Colorectal Cancer Fast Tracks: Cancer Yield and the Predictive Value of Entry Criteria
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Material
2.2. Data Collection
2.3. Statistical Analysis
3. Results
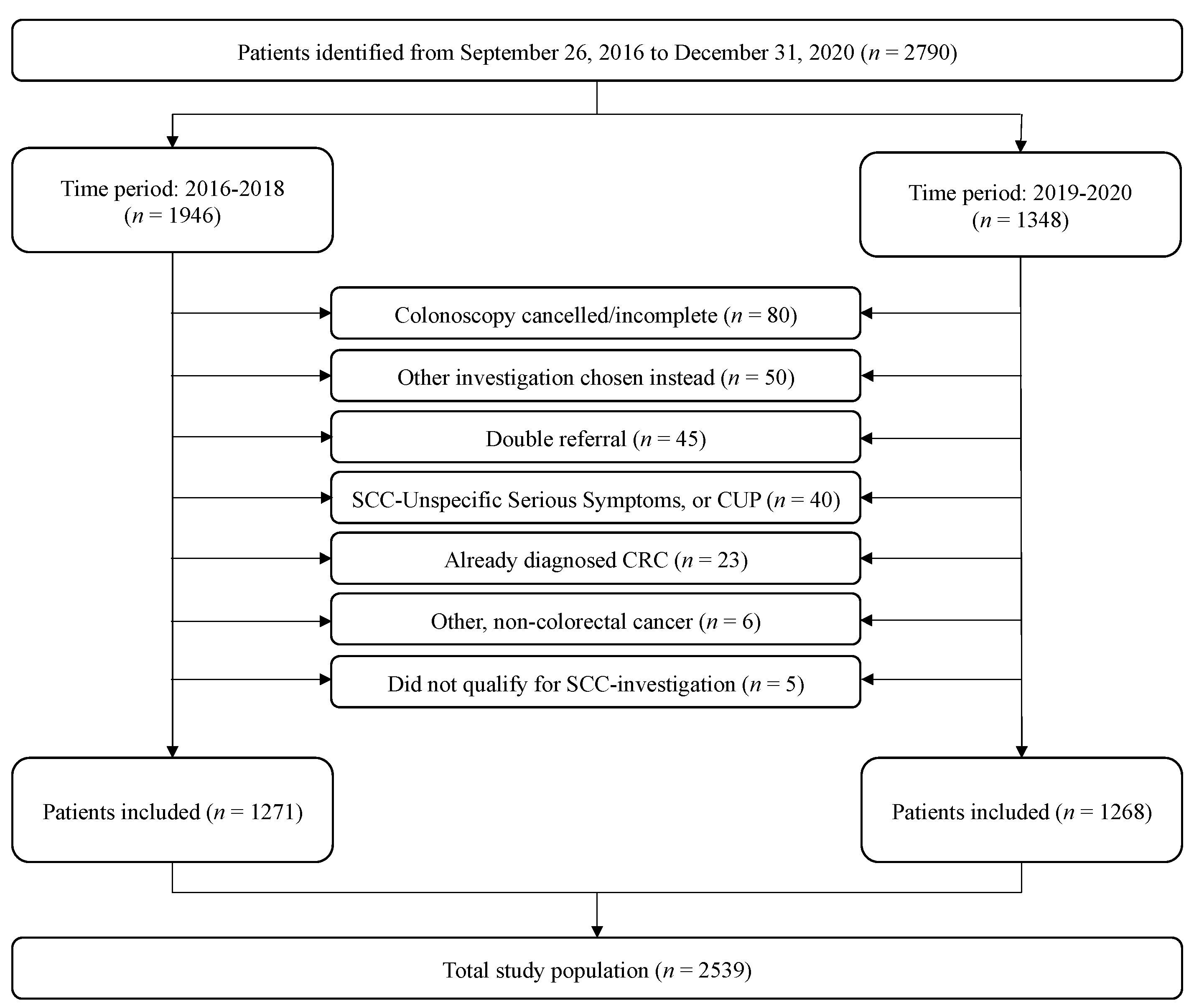
3.1. Patient Inclusion and Characteristics
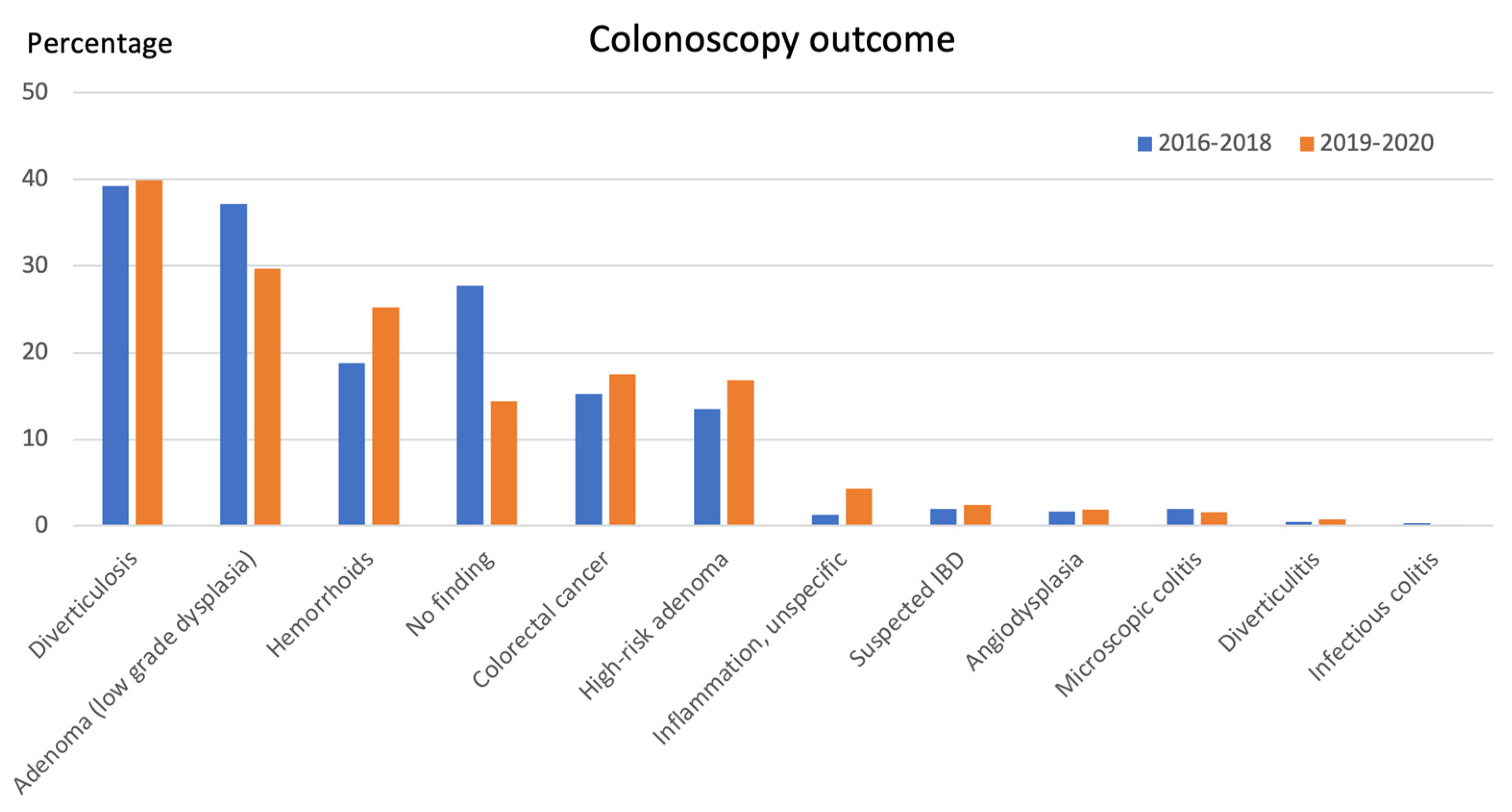
3.2. Colonoscopy Outcome
3.3. Performance of SCC-CRC Criteria
3.4. Adjusting for Confounders
3.5. FIT: The New Criterion
3.6. Laboratory Values
4. Discussion
4.1. Cancer Yield
4.2. Predictive Values of Fast-Track Criteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NICE. Suspected Cancer: Recognition and Referral. Volume 2021. National Institute for Health and Care Excellence. 23 June 2015. Available online: https://www.nice.org.uk/guidance/ng12/chapter/Recommendations-organised-by-site-of-cancer#lower-gastrointestinal-tract-cancers (accessed on 1 August 2023).
