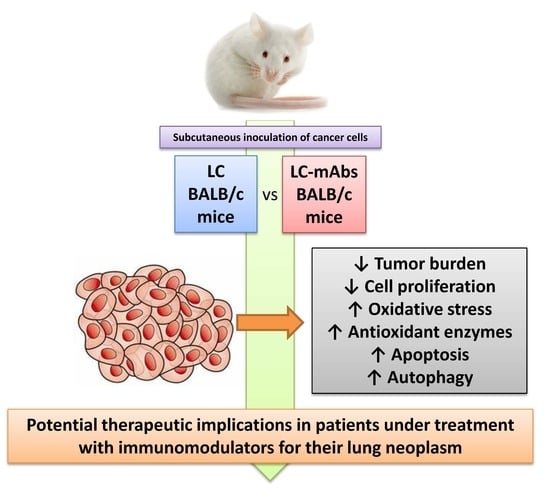
Immunotherapy with Monoclonal Antibodies in Lung Cancer of Mice: Oxidative Stress and Other Biological Events
Abstract
1. Introduction
2. Methods
2.1. Animal Experiments
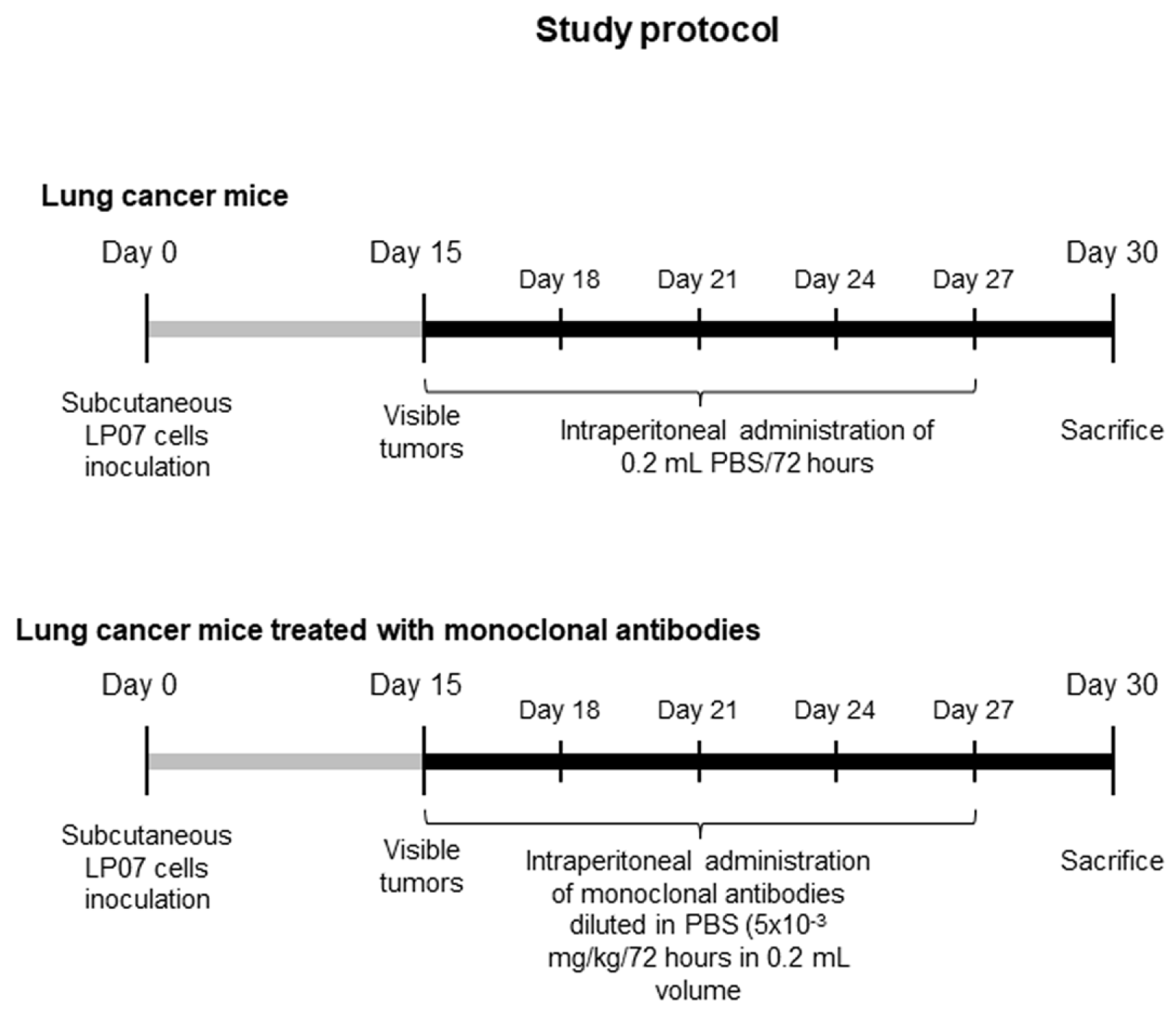
2.1.1. Experimental Design
2.1.2. In Vivo Measurements Conducted on the Animals
2.1.3. Sacrifice and Sample Collection
2.2. Molecular Biology Analyses
2.2.1. Histological Analyses of Tumor Samples
2.2.2. Immunoblotting
2.3. Statistical Analysis
3. Results
3.1. Monoclonal Antibodies Improved Tumor Burden and Body Weight in Mice
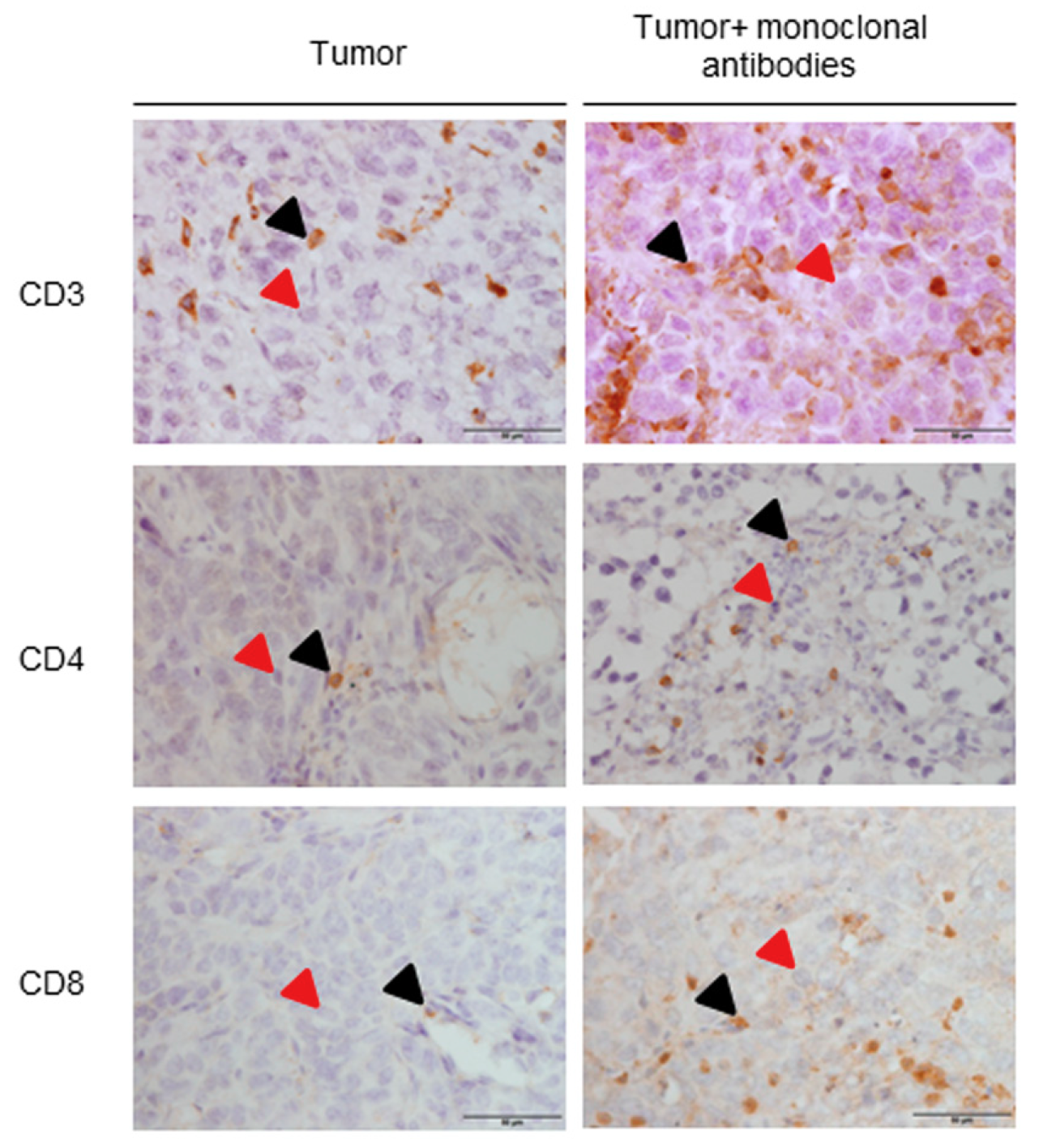
3.2. Immune Microenvironment in Response to the Immunomodulators
3.3. Tumor Oxidative Stress in Response to the Immunomodulators
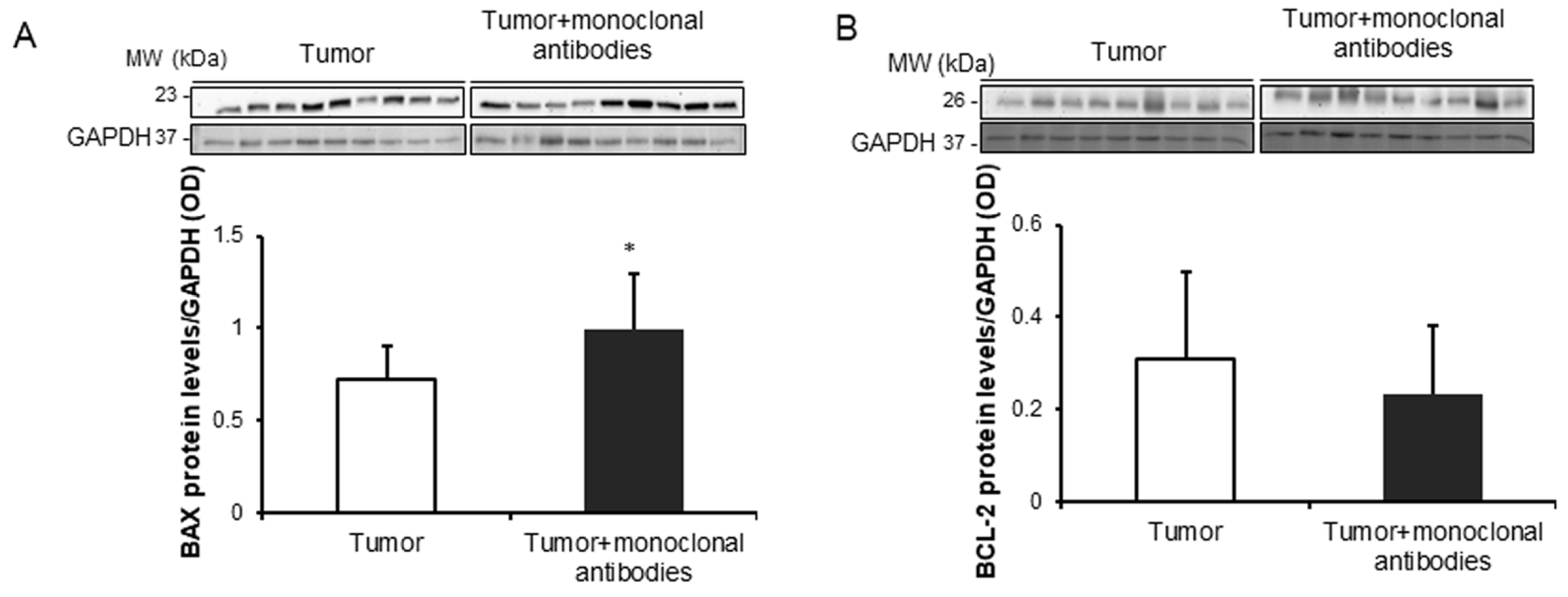
3.4. Tumor Apoptosis and Autophagy Markers in Response to Immunomodulators
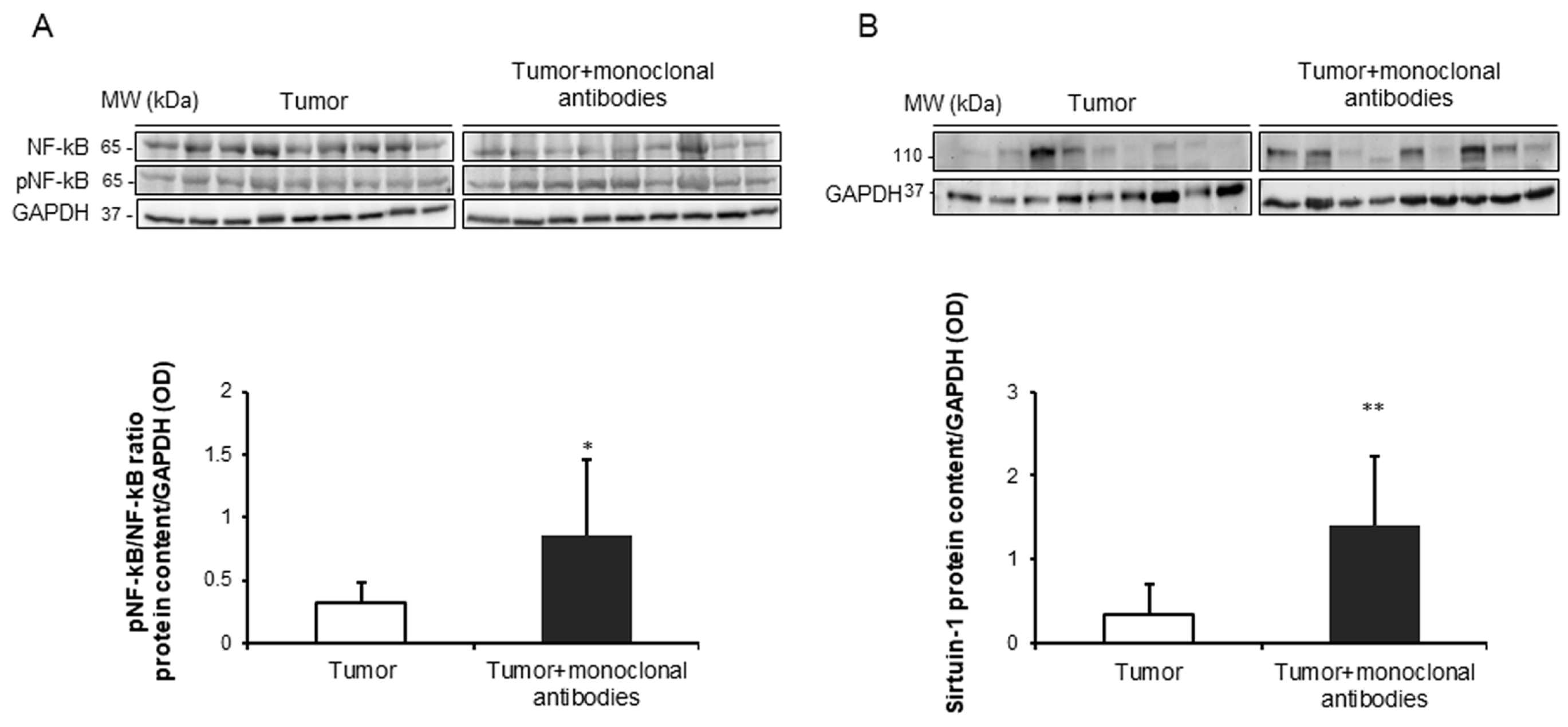
3.5. Effects of the Immunomodulators on Signaling Markers in Tumors
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.V.; Trueba, I.M.; Sanchis, J.B.; Lopez-Rodo, L.M.; Rodriguez Suarez, P.M.; de Cos Escuin, J.S.; Barreiro, E.; Henar Borrego, P.M.; Vicente, C.D.; Aldeyturriaga, J.F.; et al. Recommendations of the Spanish Society of Pneumology and Thoracic Surgery on the diagnosis and treatment of non-small-cell lung cancer. Arch. Bronconeumol. 2016, 52 (Suppl. S1), 2–62. [Google Scholar] [PubMed]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La, V.C.; Negri, E. European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann. Oncol. 2017, 28, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Soler-Cataluna, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Trigueros, J.A.; Cosio, B.G.; Casanova, C.; Antonio, R.J.; et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological Treatment of Stable Phase. Arch. Bronconeumol. 