Photoimmunotherapy of Ovarian Cancer: A Unique Niche in the Management of Advanced Disease
Abstract
:1. Introduction
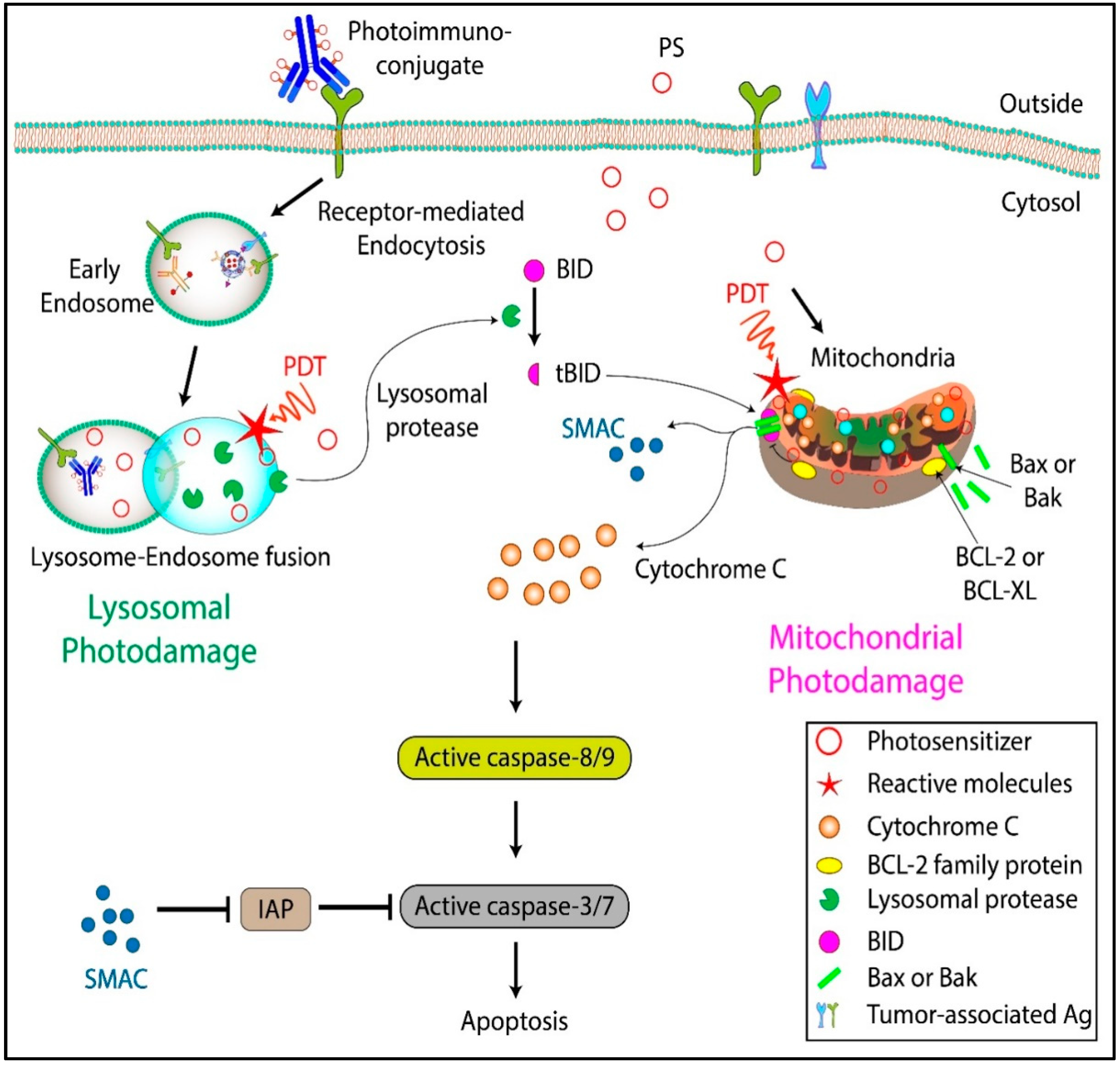
2. Photodynamic Therapy: Mechanisms
3. Photoimmunoconjugates and Photoimmunotherapy
4. PIC Design Considerations
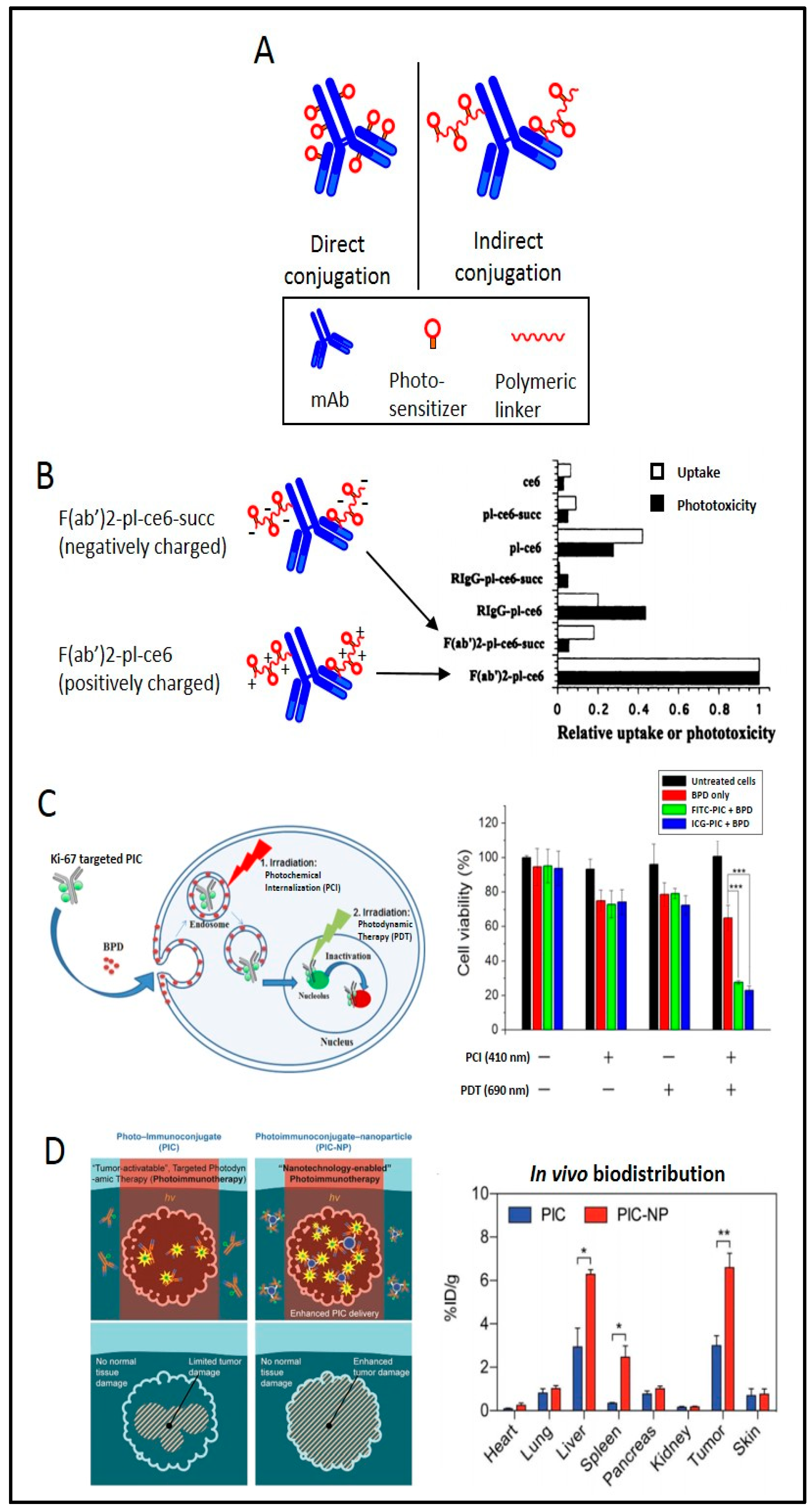
4.1. Direct vs. Indirect Conjugation
4.2. Charge-Based Enhancement of Cellular Uptake
4.3. “Multiple Epitope-Targeting” for Enhancing Efficacy
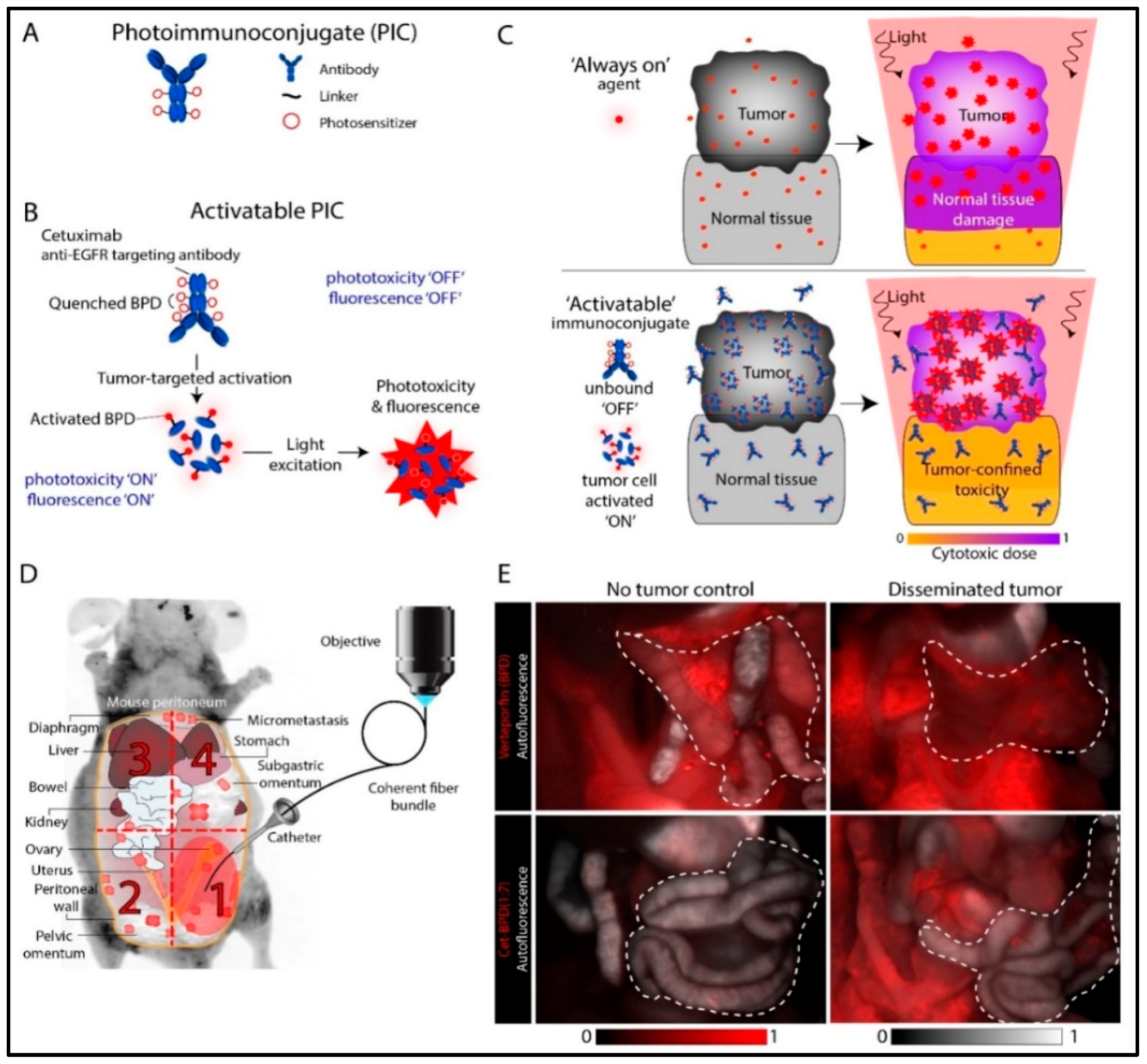
4.4. Preventing Lysosomal Degradation and Enhancing Intracellular Targeting
4.5. PIC-Based Enhancement of Nanoparticle Uptake
5. The Rationale to Target EGFR for OvCa Photoimmunotherapy
6. Other Molecular Targets for OvCa Photoimmunotherapy
6.1. Human Epidermal Growth Factor Receptor 2 (HER2)
6.2. Ki67
6.3. MUC16
6.4. Folate Receptor α (FRα)
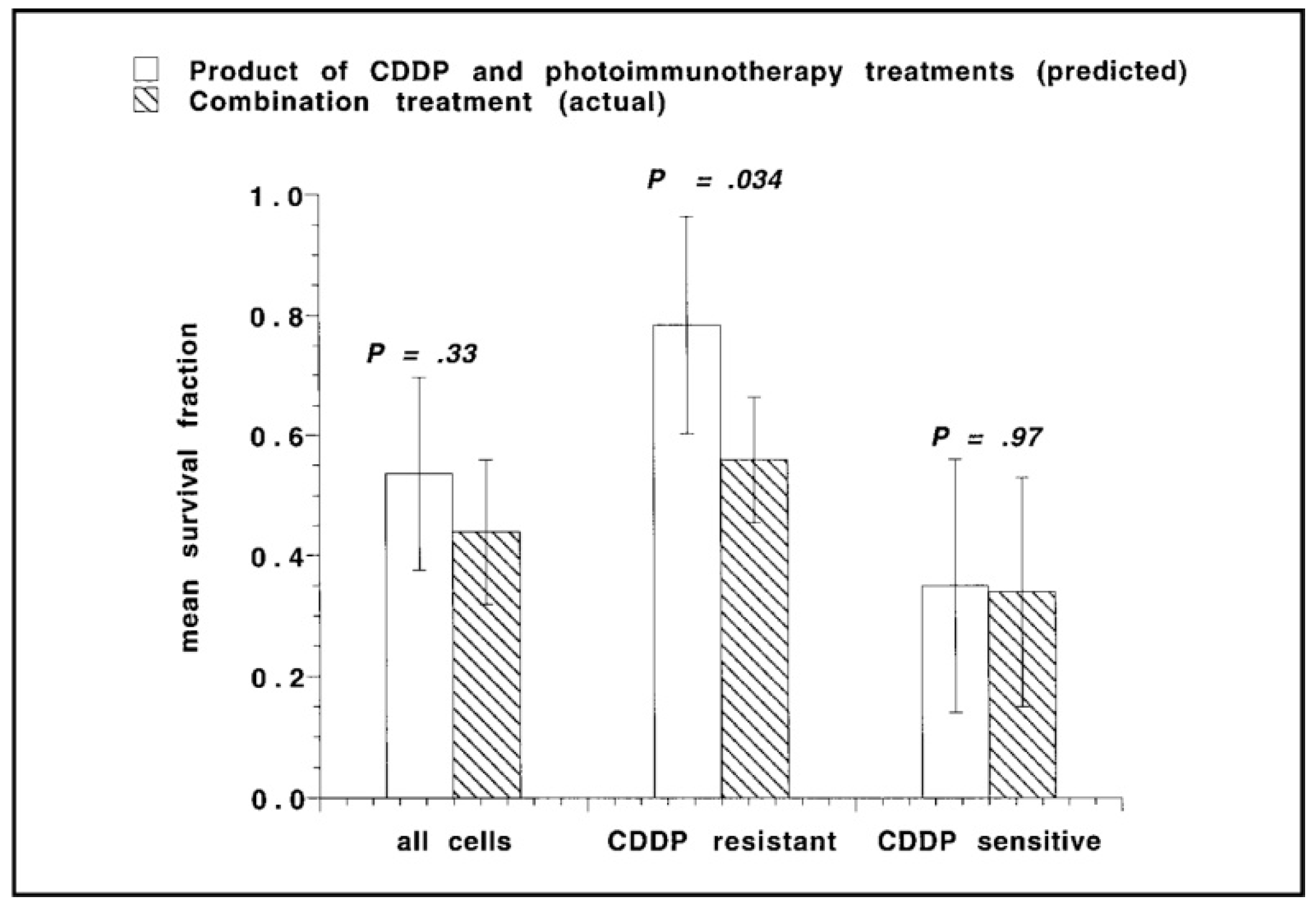
7. PIC-Based Combination Therapies
8. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BPD: | benzoporphyrin derivative |
| EGFR: | epidermal growth factor receptor |
| EOC: | epithelial ovarian cancer |
| ER: | endoplasmic reticulum |
| FDA: | Food and Drug Administration |
| FITC: | fluorescein isothiocyanate |
| GC: | Golgi complex |
| i.p.: | intraperitoneal |
| MAB: | monoclonal antibody |
| MDR: | multi-drug resistance |
| OvCa: | ovarian cancer |
| PCI: | photochemical internalization |
| PDT: | photodynamic therapy |
| PI3K: | phosphatidylinositol-3-kinase |
| PIC: | photoimmunoconjugate |
| PIT: | photoimmunotherapy |
| PF: | photofrin |
| PK: | pharmacokinetics |
| PS: | photosensitizers |
| VEGF: | vascular endothelial growth factor |
References
- Christie, E.; Bowtell, D. Acquired chemotherapy resistance in ovarian cancer. Ann. Oncol. 2017, 28, viii13–viii15. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.L.; Brenton, J.D. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol. 2011, 12, 1169–1174. [Google Scholar] [CrossRef]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, M.; Stasenko, M.; Uppal, S.; Erba, J.; Reynolds, R.K.; McLean, K. Comparison of first-line chemotherapy regimens for ovarian carcinosarcoma: A single institution case series and review of the literature. BMC Cancer 2018, 18, 172. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573. [Google Scholar] [CrossRef]
- Yap, T.A.; Carden, C.P.; Kaye, S.B. Beyond chemotherapy: Targeted therapies in ovarian cancer. Nat. Rev. Cancer 2009, 9, 167. [Google Scholar] [CrossRef]
- Hendren, S.K.; Hahn, S.M.; Spitz, F.R.; Bauer, T.W.; Rubin, S.C.; Zhu, T.; Glatstein, E.; Fraker, D.L. Phase II trial of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Ann. Surg. Oncol. 2001, 8, 65–71. [Google Scholar] [CrossRef]
- Sindelar, W.F.; DeLaney, T.F.; Tochner, Z.; Thomas, G.F.; Dachoswki, L.J.; Smith, P.D.; Friauf, W.S.; Cole, J.W.; Glatstein, E. Technique of photodynamic therapy for disseminated intraperitoneal malignant neoplasms: Phase I study. Arch. Surg. 1991, 126, 318–324. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Delaney, T.F.; Sindelar, W.F.; Tochner, Z.; Smith, P.D.; Friauf, W.S.; Thomas, G.; Dachowski, L.; Cole, J.W.; Steinberg, S.M.; Glatstein, E. Phase I study of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 445–457. [Google Scholar] [CrossRef]
- Wierrani, F.; Fiedler, D.; Grin, W.; Henry, M.; Dienes, E.; Gharehbaghi, K.; Krammer, B.; Grünberger, W. Clinical effect of meso-tetrahydroxyphenylchlorine based photodynamic therapy in recurrent carcinoma of the ovary: Preliminary results. BJOG Int. J. Obstet. Gynaecol. 1997, 104, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Tochner, Z.; Mitchell, J.; Hoekstra, H.; Smith, P.; DeLuca, A.; Barnes, M.; Harrington, F.; Manyak, M.; Russo, D.; Russo, A. Photodynamic therapy of the canine peritoneum: Normal tissue response to intraperitoneal and intravenous photofrin followed by 630 nm light. Lasers Surg. Med. 1991, 11, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Goff, B.; Hermanto, U.; Rumbaugh, J.; Blake, J.; Bamberg, M.; Hasan, T. Photoimmunotherapy and biodistribution with an OC125-chlorin immunoconjugate in an in vivo murine ovarian cancer model. Br. J. Cancer 1994, 70, 474. [Google Scholar] [CrossRef]
- Duska, L.R.; Hamblin, M.R.; Miller, J.L.; Hasan, T. Combination photoimmunotherapy and cisplatin: Effects on human ovarian cancer ex vivo. J. Natl. Cancer Inst. 1999, 91, 1557–1563. [Google Scholar] [CrossRef]
- Molpus, K.L.; Hamblin, M.R.; Rizvi, I.; Hasan, T. Intraperitoneal Photoimmunotherapy of Ovarian Carcinoma Xenografts in Nude Mice Using Charged Photoimmunoconjugates. Gynecol. Oncol. 2000, 76, 397–404. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Miller, J.L.; Hasan, T. Effect of charge on the interaction of site-specific photoimmunoconjugates with human ovarian cancer cells. Cancer Res. 1996, 56, 5205–5210. [Google Scholar]
