Scardino, P.; Shippy, A. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J. Urol. 2008, 179, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Nihei, K.; Ogino, T.; Onozawa, M.; Murayama, S.; Fuji, H.; Murakami, M.; Hishikawa, Y. Multi-institutional phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. N. Engl. J. Med. 2011, 81, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, N.P.; Hoppe, B.S.; Romaine, C.; Nichols, R.C.; William, M.; Mendenhall, W.M.; Morris, C.G.; Li, Z.; Su, Z.; Williams, C.R.; et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Smith, T.L.; Henderson, R.H.; Hoppe, B.S.; Mendenhall, W.M.; Nichols, R.C.; Morris, C.G.; Williams, C.R.; Su, Z.; Li, Z.; et al. Five-year biochemical results, toxicity, and patient-reported quality of life after delivery of dose-escalated image guided proton therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Demizu, Y.; Terashima, K.; Fujii, O.; Jin, D.; Niwa, Y.; Daimon, T.; Murakami, M.; Fuwa, N.; Okimoto, T. Long-term outcomes in patients treated with proton therapy for localized prostate cancer. Cancer Med. 2017, 6, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Ishikawa, H.; Takagi, M.; Okimoto, T.; Murayama, S.; Akimoto, T.; Wada, H.; Arimura, T.; Sato, Y.; Araya, M.; et al. Long-term outcomes of proton beam therapy for prostate cancer in Japan: Retrospective analysis of a multi-institutional survey. Cancer Med. 2018, 7, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Cahlon, O.; Zelefsky, M.J.; Shippy, A.; Chan, H.; Fuks, Z.; Yamada, Y.; Hunt, M.; Greenstein, S.; Amols, H. Ultra-high dose (86.4Gy) IMRT for localized prostate cancer: Toxicity and biochemical outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Pei, X.; Yamada, J.; Kollmeier, M.A.; Cox, B.; Zelefsky, M.J. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Kupelian, P.A.; Willoughby, T.; Reddy, C.A.; Klein, E.A.; Mahadevan, A. Hypofractionated intensity-modulated radiotherapy (70Gy at 2.5Gy per fraction) for localized prostate cancer: Cleveland clinic experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Tsuji, H.; Kamada, T.; Akakura, K.; Suzuki, H.; Shimazaki, J.; Tsujii, H.; The Working Group for Genitourinary Tumors. Carbon-ion radiation therapy for prostate cancer. Int. J. Urol. 2012, 19, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.J.; Koyama, T.; Fan, K.H.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Potosky, A.L.; Stanford, J.L.; Stroup, A.M.; et al. Long-term functional outcomes after treatment for localized prostate cancer. N. Engl. J. Med. 2013, 368, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Mishra, M.V.; Mehta, M.P. A systemic review of the cost and cost-effectiveness studies of proton beam therapy. Cancer 2016, 122, 1483–1501. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Simone, C.B., 2nd; Mishra, M.V. Quality of life and patient-reported outcomes following proton radiation therapy: A systemic review. J. Natl. Cancer Inst. 2018, 110, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Meves, A.; Bigby, M. Case reports and case series from Lancet had significant impact on medical literature. J. Clin. Epidemiol. 2005, 58, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, B.; Dijkman, B.; Einhorn, T.A.; Bhandari, M. How to design a good case series. J. Bone Joint Surg. Am. 2009, 91, 21–26. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Fondurulia, J.; Chen, M.H.; Kaplan, I.; Beard, C.J.; Tomaszewski, J.E.; Renshaw, A.A.; Wein, A.; et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J. Clin. Oncol. 1999, 17, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, K.; Murakami, M.; Hishikawa, Y.; Abe, M.; Akagi, T.; Yanou, T.; Kagiya, G.; Furusawa, Y.; Ando, K.; Nojima, K.; et al. Preclinical biological assessment of proton and carbon ion beams at Hyogo Ion Beam Medical Center. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 928–938. [Google Scholar] [CrossRef]