2017, 53, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch. Bronconeumol. 2017, 53, 128–149. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, F.V.; Trueba, I.M.; Sanchis, J.B.; López-Rodó, L.M.; Suárez, P.M.R.; de Cos Escuin, J.S.; Barreiro, E.; Pintado, M.H.B.; Vicente, C.D.; Aldeyturriaga, J.F.; et al. Executive summary of the SEPAR recommendations for the diagnosis and treatment of non-small cell lung cancer. Arch. Bronconeumol. 2016, 52, 378–388. [Google Scholar] [CrossRef]
- Conway, E.M.; Pikor, L.A.; Kung, S.H.; Hamilton, M.J.; Lam, S.; Lam, W.L.; Bennewith, K.L. Macrophages, Inflammation, and Lung Cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Jimenez, M.; Curull, V.; Pijuan, L.; Sanchez-Font, A.; Rivera-Ramos, H.; Rodriguez-Fuster, A.; Aguilo, R.; Gea, J.; Barreiro, E. Systemic and Tumor Th1 and Th2 Inflammatory Profile and Macrophages in Lung Cancer: Influence of Underlying Chronic Respiratory Disease. J. Thorac. Oncol. 2017, 12, 235–248. [Google Scholar] [CrossRef]
- O’Byrne, K.J.; Dalgleish, A.G. Chronic immune activation and inflammation as the cause of malignancy. Br. J. Cancer 2001, 85, 473–483. [Google Scholar] [CrossRef]
- O’Callaghan, D.S.; O’Donnell, D.; O’Connell, F.; O’Byrne, K.J. The role of inflammation in the pathogenesis of non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 2024–2036. [Google Scholar] [CrossRef]
- Barreiro, E.; Fermoselle, C.; Mateu-Jimenez, M.; Sanchez-Font, A.; Pijuan, L.; Gea, J.; Curull, V. Oxidative stress and inflammation in the normal airways and blood of patients with lung cancer and COPD. Free Radic. Biol. Med. 2013, 65, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Jimenez, M.; Fermoselle, C.; Rojo, F.; Mateu, J.; Pena, R.; Urtreger, A.J.; Diament, M.J.; Joffe, E.D.; Pijuan, L.; Herreros, A.G.; et al. Pharmacological Approaches in an Experimental Model of