- Tochner, Z.A.; Hahn, S.; Glatstein, E. Photoimmunotherapy and ovarian cancer: An improbable fiction or a palpable hit? J. Natl. Cancer Inst. 1999, 91, 1526–1527. [Google Scholar] [CrossRef]
- Duska, L.; Hamblin, M.; Bamberg, M.; Hasan, T. Biodistribution of charged F (ab’) 2 photoimmunoconjugates in a xenograft model of ovarian cancer. Br. J. Cancer 1997, 75, 837. [Google Scholar] [CrossRef]
- Goff, B.A.; Bamberg, M.; Hasan, T. Photoimmunotherapy of human ovarian carcinoma cells ex vivo. Cancer Res. 1991, 51, 4762–4767. [Google Scholar]
- DeWitt, J.M.; Sandrasegaran, K.; O’Neil, B.; House, M.G.; Zyromski, N.J.; Sehdev, A.; Perkins, S.M.; Flynn, J.; McCranor, L.; Shahda, S. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest. Endosc. 2019, 89, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.; Hasan, T.; Pogue, B. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Wagner, U.; Oehr, P.; Krebs, D. Clinical use of photodynamic therapy in gynecologic tumor patients—Antibody-targeted photodynamic laser therapy as a new oncologic treatment procedure. Zent. Fur Gynakol. 1992, 114, 307–311. [Google Scholar]
- Schmidt, S.; Wagner, U.; Schultes, B.; Oehr, P.; Decleer, W.; Ertmer, W.; Lubaschowski, H.; Biersack, H.J.; Krebs, D. [Photodynamic laser therapy with antibody-bound dyes. A new procedure in therapy of gynecologic malignancies]. Fortschr. Der Med. 1992, 110, 298–301. [Google Scholar]
- Löning, M.; Diddens, H.; Küpker, W.; Diedrich, K.; Hüttmann, G. Laparoscopic fluorescence detection of ovarian carcinoma metastases using 5-aminolevulinic acid-induced protoporphyrin IX. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2004, 100, 1650–1656. [Google Scholar]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging fundamentals of photochemically-induced Immunogenic Cell Death to the Enrichment of T Cell Repertoire. Photochem. Photobiol. 2019. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef]
- Obaid, G.; Broekgaarden, M.; Bulin, A.-L.; Huang, H.-C.; Kuriakose, J.; Liu, J.; Hasan, T. Photonanomedicine: A convergence of photodynamic therapy and nanotechnology. Nanoscale 2016, 8, 12471–12503. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three—Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N.L. Cell death pathways associated with photodynamic therapy: An update. Photochem. Photobiol. 2018, 94, 213–218. [Google Scholar] [CrossRef]
- Kessel, D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Autophagic death probed by photodynamic therapy. Autophagy 2015, 11, 1941–1943. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers (Basel) 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Luo, Y.; Deng, Y.; Chang, C. The role of subcellular localization in initiation of apoptosis by photodynamic therapy. Photochem. Photobiol. 1997, 65, 422–426. [Google Scholar] [CrossRef]
- Wyld, L.; Tomlinson, M.; Reed, M.; Brown, N. Aminolaevulinic acid-induced photodynamic therapy: Cellular responses to glucose starvation. Br. J. Cancer 2002, 86, 1343. [Google Scholar] [CrossRef]
- Inai, M.; Honda, N.; Hazama, H.; Akter, S.; Fuse, S.; Nakamura, H.; Nishikawa, T.; Kaneda, Y.; Awazu, K. Photodynamic therapy using a cytotoxic photosensitizer porphyrus envelope that targets the cell membrane. Photodiagnosis Photodyn. Ther. 2017, 20, 238–245. [Google Scholar] [CrossRef]
- Ahn, W.; Bae, S.; Huh, S.; Lee, J.; Namkoong, S.; Han, S.-J.; Kim, C.; Kim, J.-K.; Kim, Y.-W. Necrosis-like death with plasma membrane damage against cervical cancer cells by photodynamic therapy. Int. J. Gynecol. Cancer 2004, 14, 475–482. [Google Scholar] [CrossRef]
- Cristóbal, J.; Stockert, J.C.; Villanueva, A.; Rello-Varona, S.; Juarranz, A.; Cañete, M. Caspase-2: a possible trigger of apoptosis induced in A-549 tumor cells by ZnPc photodynamic treatment. Int. J. Oncol. 2006, 28, 1057–1063. [Google Scholar] [CrossRef] [Green Version]
- Gomes-da-Silva, L.C.; Zhao, L.; Bezu, L.; Zhou, H.; Sauvat, A.; Liu, P.; Durand, S.; Leduc, M.; Souquere, S.; Loos, F. Photodynamic therapy with redaporfin targets the endoplasmic reticulum and Golgi apparatus. Embo J. 2018, 37, e98354. [Google Scholar] [CrossRef]
- Ball, D.J.; Luo, Y.; Kessel, D.; Griffiths, J.; Brown, S.B.; Vernon, D.I. The induction of apoptosis by a positively charged methylene blue derivative. J. Photochem. Photobiol. B: Biol. 1998, 42, 159–163. [Google Scholar] [CrossRef]
- Celli, J.P.; Solban, N.; Liang, A.; Pereira, S.P.; Hasan, T. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers Surg. Med. 2011, 43, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-R.C.; Luo, Y.; Li, G.; Kessel, D. Enhanced apoptotic response to photodynamic therapy after bcl-2 transfection. Cancer Res. 1999, 59, 3429–3432. [Google Scholar] [PubMed]