- Fiorino, C.; Fellin, G.; Rancati, T.; Vavassori, V.; Bianchi, C.; Borca, V.C.; Girelli, G.; Mapelli, M.; Menegotti, L.; Nava, S.; et al. Clinical and dosimetric predictors of late rectal syndrome after 3-D CRT for localized prostate cancer: Preliminary results of a multicenter prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.V.; Denekamp, J.; Fowler, J.F. A review of α/β ratios for experimental tumors: Implications for clinical studies of altered fractionation. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 87–96. [Google Scholar] [CrossRef]
- Abramowitz, M.C.; Li, T.; Buyyounouski, M.K.; Ross, E.; Uzzo, R.G.; Pollack, A.; Horwitz, E.M. The phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer 2008, 112, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.T.; Dunn, R.L.; Litwin, M.S.; Sandler, H.M.; Sanda, M.G. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000, 56, 899–905. [Google Scholar] [CrossRef]

| Factors | Items | Value | Unit | |
|---|---|---|---|---|
| Age | Median | 65 | years | |
| Range | 39–86 | years | ||
| Performance status (ECOG) | 0 | 200 | (98%) | |
| 1 | 4 | (2%) | ||
| Follow-up time | Median | 52 | months | |
| Range | 24–76 | months | ||
| Initial PSA (ng/ml) | <10 | 140 | (69%) | |
| 10–20 | 44 | (22%) | ||
| ≥20 | 20 | (10%) | ||
| Gleason score | 6 | 18 | (9%) | |
| 7 | 125 | (61%) | ||
| 8 | 41 | (20%) | ||
| ≥9 | 20 | (10%) | ||
| Clinical T stage (UICC) | T1c | 88 | (43%) | |
| T2a | 70 | (34%) | ||
| T2b | 11 | (5%) | ||
| T2c | 18 | (9%) | ||
| T3a | 14 | (7%) | ||
| T3b | 3 | (1%) | ||
| Risk stratification | Intermediate | 112 | (55%) | |
| High | 92 | (45%) | ||
| Prescription dose (GyE) | 70 | 26 | (Intermediate, 13%) | |
| 35 | (High, 17%) | |||
| 74 | 85 | (42%) | ||
| 78 | 58 | (28%) | ||
| Anticoagulants | Yes | 30 | (15%) | |
| No | 174 | (85%) | ||
| HbA1c | <6.5 | 177 | (87%) | |
| ≥6.5 | 27 | (13%) | ||
| Percentage of positive biopsy cores | Median | 25 | % | |
| Range | 5–100 | % | ||
| Perineural invasion in biopsy specimens | Yes | 11 | (5%) | |
| No | 193 | (95%) | ||
| CTV D95 | Median | 74 | GyE | |
| Range | 69–78 | GyE | ||
| PTV D95 | Median | 59 | GyE | |
| Range | 38–67 | GyE | ||
| CTV V95 | Median | 100 | % | |
| Range | 98–100 | % | ||
| PTV V95 | Median | 79 | % | |
| Range | 63–88 | % | ||
| GU toxicity (grade 2) | Late | Median | 34 | months |
| Range | 7–46 | months | ||
| Acute/Late | Retention | 30/3 | (15%/1%) | |
| Frequency | 21/1 | (10%/<1%) | ||
| Pain | 10/0 | (5%/0%) | ||
| Events | Urgency | 4/0 | (2%/0%) | |
| Hematuria | 0/4 | (0%/2%) | ||
| Incontinence | 0/1 | (0%/<1%) | ||
| GI toxicity (grade 2) | Late | Median | 12 | months |
| Range | 6–24 | months | ||
| Events | Acute/Late | Hemorrhage | 0/8 | (0%/4%) |
| PSA nadir (Value) | 70(GyE) | Median | 0.5 | ng/ml |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| 5-Year PFS | p Value | Hazard Ratio (95% CI) | p Value | |
| Age | ||||
| <60 vs. ≥60 | 92% vs. 91% | 0.989 | – | – |
| <70 vs. ≥70 | 89% vs. 94% | 0.843 | 1.595 (0.482–5.282) | 0.445 |
| PSA | ||||
| <10 vs. ≥10 | 93% vs. 86% | 0.052 | – | – |
| <12 vs. ≥12 | 93% vs. 82% | 0.019 | 0.185 (0.061–0.555) | 0.003 |
| <20 vs. ≥20 | 92% vs. 79% | 0.055 | – | – |