- Manes, G.; Saibeni, S.; Pellegrini, L.; Picascia, D.; Pace, F.; Schettino, M.; Bezzio, C.; de Nucci, G.; Hassan, C.; Repici, A.; et al. Improvement in appropriateness and diagnostic yield of fast-track endoscopy during the COVID-19 pandemic in Northern Italy. Endoscopy 2021, 53, 162–165. [Google Scholar] [CrossRef]
- Helsedirektoratet. Nasjonalt Handlingsprogram Med Retningslinjer for Diagnostikk, Behandling og Oppfølging av Kreft i Tykktarm og Endetarm. Helsedirektoratet.no. 2019. Available online: https://www.helsedirektoratet.no/retningslinjer/kreft-i-tykktarm-og-endetarm-handlingsprogram (accessed on 1 August 2023).
- Sundhedsstyrelsen. Pakkeforløb for Kræft i tyk- og Endetarm [Cancer Package for Colorectal Cancer] 2022. Available online: https://www.sst.dk/da/udgivelser/2022/pakkeforloeb-for-kraeft-i-tyk-og-endetarm (accessed on 1 August 2023).
- RCC. Standardiserat Vårdförlopp Tjock- och Ändtarmscancer. Volume 2021. Regionala Cancercentrum i Samverkan. Available online: https://kunskapsbanken.cancercentrum.se/diagnoser/tjock-och-andtarmscancer/vardforlopp/ (accessed on 1 August 2023).
- Cartié, H.V.; Camp, J.C.; Sáenz, R.A.O.; Negre, J.L.L.; Garriga, P.J.G.; Fraile, J.J.; Bosch, J.H.; Pradell, C.S.; Alsina, S.T.; Bosch, J.U.; et al. Results of implementation of a fast track pathway for diagnosis of colorectal cancer. Rev. Esp. Enferm. Dig. 2011, 103, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Montón-Bueno, J.; Simon, S.; Ortega, B.; Moragon, S.; Roselló, S.; Insa, A.; Navarro, J.; Sanmartín, A.; Julve, A.; et al. Ten-year assessment of a cancer fast-track programme to connect primary care with oncology: Reducing time from initial symptoms to diagnosis and treatment initiation. ESMO Open 2021, 6, 100148. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Maclean, W.; Singh, R.; Mackenzie, P.; White, D.; Benton, S.; Stebbing, J.; Rockall, T.; Jourdan, I. The two-week rule colorectal cancer pathway: An update on recent practice, the unsustainable burden on diagnostics and the role of faecal immunochemical testing. Ann. R. Coll. Surg. Engl. 2020, 102, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; O’Leary, D.; Heath, I.; Wood, L.F.; Ellis, B.; Flashman, K.; Smart, N.; Nicholls, J.; Mortensen, N.; Finan, P.; et al. Have large increases in fast track referrals improved bowel cancer outcomes in UK? BMJ 2020, 371, m3273. [Google Scholar] [CrossRef]
- Thompson, M.; O’Leary, D.; Heath, I.; Wood, L.F.; Ellis, B.; Flashman, K.; Nicholls, J.; Mortensen, N.; Finan, P.; Senapati, A.; et al. Systematic review with meta-analysis of over 90,000 patients. Does fast-track review diagnose colorectal cancer earlier? Aliment. Pharmacol. Ther. 2019, 50, 348–372. [Google Scholar]
- Jensen, H.; Tørring, M.L.; Olesen, F.; Overgaard, J.; Fenger-Grøn, M.; Vedsted, P. Diagnostic intervals before and after implementation of cancer patient pathways—A GP survey and registry based comparison of three cohorts of cancer patients. BMC Cancer 2015, 15, 308. [Google Scholar] [CrossRef]
- Andersson, E.; Nyhlin, N.; van Nieuwenhoven, M.A. The effectiveness of the colorectal cancer referral pathway—Identification of colorectal cancer in a Swedish region. Scand. J. Gastroenterol. 2021, 56, 552–558. [Google Scholar] [CrossRef]
- RCC. Väntetider i Standardiserade Vårdförlopp (SVF). Regionala Cancercentrum i Samverkan. 12 May 2021. Available online: https://www.cancercentrum.se/samverkan/vara-uppdrag/statistik/svf-statistik/vantetider-i-svf/ (accessed on 1 August 2023).