- Gibson, S.L.; Al-Shawi, M.K.; Senior, A.E.; Hilf, R. Inhibition of the ATPase activity of P-glycoprotein by porphyrin photosensitization of multidrug-resistant cells in vitro. Photochem. Photobiol. 1995, 61, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Luo, Y.; Mathieu, P.; Reiners Jr, J.J. Determinants of the apoptotic response to lysosomal photodamage. Photochem. Photobiol. 2000, 71, 196–200. [Google Scholar] [CrossRef]
- Berg, K.; Moan, J. Lysosomes and microtubules as targets for photochemotherapy of cancer. Photochem. Photobiol. 1997, 65, 403–409. [Google Scholar] [CrossRef]
- Wood, S.R.; Holroyd, J.A.; Brown, S.B. The subcellular localization of Zn (ll) phthalocyanines and their redistribution on exposure to light. Photochem. Photobiol. 1997, 65, 397–402. [Google Scholar] [CrossRef]
- Rizvi, I.; Obaid, G.; Bano, S.; Hasan, T.; Kessel, D. Photodynamic therapy: Promoting in vitro efficacy of photodynamic therapy by liposomal formulations of a photosensitizing agent. Lasers Surg. Med. 2018. [Google Scholar] [CrossRef]
- Acedo, P.; Stockert, J.; Cañete, M.; Villanueva, A. Two combined photosensitizers: A goal for more effective photodynamic therapy of cancer. Cell Death Dis. 2014, 5, e1122. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, I.; Nath, S.; Obaid, G.; Ruhi, M.K.; Moore, K.; Bano, S.; Kessel, D.; Hasan, T. A Combination of Visudyne and a Lipid-anchored Liposomal Formulation of Benzoporphyrin Derivative Enhances Photodynamic Therapy Efficacy in a 3D Model for Ovarian Cancer. Photochem. Photobiol. 2019, 95, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.; Moore, K. Photodynamic Therapy in a 3D Model of Ovarian Cancer. Bio-Protocl 2019, 9, e3314. [Google Scholar] [CrossRef]
- Sato, K.; Hanaoka, H.; Watanabe, R.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy in the treatment of disseminated peritoneal ovarian cancer. Mol. Cancer Ther. 2015, 14, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Donovan, J.M. Release of prostaglandin E2 from cells by photodynamic treatment in vitro. Cancer Res. 1989, 49, 6896–6900. [Google Scholar] [PubMed]
- Moan, J.; Christensen, T. Photodynamic effects on human cells exposed to light in the presence of hematoporphyrin. Localization of the active dye. Cancer Lett. 1981, 11, 209–214. [Google Scholar] [CrossRef]
- Snyder, J.W.; Greco, W.R.; Bellnier, D.A.; Vaughan, L.; Henderson, B.W. Photodynamic therapy: A means to enhanced drug delivery to tumors. Cancer Res 2003, 63, 8126–8131. [Google Scholar]
- Kishimoto, S.; Bernardo, M.; Saito, K.; Koyasu, S.; Mitchell, J.B.; Choyke, P.L.; Krishna, M.C. Evaluation of oxygen dependence on in vitro and in vivo cytotoxicity of photoimmunotherapy using IR-700–antibody conjugates. Free Radic. Biol. Med. 2015, 85, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [Green Version]
- Vrouenraets, M.B.; Visser, G.W.; Stewart, F.A.; Stigter, M.; Oppelaar, H.; Postmus, P.E.; Snow, G.B.; van Dongen, G.A. Development of meta-tetrahydroxyphenylchlorin-monoclonal antibody conjugates for photoimmunotherapy. Cancer Res. 1999, 59, 1505–1513. [Google Scholar]
- GoMER, C.J.; LUNA, M.; FERRARIO, A.; RUCKER, N. Increased transcription and translation of heme oxygenase in Chinese hamster fibroblasts following photodynamic stress or Photofrin II incubation. Photochem. Photobiol. 1991, 53, 275–279. [Google Scholar] [CrossRef]
- Luna, M.C.; Wong, S.; Gomer, C.J. Photodynamic therapy mediated induction of early response genes. Cancer Res. 1994, 54, 1374–1380. [Google Scholar]
- Tao, J.-s.; Sanghera, J.S.; Pelech, S.L.; Wong, G.; Levy, J.G. Stimulation of stress-activated protein kinase and p38 HOG1 kinase in murine keratinocytes following photodynamic therapy with benzoporphyrin derivative. J. Biol. Chem. 1996, 271, 27107–27115. [Google Scholar] [CrossRef] [Green Version]
- Gomer, C.J.; Ryter, S.W.; Ferrario, A.; Rucker, N.; Wong, S.; Fisher, A.M. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996, 56, 2355–2360. [Google Scholar] [PubMed]
- Verrico, A.; Moore, J.V. Expression of the collagen-related heat shock protein HSP47 in fibroblasts treated with hyperthermia or photodynamic therapy. Br. J. Cancer 1997, 76, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gollnick, S.O.; Liu, X.; Owczarczak, B.; Musser, D.A.; Henderson, B.W. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997, 57, 3904–3909. [Google Scholar] [PubMed]
- Kick, G.; Messer, G.; Goetz, A.; Plewig, G.; Kind, P. Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-κB DNA binding. Cancer Res. 1995, 55, 2373–2379. [Google Scholar]
- Herman, S.; Kalechman, Y.; Gafter, U.; Sredni, B.; Malik, Z. Photofrin II induces cytokine secretion by mouse spleen cells and human peripheral mononuclear cells. Immunopharmacology 1996, 31, 195–204. [Google Scholar] [CrossRef]