Non-Small Cell Lung Cancer: Effects on Tumor Biology. Curr. Pharm. Des. 2016, 22, 5300–5310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mateu-Jimenez, M.; Sanchez-Font, A.; Rodriguez-Fuster, A.; Aguilo, R.; Pijuan, L.; Fermoselle, C.; Gea, J.; Curull, V.; Barreiro, E. Redox Imbalance in Lung Cancer of Patients with Underlying Chronic Respiratory Conditions. Mol. Med. 2016, 22, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Bustamante, V.; Curull, V.; Gea, J.; Lopez-Campos, J.L.; Munoz, X. Relationships between chronic obstructive pulmonary disease and lung cancer: Biological insights. J. Thorac. Dis. 2016, 8, E1122–E1135. [Google Scholar] [CrossRef] [PubMed]
- Pore, M.M.; Hiltermann, T.J.; Kruyt, F.A. Targeting apoptosis pathways in lung cancer. Cancer Lett. 2013, 332, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Jimenez, M.; Curull, V.; Rodriguez-Fuster, A.; Aguilo, R.; Sanchez-Font, A.; Pijuan, L.; Gea, J.; Barreiro, E. Profile of epigenetic mechanisms in lung tumors of patients with underlying chronic respiratory conditions. Clin. Epigenet. 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Wei, H.; Yip, Y.Y.; Feng, Q.; He, K.; Popov, V.; Hellstrom, I.; Hellstrom, K.E. Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation. J. Immunother. 2013, 36, 248–257. [Google Scholar] [CrossRef]
- Dai, M.; Yip, Y.Y.; Hellstrom, I.; Hellstrom, K.E. Curing mice with large tumors by locally delivering combinations of immunomodulatory antibodies. Clin. Cancer Res. 2015, 21, 1127–1138. [Google Scholar] [CrossRef]
- Tsou, P.; Katayama, H.; Ostrin, E.J.; Hanash, S.M. The Emerging Role of B Cells in Tumor Immunity. Cancer Res. 2016, 76, 5597–5601. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, A.; Dutt, S.; Chester, C.; Kim, J.; Kohrt, H.E. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin. Cancer Res. 2015, 21, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.T.; Ahmed, R.; Okazaki, T. Role of PD-1 in regulating T-cell immunity. Curr. Top. Microbiol. Immunol. 2011, 350, 17–37. [Google Scholar] [PubMed]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Forsthuber, T.G.; Cimbora, D.M.; Ratchford, J.N.; Katz, E.; Stuve, O. B cell-based therapies in CNS autoimmunity: Differentiating CD19 and CD20 as therapeutic targets. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418761697. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Enk, A. Therapeutic use of anti-CTLA-4 antibodies. Int. Immunol. 2015, 27, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.; Robert, C. Immune Checkpoint Inhibitors. Prog. Tumor Res. 2015, 42, 55–66. [Google Scholar] [PubMed]
- Rolfo, C.; Caglevic, C.; Santarpia, M.; Araujo, A.; Giovannetti, E.; Gallardo, C.D.; Pauwels, P.; Mahave, M. Immunotherapy in NSCLC: A Promising and Revolutionary Weapon. Adv. Exp. Med. Biol. 2017, 995, 97–125. [Google Scholar] [PubMed]
- Fernandez-Bussy, S.; Pires, Y.; Labarca, G.; Vial, M.R. PD-L1 Expression in a Non-Small Cell Lung Cancer Specimen Obtained by EBUS-TBNA. Arch. Bronconeumol. 2018, 54, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Moliner, L.; Fernandez, C.; Clave, S.; Arriola, E. Accurate Identification of Predictive Biomarkers of Response to Targeted Therapies in Lung Cancer with Next Generation Sequencing. Arch. Bronconeumol. 2019, 55, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.; Arriola, E. Immunotherapy is Here to Stay: A New Treatment Paradigm in Lung Cancer. Arch. Bronconeumol. 