| Gleason score | ||||
| ≤6 vs. ≥7 | 100% vs. 90% | 0.535 | – | – |
| ≤7 vs. ≥8 | 94% vs. 84% | 0.005 | 0.122 (0.034–0.441) | 0.001 |
| Percentage of positive biopsy cores (%) | ||||
| <20 vs. ≥20 | 94% vs. 89% | 0.342 | – | – |
| <30 vs. ≥30 | 94% vs. 87% | 0.146 | 0.546 (0.199–1.501) | 0.241 |
| Perineural invasion in biopsy specimens | ||||
| Yes vs. No | 91% vs. 91% | 0.955 | 1.992 (0.209–18.995) | 0.549 |
| Clinical T stage (UICC) | ||||
| 1 vs. ≥2 | 98% vs. 86% | 0.011 | – | – |
| ≤2 vs. 3 | 92% vs. 76% | 0.015 | 0.221 (0.063–0.778) | 0.019 |
| Dose (GyE/fraction) | ||||
| 70/28 vs. 74/37 | 93% vs. 92% | 0.281 | 1.822 (0.362–9.172) | 0.467 |
| 74/37 vs. 78/39 | 92% vs. 88% | 0.296 | 3.231 (0.778–13.413) | 0.106 |
| 70/28 vs. 78/39 | 93% vs. 88% | 0.325 | 1.773 (0.460–6.841) | 0.406 |
| 70/28 vs. 74/37 & 78/39 | 93% vs. 91% | 0.966 | – | – |
| PTV D95 (GyE) | ||||
| <60 vs. ≥60 | 89% vs. 91% | 0.634 | 1.197 (0.350–4.089) | 0.775 |
| PTV V95 (%) | ||||
| <80 vs. ≥80 | 90% vs. 92% | 0.834 | 0.433 (0.137–1.363) | 0.152 |
| Currency | Range | PBT (Until March 2018) | EBRT (IMRT) + ADT 2.5y | BT + EBRT (3D CRT) + ADT 2y | PBT (Since April 2018) |
|---|---|---|---|---|---|
| Japanese yen | Minimum | ¥2,600,000- | ¥2,522,100- | ¥2,591,380- | ¥1,600,000- |
| Maximum | ¥3,000,000- | ¥3,123,280- | ¥3,158,040- | ||
| US dollar | Minimum | $21,667- | $21,018- | $21,595- | $13,333- |
| Maximum | $25,000- | $26,027- | $26,317- |
| Reports | Year | EBRT | Patients (Number) | Median f/u (Months) | Dose (Gy(E)) | Fraction | ADT (%) | 5-Year Recurrence-Free Survival (%) | Gr 2 (Gr 3) Toxicity (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intermediate Risk | High Risk | Acute GU | Late GU | Late GI | ||||||||
| Kupelian et al. [36] | 2007 | IMRT | 508 | 45 | 70 | 28 | 60 * | 83 | 72 | – | 7.0 | 6.0 |
| Cahlon et al. [34] | 2008 | IMRT | 378 | 53 | 86.4 | 48 | 66 * | 85 | 70 | 22.0 (0.6) | 12.6 (2.5) | 3.4 (0.4) |
| Ishikawa et al. [37] | 2012 | Carbon | 768 | 43 | 57.6–66 | 16–20 | 100 | 97 | 88 | – | 6.2 (0.1) | 1.9 (0.0) |
| Spratt et al. [35] | 2013 | IMRT | 806 | 66 | 86.4 | 48 | 66 | 86 ** | 68 ** | – | 18.9 (2.2) | 3.7 (0.7) |
| Mendenhall et al. [30] | 2014 | Proton | 122 | 62 | 78–82 | 39–41 | 37 | 99 | 76 | (0.0) | (0.9) | (0.5) |
| Bryant et al. [31] | 2016 | Proton | 780 | 66 | 72–80 | 36–43 | 27 | 94 | 74 | (1.8) | (2.9) | (0.6) |
| Takagi et al. [32] | 2017 | Proton | 1126 | 70 | 74–78 | 37–39 | 64 | 91 | 86 | – | 1.9 (0.1) | 3.8 (0.1) |
| Iwata et al. [33] | 2018 | Proton | 1076 | 69 | 63–80 | 21–40 | 68 | 91 | 83 | – | (0.3) | (0.5) |
| Our study | 2018 | Proton | 204 | 52 | 70–78 | 28–39 | 0 | 97 | 83 | 23.5 (0.0) | 3.4 (0.0) | 3.9 (0.0) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arimura, T.; Yoshiura, T.; Matsukawa, K.; Kondo, N.; Kitano, I.; Ogino, T. Proton Beam Therapy Alone for Intermediate- or High-Risk Prostate Cancer: An Institutional Prospective Cohort Study. Cancers 2018, 10, 116. https://doi.org/10.3390/cancers10040116
Arimura T, Yoshiura T, Matsukawa K, Kondo N, Kitano I, Ogino T. Proton Beam Therapy Alone for Intermediate- or High-Risk Prostate Cancer: An Institutional Prospective Cohort Study. Cancers. 2018; 10(4):116. https://doi.org/10.3390/cancers10040116
Chicago/Turabian StyleArimura, Takeshi, Takashi Yoshiura, Kyoko Matsukawa, Naoaki Kondo, Ikumi Kitano, and Takashi Ogino. 2018. "Proton Beam Therapy Alone for Intermediate- or High-Risk Prostate Cancer: An Institutional Prospective Cohort Study" Cancers 10, no. 4: 116. https://doi.org/10.3390/cancers10040116