- Al-Laith, M.; Matthews, E.K.; Cui, Z.J. Photodynamic drug action on isolated rat pancreatic acini: Mobilization of arachidonic acid and prostaglandin production. Biochem. Pharmacol. 1993, 46, 567–573. [Google Scholar] [CrossRef]
- Bachowski, G.J.; Korytowski, W.; Girotti, A.W. Characterization of lipid hydroperoxides generated by photodynamic treatment of leukemia cells. Lipids 1994, 29, 449–459. [Google Scholar] [CrossRef]
- Andrejevic-Blant, S.; Hadjur, C.; Ballini, J.; Wagnieres, G.; Fontolliet, C.; Van den Bergh, H.; Monnier, P. Photodynamic therapy of early squamous cell carcinoma with tetra (m-hydroxyphenyl) chlorin: Optimal drug-light interval. Br. J. Cancer 1997, 76, 1021. [Google Scholar] [CrossRef] [Green Version]
- Henderson, B.W.; Waldow, S.M.; Mang, T.S.; Potter, W.R.; Malone, P.B.; Dougherty, T.J. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985, 45, 572–576. [Google Scholar]
- Zaidi, S.I.; Oleinick, N.L.; Zaim, M.T.; Mukhtar, H. Apoptosis during photodynamic therapy-induced ablation of RIF-1 tumors in C3H mice: Electron microscopic, histopathologic and biochemical evidence. Photochem. Photobiol. 1993, 58, 771–776. [Google Scholar] [CrossRef]
- Fingar, V.H.; Wieman, T.J.; Doak, K.W. Role of thromboxane and prostacyclin release on photodynamic therapy-induced tumor destruction. Cancer Res. 1990, 50, 2599–2603. [Google Scholar] [PubMed]
- Winsborrow, B.G.; Grondey, H.; Savoie, H.; Fyfe, C.A.; Dolphin, D. Magnetic resonance imaging evaluation of photodynamic therapy-induced hemorrhagic necrosis in the murine M1 tumor model. Photochem. Photobiol. 1997, 66, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M. Induction of tumor immunity by photodynamic therapy. J. Clin. Laser Med. Surg. 1996, 14, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Maeding, N.; Verwanger, T.; Krammer, B. Boosting tumor-specific immunity using PDT. Cancers 2016, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Kercher, E.M.; Nath, S.; Rizvi, I.; Spring, B.Q. Cancer Cell-targeted and Activatable Photoimmunotherapy Spares T Cells in a 3D Co-culture Model. Photochem. Photobiol. 2019. [Google Scholar] [CrossRef]
- Gollnick, S.O.; Vaughan, L.; Henderson, B.W. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res 2002, 62, 1604–1608. [Google Scholar]
- Macatonia, S.E.; Hosken, N.A.; Litton, M.; Vieira, P.; Hsieh, C.-S.; Culpepper, J.A.; Wysocka, M.; Trinchieri, G.; Murphy, K.M.; O’Garra, A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995, 154, 5071–5079. [Google Scholar]
- Wang, D.; Wang, T.; Yu, H.; Feng, B.; Zhou, L.; Zhou, F.; Hou, B.; Zhang, H.; Luo, M.; Li, Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 2019, 4, eaau6584. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core–Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hady, E.-S.; Martin-Hirsch, P.; Duggan-Keen, M.; Stern, P.L.; Moore, J.V.; Corbitt, G.; Kitchener, H.C.; Hampson, I.N. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001, 61, 192–196. [Google Scholar] [PubMed]
- Winters, U.; Daayana, S.; Lear, J.T.; Tomlinson, A.E.; Elkord, E.; Stern, P.L.; Kitchener, H.C. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin. Cancer Res. 2008, 14, 5292–5299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Carmen, M.G.; Rizvi, I.; Chang, Y.; Moor, A.C.; Oliva, E.; Sherwood, M.; Pogue, B.; Hasan, T. Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. J Natl Cancer Inst 2005, 97, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Spring, B.Q.; Bano, S.; Hasan, T. Activatable clinical fluorophore-quencher antibody pairs as dual molecular probes for the enhanced specificity of image-guided surgery. J. Biomed. Opt. 2017, 22, 121607. [Google Scholar] [CrossRef] [PubMed]
- Urano, Y.; Asanuma, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 2009, 15, 104. [Google Scholar] [CrossRef] [Green Version]
- Spring, B.Q.; Abu-Yousif, A.O.; Palanisami, A.; Rizvi, I.; Zheng, X.; Mai, Z.; Anbil, S.; Sears, R.B.; Mensah, L.B.; Goldschmidt, R.; et al. Selective treatment and monitoring of disseminated cancer micrometastases in vivo using dual-function, activatable immunoconjugates. Proc. Natl. Acad. Sci. USA 2014, 111, E933–E942. [Google Scholar] [CrossRef] [Green Version]
- Patterson, M.S.; Wilson, B.C.; Graff, R. In vivo tests of the concept of photodynamic threshold dose in normal rat liver photosensitized by aluminum chlorosulphonated phthalocyanine. Photochem. Photobiol. 1990, 51, 343–349. [Google Scholar] [CrossRef]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef]
- Folli, S.; Westermann, P.; Braichotte, D.; Pèlegrin, A.; Wagnières, G.; van den Bergh, H.; Mach, J.-P. Antibody-indocyanin conjugates for immunophotodetection of human squamous cell carcinoma in nude mice. Cancer Res. 1994, 54, 2643–2649. [Google Scholar]