2019, 55, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Jimenez, M.; Cucarull-Martinez, B.; Yelamos, J.; Barreiro, E. Reduced tumor burden through increased oxidative stress in lung adenocarcinoma cells of PARP-1 and PARP-2 knockout mice. Biochimie 2016, 121, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Habtetsion, T.; Ding, Z.C.; Pi, W.; Li, T.; Lu, C.; Chen, T.; Xi, C.; Spartz, H.; Liu, K.; Hao, Z.; et al. Alteration of Tumor Metabolism by CD4+ T Cells Leads to TNF-alpha-Dependent Intensification of Oxidative Stress and Tumor Cell Death. Cell Metab. 2018, 28, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Maj, T.; Wang, W.; Crespo, J.; Zhang, H.; Wang, W.; Wei, S.; Zhao, L.; Vatan, L.; Shao, I.; Szeliga, W.; et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 2017, 18, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Cabrera, A.; Fermoselle, C.; Urtreger, A.J.; Mateu-Jimenez, M.; Diament, M.J.; de Kier Joffe, E.D.; Sandri, M.; Barreiro, E. Pharmacological strategies in lung cancer-induced cachexia: Effects on muscle proteolysis, autophagy, structure, and weakness. J. Cell Physiol. 2014, 229, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Cabrera, A.; Fermoselle, C.; Salmela, I.; Yelamos, J.; Barreiro, E. MicroRNA expression and protein acetylation pattern in respiratory and limb muscles of Parp-1(−/−) and Parp-2(−/−) mice with lung cancer cachexia. Biochim. Biophys. Acta 2015, 1850, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Cabrera, A.; Mateu-Jimenez, M.; Langohr, K.; Fermoselle, C.; Garcia-Arumi, E.; Andreu, A.L.; Yelamos, J.; Barreiro, E. Role of Parp Activity in Lung Cancer-induced Cachexia: Effects on Muscle Oxidative Stress, Proteolysis, Anabolic Markers and Phenotype. J. Cell Physiol. 2017, 232, 3744–3761. [Google Scholar] [CrossRef]
- Diament, M.J.; Garcia, C.; Stillitani, I.; Saavedra, V.M.; Manzur, T.; Vauthay, L.; Klein, S. Spontaneous murine lung adenocarcinoma (P07): A new experimental model to study paraneoplastic syndromes of lung cancer. Int. J. Mol. Med. 1998, 2, 45–50. [Google Scholar] [CrossRef]