- Rosenthal, E.L.; Warram, J.M.; De Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G. Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.A.; Choyke, P.L.; Kobayashi, H. Fluorescence-guided surgery. Front. Oncol. 2017, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- COGLIATI, T.; BRUSA, P.; CANEVARI, S.; CALDERA, M. Preparation and Biological Characterization of Conjugates Consisting of Ricin and a Tumor-Specific Non-Internalizing. Anticancer Res. 1991, 11, 417–422. [Google Scholar]
- Ozzello, L.; De Rosa, C.; Blank, E.; Cantell, K.; Ceriani, R.; Habif, D. The use of natural interferon alpha conjugated to a monoclonal antibody anti mammary epithelial mucin (Mc5) for the treatment of human breast cancer xenografts. Breast Cancer Res. Treat. 1993, 25, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Soloway, A.H.; Barth, R.F.; Mafune, N.; Adams, D.M.; Knoth, W.H. Boron neutron capture therapy: Linkage of a boronated macromolecule to monoclonal antibodies directed against tumor-associated antigens. J. Med. Chem. 1989, 32, 2326–2330. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Adams, D.M.; Soloway, A.H.; Alam, F.; Darby, M.V. Boronated starburst dendrimer-monoclonal antibody immunoconjugates: Evaluation as a potential delivery system for neutron capture therapy. Bioconj. Chem. 1994, 5, 58–66. [Google Scholar] [CrossRef]
- Oseroff, A.R.; Ohuoha, D.; Hasan, T.; Bommer, J.C.; Yarmush, M.L. Antibody-targeted photolysis: Selective photodestruction of human T-cell leukemia cells using monoclonal antibody-chlorin e6 conjugates. Proc. Natl. Acad. Sci. 1986, 83, 8744–8748. [Google Scholar] [CrossRef] [Green Version]
- Steele, J.K.; Liu, D.; Stammers, A.T.; Whitney, S.; Levy, J.G. Suppressor deletion therapy: Selective elimination of T suppressor cells in vivo using a hematoporphyrin conjugated monoclonal antibody permits animals to reject syngeneic tumor cells. Cancer Immunol. Immunother. 1988, 26, 125–131. [Google Scholar] [CrossRef]
- Mew, D.; Lum, V.; Wat, C.; Towers, G.; Sun, C.C.; Walter, R.; Wright, W.; Berns, M.; Levy, J. Ability of specific monoclonal antibodies and conventional antisera conjugated to hematoporphyrin to label and kill selected cell lines subsequent to light activation. Cancer Res. 1985, 45, 4380–4386. [Google Scholar]
- Goff, B.A.; Bachor, R.; Kollias, N.; Hasan, T. Effects of photodynamic therapy with topical application of 5-aminolevulinic acid on normal skin of hairless guinea pigs. J. Photochem. Photobiol. B Biol. 1992, 15, 239–251. [Google Scholar] [CrossRef]
- Hasan, T.; Lin, C.; Lin, A. Laser-induced selective cytotoxicity using monoclonal antibody-chromophore conjugates. Prog. Clin. Biol. Res. 1989, 288, 471. [Google Scholar]
- Hasan, T.; Lin, A.; Yarmush, D.; Oseroff, A.; Yarmush, M. Monoclonal antibody-chromophore conjugates as selective phototoxins. J. Control. Release 1989, 10, 107–117. [Google Scholar] [CrossRef]
- Hasan, T.; Sherwood, M.; Anderson, T.; Bamberg, M.; Flotte, T.J.; Zurawski, V.R. Cellular response of ovarian carcinoma cells to antibody-photosensitizer-mediated injury. In Proceedings of the Photodynamic Therapy: Mechanisms II; pp. 136–144. Available online: https://spie.org/Publications/Proceedings/Volume/1203?SSO=1 (accessed on 26 November 2019).
- Jiang, F.N.; Liu, D.J.; Neyndorff, H.; Chester, M.; Jiang, S.-y.; Levy, J.G. Photodynamic killing of human squamous cell carcinoma cells using a monoclonal antibody-photosensitizer conjugate. JNCI J. Natl. Cancer Inst. 1991, 83, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Krinick, N.; Sun, Y.; Joyner, D.; Spikes, J.; Straight, R.; Kopeček, J. A polymeric drug delivery system for the simultaneous delivery of drugs activatable by enzymes and/or light. J. Biomater. Sci. Polym. Ed. 1994, 5, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-M.; Fischman, A.; Stevens, E.; Lee, T.; Strong, L.; Tompkins, R.; Yarmush, M. Sn-chlorin e6 antibacterial immunoconjugates. An in vitro and in vivo analysis. J. Immunol. Methods 1992, 156, 85–99. [Google Scholar]
- Morgan, J.; Gray, A.; Huehns, E. Specific targeting and toxicity of sulphonated aluminium phthalocyanine photosensitised liposomes directed to cells by monoclonal antibody in vitro. Br. J. Cancer 1989, 59, 366. [Google Scholar] [CrossRef] [Green Version]
- Sandland, J.; Boyle, R.W. Photosensitizer Antibody–Drug Conjugates: Past, Present, and Future. Bioconj. Chem. 2019, 30, 975–993. [Google Scholar] [CrossRef]
- Wang, S.; Hüttmann, G.M.; Rudnitzki, F.; Diddens-Tschoeke, H.; Zhang, Z.; Rahmanzadeh, R. Indocyanine green as effective antibody conjugate for intracellular molecular targeted photodynamic therapy. J. Biomed. Opt. 2016, 21, 078001. [Google Scholar] [CrossRef]
- Goff, B.A.; Blake, J.; Bamberg, M.P.; Hasan, T. Treatment of ovarian cancer with photodynamic therapy and immunoconjugates in a murine ovarian cancer model. Br. J. Cancer 1996, 74, 1194. [Google Scholar] [CrossRef] [Green Version]
- Pogrebniak, H.; Matthews, W.; Black, C.; Russo, A.; Mitchell, J.; Smith, P.; Roth, J.; Pass, H. Targetted phototherapy with sensitizer-monoclonal antibody conjugate and light. Surg. Oncol. 1993, 2, 31–42. [Google Scholar] [CrossRef]
- Del Governatore, M.; Hamblin, M.R.; Shea, C.R.; Rizvi, I.; Molpus, K.G.; Tanabe, K.K.; Hasan, T. Experimental photoimmunotherapy of hepatic metastases of colorectal cancer with a 17.1 A chlorine6 immunoconjugate. Cancer Res. 2000, 60, 4200–4205. [Google Scholar]
- Pogue, B.W.; Elliott, J.T.; Kanick, S.C.; Davis, S.C.; Samkoe, K.S.; Maytin, E.V.; Pereira, S.P.; Hasan, T. Revisiting photodynamic therapy dosimetry: Reductionist & surrogate approaches to facilitate clinical success. Phys. Med. Biol. 2016, 61, R57. [Google Scholar] [PubMed] [Green Version]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315. [Google Scholar] [CrossRef]
- Hamblin, M.; Miller, J.; Rizvi, I.; Loew, H.; Hasan, T. Pegylation of charged polymer-photosensitiser conjugates: Effects on photodynamic efficacy. Br. J. Cancer 2003, 89, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hongrapipat, J.; Kopecková, P.; Liu, J.; Prakongpan, S.; Kopecek, J. Combination chemotherapy and photodynamic therapy with Fab′ fragment targeted HPMA copolymer conjugates in human ovarian carcinoma cells. Mol. Pharm. 2008, 5, 696–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omelyanenko, V.; Gentry, C.; Kopečková, P.; Kopeček, J. HPMA copolymer–anticancer drug–OV-TL16 antibody conjugates. II. Processing in epithelial ovarian carcinoma cells in vitro. Int. J. Cancer 1998, 75, 600–608. [Google Scholar] [CrossRef]
- Omelyanenko, V.; Kopečková, P.; Gentry, C.; Shiah, J.-G.; Kopeček, J. HPMA copolymer-anticancer drug-OV-TL16 antibody conjugates. 1. Influence of the method of synthesis on the binding affinity to OVCAR-3 ovarian carcinoma cells in vitro. J. Drug Target. 1996, 3, 357–373. [Google Scholar] [CrossRef]
- Shiah, J.-G.; Sun, Y.; Kopečková, P.; Peterson, C.; Straight, R.; Kopeček, J. Combination chemotherapy and photodynamic therapy of targetable N-(2-hydroxypropyl) methacrylamide copolymer–doxorubicin/mesochlorin e6-OV-TL 16 antibody immunoconjugates. J. Control. Release 2001, 74, 249–253. [Google Scholar] [CrossRef]
- Lu, J.M.; Peterson, C.; Guo-Shiah, J.; Gu, Z.; Peterson, C.A.; Straight, R.C.; Kopecek, J. Cooperativity between free and N-(2-hydroxypropyl) methacrylamide copolymer bound adriamycin and meso-chlorin e6 monoethylene diamine induced photodynamic therapy in human epithelial ovarian carcinoma in vitro. Int. J. Oncol. 1999, 15, 5–21. [Google Scholar] [CrossRef]
- Lu, Z.-R.; Shiah, J.-G.; Kopečková, P.; Kopeček, J. Preparation and biological evaluation of polymerizable antibody Fab′ fragment targeted polymeric drug delivery system. J. Control. Release 2001, 74, 263–268. [Google Scholar] [CrossRef]
- Fowers, K.D.; Kopeček, J. Targeting of Multidrug--Resistant Human Ovarian Carcinoma Cells With Anti-P-Glycoprotein Antibody Conjugates. Macromol. Biosci. 2012, 12, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Berguig, G.Y.; Convertine, A.J.; Shi, J.; Palanca-Wessels, M.C.; Duvall, C.L.; Pun, S.H.; Press, O.W.; Stayton, P.S. Intracellular delivery and trafficking dynamics of a lymphoma-targeting antibody–polymer conjugate. Mol. Pharm. 2012, 9, 3506–3514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Hüttmann, G.; Zhang, Z.; Vogel, A.; Birngruber, R.; Tangutoori, S.; Hasan, T.; Rahmanzadeh, R. Light-controlled delivery of monoclonal antibodies for targeted photoinactivation of Ki-67. Mol. Pharm. 2015, 12, 3272–3281. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Pigula, M.; Fang, Y.; Hasan, T. Immobilization of Photo—Immunoconjugates on Nanoparticles Leads to Enhanced Light—Activated Biological Effects. Small 2018, 14, 1800236. [Google Scholar] [CrossRef]
- Soukos, N.S.; Hamblin, M.R.; Hasan, T. The Effect of Charge on Cellular Uptake and Phototoxicity of Polylysine Chlorine6Conjugates. Photochem. Photobiol. 1997, 65, 723–729. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Kang, Y.S.; Yang, J.; Buciak, J.L. Enhanced cellular uptake and in vivo biodistribution of a monoclonal antibody following cationization. J. Pharm. Sci. 1995, 84, 943–948. [Google Scholar] [CrossRef]