- Diament, M.J.; Peluffo, G.D.; Stillitani, I.; Cerchietti, L.C.; Navigante, A.; Ranuncolo, S.M.; Klein, S.M. Inhibition of tumor progression and paraneoplastic syndrome development in a murine lung adenocarcinoma by medroxyprogesterone acetate and indomethacin. Cancer Invest. 2006, 24, 126–131. [Google Scholar] [CrossRef]
- Urtreger, A.J.; Diament, M.J.; Ranuncolo, S.M.; Vidal, D.C.; Puricelli, L.I.; Klein, S.M.; Bal de Kier Joffe, E.D. New murine cell line derived from a spontaneous lung tumor induces paraneoplastic syndromes. Int. J. Oncol. 2001, 18, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Cabrera, A.; Gea, J.; Barreiro, E. Short- and Long-Term Hindlimb Immobilization and Reloading: Profile of Epigenetic Events in Gastrocnemius. J. Cell Physiol. 2016, 232, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Degracia, A.; Granado-Martinez, P.; Millan-Sanchez, A.; Tang, J.; Pons-Carreto, A.; Barreiro, E. Reduced lung cancer burden by selective immunomodulators elicits improvements in muscle proteolysis and strength in cachectic mice. J. Cell Physiol. 2019, 234, 18041–18052. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.M.; Cao, C.; Kim, K.W.; Willey, C.D.; Geng, L.; Xiao, D.; Wang, H.; Sandler, A.; Johnson, D.H.; Colevas, A.D.; et al. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin. Cancer Res. 2007, 13, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Wang, L.; Wu, K.; Li, W.; Zhao, E.; Cui, T.; Wei, S.; Liu, Y.; Wang, Y.; Vatan, L.; et al. Inflammatory regulatory T cells in the microenvironments of ulcerative colitis and colon carcinoma. Oncoimmunology 2016, 5, e1105430. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kryczek, I.; Dostal, L.; Lin, H.; Tan, L.; Zhao, L.; Lu, F.; Wei, S.; Maj, T.; Peng, D.; et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016, 165, 1092–1105. [Google Scholar] [CrossRef]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer Activity of Sulforaphane. The Epigenetic Mechanisms and the Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, N.N.; Luketich, J.D.; Opest, A.; Visus, C.; Meyer, E.M.; Landreneau, R.; Schuchert, M.J. Inhibition of poly(ADP-ribose) polymerase (PARP) induces apoptosis in lung cancer cell lines. Cancer Invest. 2011, 29, 608–616. [Google Scholar] [CrossRef]
- Gaymes, T.J.; Shall, S.; MacPherson, L.J.; Twine, N.A.; Lea, N.C.; Farzaneh, F.; Mufti, G.J. Inhibitors of poly ADP-ribose polymerase (PARP) induce apoptosis of myeloid leukemic cells: Potential for therapy of myeloid leukemia and myelodysplastic syndromes. Haematologica 2009, 94, 638–646. [Google Scholar] [CrossRef][Green Version]
- Karpel-Massler, G.; Pareja, F.; Aime, P.; Shu, C.; Chau, L.; Westhoff, M.A.; Halatsch, M.E.; Crary, J.F.; Canoll, P.; Siegelin, M.D. PARP inhibition restores extrinsic apoptotic sensitivity in glioblastoma. PLoS ONE 2014, 9, e114583. [Google Scholar] [CrossRef]
- Huang, R.; Xu, Y.; Wan, W.; Shou, X.; Qian, J.; You, Z.; Liu, B.; Chang, C.; Zhou, T.; Lippincott-Schwartz, J.; et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 2015, 57, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.; Huang, P.K.; Yang, D.; Joosten, L.A.; Van Der Meer, J.W.; Oppenheim, J.J.; Netea, M.G.; Cheng, S.C. High-Mobility Group Nucleosome-Binding Protein 1 as Endogenous Ligand Induces Innate Immune Tolerance in a TLR4-Sirtuin-1 Dependent Manner in Human Blood Peripheral Mononuclear Cells. Front. Immunol. 2018, 9, 526. [Google Scholar] [CrossRef] [PubMed]




| Monoclonal Antibodies | Targets | Function |
|---|---|---|
| Anti-PD-1 | PD-1 | PD-1 receptor is expressed in activated T cells and induces immune tolerance by repressing T cell effector function [23,24]. |
| Anti-CTLA-4 | CTLA-4 | CTLA-4 receptor is expressed in T cells and induces immune tolerance by repressing antigen presentation [23,25]. |