- Savellano, M.D.; Pogue, B.W.; Hoopes, P.J.; Vitetta, E.S.; Paulsen, K.D. Multiepitope HER2 targeting enhances photoimmunotherapy of HER2-overexpressing cancer cells with pyropheophorbide-a immunoconjugates. Cancer Res. 2005, 65, 6371–6379. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.S.; Liu, Z.; Bartlett, D.L. Oncolytic Immunotherapy: Dying the Right Way is a Key to Eliciting Potent Antitumor Immunity. Front Oncol 2014, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Rahmanzadeh, R.; Rai, P.; Celli, J.P.; Rizvi, I.; Baron-Lühr, B.; Gerdes, J.; Hasan, T. Ki-67 as a molecular target for therapy in an in vitro three-dimensional model for ovarian cancer. Cancer Res. 2010, 70, 9234–9242. [Google Scholar] [CrossRef] [Green Version]
- Obaid, G.; Bano, S.; Mallidi, S.; Broekgaarden, M.; Kuriakose, J.; Silber, Z.; Bulin, A.L.; Wang, Y.; Mai, Z.; Jin, W.; et al. Impacting Pancreatic Cancer Therapy in Heterotypic in Vitro Organoids and in Vivo Tumors with Specificity-Tuned, NIR-Activable Photoimmunonanoconjugates: Towards Conquering Desmoplasia? Nano Lett. 2019, 19, 7573–7587. [Google Scholar] [CrossRef]
- Rizvi, I.; Dinh, T.A.; Yu, W.; Chang, Y.; Sherwood, M.E.; Hasan, T. Photoimmunotherapy and irradiance modulation reduce chemotherapy cycles and toxicity in a murine model for ovarian carcinomatosis: Perspective and results. Israel J. Chem. 2012, 52, 776–787. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Ogata, F.; Nagaya, T.; Nakamura, Y.; Sato, K.; Okuyama, S.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Near-infrared photoimmunotherapy: A comparison of light dosing schedules. Oncotarget 2017, 8, 35069. [Google Scholar] [CrossRef] [PubMed]
- Gillenwater, A.M.; Cognetti, D.; Johnson, J.M.; Curry, J.; Kochuparambil, S.T.; McDonald, D.; Fidler, M.J.; Stenson, K.; Vasan, N.; Razaq, M. RM-1929 photo-immunotherapy in patients with recurrent head and neck cancer: Results of a multicenter phase 2a open-label clinical trial; American Society of Clinical Oncology: Alexandria, VA, USA, 2018. [Google Scholar]
- Fan, Z.; Mendelsohn, J. Therapeutic application of anti-growth factor receptor antibodies. Curr. Opin. Oncol. 1998, 10, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, J. Use of an antibody to target geldanamycin; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Mendelsohn, J. Jeremiah Metzger Lecture. Targeted cancer therapy. Trans. Am. Clin. Climatol. Assoc. 2000, 111, 95. [Google Scholar] [PubMed]
- Mendelsohn, J. Blockade of receptors for growth factors: An anticancer therapy—the fourth annual Joseph, H. Burchenal American Association for Cancer Research Clinical Research Award Lecture. Clin. Cancer Res. 2000, 6, 747–753. [Google Scholar]
- Ciardiello, F.; Tortora, G. A novel approach in the treatment of cancer: Targeting the epidermal growth factor receptor. Clin. Cancer Res. 2001, 7, 2958–2970. [Google Scholar]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463. [Google Scholar] [CrossRef]
- Wells, A. Tumor invasion: Role of growth factor-induced cell motility. Adv. Cancer Res. 1999, 78, 31–101. [Google Scholar]
- Teplinsky, E.; Muggia, F. EGFR and HER2: Is there a role in ovarian cancer? Transl. Cancer Res 2015, 4. [Google Scholar]
- Gui, T.; Shen, K. The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiol. 2012, 36, 490–496. [Google Scholar] [CrossRef]
- Abu-Yousif, A.O.; Moor, A.C.; Zheng, X.; Savellano, M.D.; Yu, W.; Selbo, P.K.; Hasan, T. Epidermal growth factor receptor-targeted photosensitizer selectively inhibits EGFR signaling and induces targeted phototoxicity in ovarian cancer cells. Cancer Lett. 2012, 321, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. Targeting EGFR with photodynamic therapy in combination with Erbitux enhances in vivo bladder tumor response. Mol. Cancer 2009, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, N.; Kalka, K.; Mukhtar, H. In vitro and in vivo inhibition of epidermal growth factor receptor-tyrosine kinase pathway by photodynamic therapy. Oncogene 2001, 20, 2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagut, C.; Dalmases, A.; Bellosillo, B.; Crespo, M.; Pairet, S.; Iglesias, M.; Salido, M.; Gallen, M.; Marsters, S.; Tsai, S.P. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat. Med. 2012, 18, 221. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Jay, D.G. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc. Natl. Acad. Sci. 1988, 85, 5454–5458. [Google Scholar] [CrossRef] [Green Version]
- Rahmanzadeh, R.; Hüttmann, G.; Gerdes, J.; Scholzen, T. Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis. Cell Prolif. 2007, 40, 422–430. [Google Scholar] [CrossRef]
- Bullwinkel, J.; Baron-Lühr, B.; Lüdemann, A.; Wohlenberg, C.; Gerdes, J.; Scholzen, T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J. Cell. Physiol. 2006, 206, 624–635. [Google Scholar] [CrossRef]