| Anti-CD19 | CD-19 | CD-19 activates B cells [23,26]. |
| Anti-CD137 | CD-137 | CD-137 receptor activates CD8+ T and NK cells [21,23]. |
| Variables | Lung Cancer Mice | Lung Cancer + Monoclonal Antibodies Mice |
|---|---|---|
| Initial body weight (g) | 20.41 (1.22) | 20.34 (0.79) |
| Final body weight (g) | 19.35 (2.25) | 21.39 (1.57), * |
| Body weight gain (%) | −4.27 (10.47) | +5.16 (6.33), * |
| Body weight gain without tumor (%) | −15.06 (11.28) | −2.66 (8.35), * |
| Tumor weight (g) | 2.38 (0.75) | 1.57 (0.89), * |
| T Cells | Lung Cancer Mice | Lung Cancer + Monoclonal Antibodies Mice |
|---|---|---|
| CD3 + cells (%) | 8.34 (0.91) | 11.30 (0.78), *** |
| CD4 + (cells/µm2) | 1.99 × 10−6 (0.44 × 10−6) | 3.37 × 10−6 (1.49 × 10−6), * |
| CD8 + cells (%) | 5.16 (1.35) | 7.86 (1.04), *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Ramis-Cabrer, D.; Wang, X.; Barreiro, E. Immunotherapy with Monoclonal Antibodies in Lung Cancer of Mice: Oxidative Stress and Other Biological Events. Cancers 2019, 11, 1301. https://doi.org/10.3390/cancers11091301
Tang J, Ramis-Cabrer D, Wang X, Barreiro E. Immunotherapy with Monoclonal Antibodies in Lung Cancer of Mice: Oxidative Stress and Other Biological Events. Cancers. 2019; 11(9):1301. https://doi.org/10.3390/cancers11091301
Chicago/Turabian StyleTang, Jun, Daniel Ramis-Cabrer, Xuejie Wang, and Esther Barreiro. 2019. "Immunotherapy with Monoclonal Antibodies in Lung Cancer of Mice: Oxidative Stress and Other Biological Events" Cancers 11, no. 9: 1301. https://doi.org/10.3390/cancers11091301
APA StyleTang, J., Ramis-Cabrer, D., Wang, X., & Barreiro, E. (2019). Immunotherapy with Monoclonal Antibodies in Lung Cancer of Mice: Oxidative Stress and Other Biological Events. Cancers, 11(9), 1301. https://doi.org/10.3390/cancers11091301