- Debnath, J.; Mills, K.R.; Collins, N.L.; Reginato, M.J.; Muthuswamy, S.K.; Brugge, J.S. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 2002, 111, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Tangutoori, S.; Spring, B.Q.; Mai, Z.; Palanisami, A.; Mensah, L.B.; Hasan, T. Simultaneous delivery of cytotoxic and biologic therapeutics using nanophotoactivatable liposomes enhances treatment efficacy in a mouse model of pancreatic cancer. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Mu, H.; Wang, Y.; Chu, Y.; Jiang, Y.; Hua, H.; Chu, L.; Wang, K.; Wang, A.; Liu, W.; Li, Y. Multivesicular liposomes for sustained release of bevacizumab in treating laser-induced choroidal neovascularization. Drug Deliv. 2018, 25, 1372–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Yao, S.; Cao, Q.; Xia, M.; Liu, J.; He, M. The prognostic value of Ki67 in ovarian high-grade serous carcinoma: An 11-year cohort study of Chinese patients. Oncotarget 2017, 8, 107877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, B.H.; Gipson, I.K. Focus on molecules: Human mucin MUC16. Exp. Eye Res. 2008, 87, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45. [Google Scholar] [CrossRef]
- Konishi, I.; Fendrick, J.L.; Parmley, T.H.; Quirk JR, J.G.; O’Brien, T.J. Epidermal Growth Factor Enhances Secretion of the Ovarian Tumor-Associated Cancer Antigen CAI25 From the Human Amnion WISH Cell Line. J. Soc. Gynecol. Investig. 1994, 1, 89–96. [Google Scholar] [CrossRef]
- Thériault, C.; Pinard, M.; Comamala, M.; Migneault, M.; Beaudin, J.; Matte, I.; Boivin, M.; Piché, A.; Rancourt, C. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol. Oncol. 2011, 121, 434–443. [Google Scholar] [CrossRef]
- Bast, R.C.; Feeney, M.; Lazarus, H.; Nadler, L.; Colvin, R.; Knapp, R. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef] [Green Version]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13, 129. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-L.; Chang, M.-C.; Huang, C.-Y.; Chiang, Y.-C.; Lin, H.-W.; Chen, C.-A.; Hsieh, C.-Y.; Cheng, W.-F. Serous ovarian carcinoma patients with high alpha-folate receptor had reducing survival and cytotoxic chemo-response. Mol. Oncol. 2012, 6, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Azaïs, H.; Frochot, C.; Grabarz, A.; Khodja, S.B.; Colombeau, L.; Delhem, N.; Mordon, S.; Collinet, P. Specific folic-acid targeted photosensitizer. The first step toward intraperitoneal photodynamic therapy for epithelial ovarian cancer. Gynecol. Obstet. Fertil. Senol. 2017, 45, 190–196. [Google Scholar]
- Azais, H.; Queniat, G.; Bonner, C.; Kerdraon, O.; Tardivel, M.; Jetpisbayeva, G.; Frochot, C.; Betrouni, N.; Collinet, P.; Mordon, S. Fischer 344 rat: A preclinical model for epithelial ovarian cancer folate-targeted therapy. Int. J. Gynecol. Cancer 2015, 25, 1194–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azaïs, H.; Schmitt, C.; Tardivel, M.; Kerdraon, O.; Stallivieri, A.; Frochot, C.; Betrouni, N.; Collinet, P.; Mordon, S. Assessment of the specificity of a new folate-targeted photosensitizer for peritoneal metastasis of epithelial ovarian cancer to enable intraperitoneal photodynamic therapy. A preclinical study. Photodiagnosis Photodyn. Ther. 2016, 13, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.-C.; Mallidi, S.; Liu, J.; Chiang, C.-T.; Mai, Z.; Goldschmidt, R.; Ebrahim-Zadeh, N.; Rizvi, I.; Hasan, T. Photodynamic therapy synergizes with irinotecan to overcome compensatory mechanisms and improve treatment outcomes in pancreatic cancer. Cancer Res. 2016, 76, 1066–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.R.; Kim, S.; Choi, J.W.; Choi, S.Y.; Lee, S.-H.; Kim, H.; Hahn, S.K.; Koh, G.Y.; Yun, S.H. Bioluminescence-activated deep-tissue photodynamic therapy of cancer. Theranostics 2015, 5, 805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-ray induced photodynamic therapy: A combination of radiotherapy and photodynamic therapy. Theranostics 2016, 6, 2295. [Google Scholar] [CrossRef]
- Larue, L.; Mihoub, A.B.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using x-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 2013, 3, 317. [Google Scholar] [CrossRef] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, S.; Saad, M.A.; Pigula, M.; Swain, J.W.R.; Hasan, T. Photoimmunotherapy of Ovarian Cancer: A Unique Niche in the Management of Advanced Disease. Cancers 2019, 11, 1887. https://doi.org/10.3390/cancers11121887
Nath S, Saad MA, Pigula M, Swain JWR, Hasan T. Photoimmunotherapy of Ovarian Cancer: A Unique Niche in the Management of Advanced Disease. Cancers. 2019; 11(12):1887. https://doi.org/10.3390/cancers11121887
Chicago/Turabian StyleNath, Shubhankar, Mohammad Ahsan Saad, Michael Pigula, Joseph W.R. Swain, and Tayyaba Hasan. 2019. "Photoimmunotherapy of Ovarian Cancer: A Unique Niche in the Management of Advanced Disease" Cancers 11, no. 12: 1887. https://doi.org/10.3390/cancers11121887