- Hassan, C.; Antonelli, G.; Dumonceau, J.-M.; Regula, J.; Bretthauer, M.; Chaussade, S.; Dekker, E.; Ferlitsch, M.; Gimeno-Garcia, A.; Jover, R.; et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2020. Endoscopy 2020, 52, 687–700. [Google Scholar] [CrossRef]
- Martínez, M.T.; González, I.; Tarazona, N.; Roselló, S.; Saiz, R.; Sanmartín, A.; Martínez-Agulló, Á.; Caballero, A.; Mas, P.; Franco, J.; et al. Implementation and assessment of a fast-track programme to improve communication between primary and specialized care in patients with suspected cancer: How to shorten time between initial symptoms of cancer, diagnosis and initiation of treatment. Clin. Transl. Oncol. 2015, 17, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.; Espinàs, J.A.; Font, R.; Argimon, J.M.; Borràs, J.M. Implementing a Cancer Fast-track Programme between primary and specialised care in Catalonia (Spain): A mixed methods study. Br. J. Cancer 2011, 105, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Astin, M.; Griffin, T.; Neal, R.D.; Rose, P.; Hamilton, W. The diagnostic value of symptoms for colorectal cancer in primary care: A systematic review. Br. J. Gen. Pract. 2011, 61, e231–e243. [Google Scholar] [CrossRef]
- Mashlab, S.; Large, P.; Laing, W.; Ng, O.; D’auria, M.; Thurston, D.; Thomson, S.; Acheson, A.; Humes, D.; Banerjea, A.; et al. Anaemia as a risk stratification tool for symptomatic patients referred via the two-week wait pathway for colorectal cancer. Ann. R. Coll. Surg. Engl. 2018, 100, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Virdee, P.S.; Marian, I.R.; Mansouri, A.; Elhussein, L.; Kirtley, S.; Holt, T.; Birks, J. The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal. Cancers 2020, 12, 2348. [Google Scholar] [CrossRef] [PubMed]
- Atkin, W.; Wooldrage, K.; Shah, U.; Skinner, K.; Brown, J.P.; Hamilton, W.; Kralj-Hans, I.; Thompson, M.R.; Flashman, K.G.; Halligan, S.; et al. Is whole-colon investigation by colonoscopy, computerised tomography colonography or barium enema necessary for all patients with colorectal cancer symptoms, and for which patients would flexible sigmoidoscopy suffice? A retrospective cohort study. Health Technol. Assess 2017, 21, 1–80. [Google Scholar] [CrossRef]
- Huggenberger, I.K.; Andersen, J.S. Predictive value of the official cancer alarm symptoms in general practice—A systematic review. Dan. Med. J. 2015, 62, A5034. [Google Scholar]
- Högberg, C.; Karling, P.; Rutegård, J.; Lilja, M. Patient-reported and doctor-reported symptoms when faecal immunochemical tests are requested in primary care in the diagnosis of colorectal cancer and inflammatory bowel disease: A prospective study. BMC Fam. Pract. 2020, 21, 129. [Google Scholar] [CrossRef]
- Rasmussen, S.; Haastrup, P.F.; Balasubramaniam, K.; Elnegaard, S.; Christensen, R.D.; Storsveen, M.M.; Søndergaard, J.; Jarbøl, D.E. Predictive values of colorectal cancer alarm symptoms in the general population: A nationwide cohort study. Br. J. Cancer 2019, 120, 595–600. [Google Scholar] [CrossRef]
- Tong, G.-X.; Chai, J.; Cheng, J.; Xia, Y.; Feng, R.; Zhang, L.; Wang, D.-B. Diagnostic value of rectal bleeding in predicting colorectal cancer: A systematic review. Asian Pac. J. Cancer Prev. 2014, 15, 1015–1021. [Google Scholar] [CrossRef]
- Högberg, C.; Gunnarsson, U.; Jansson, S.; Thulesius, H.; Cronberg, O.; Lilja, M. Diagnosing colorectal cancer in primary care: Cohort study in Sweden of qualitative faecal immunochemical tests, haemoglobin levels, and platelet counts. Br. J. Gen. Pract. 2020, 70, e843–e851. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Klimovskij, M.; Harshen, R. Accuracy of faecal immunochemical testing in patients with symptomatic colorectal cancer. BJS Open 2020, 4, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
| Symptom/Sign | Sweden | Norway | Denmark | UK | Italy | Catalonia (Spain) | Valencia (Spain) |
|---|---|---|---|---|---|---|---|
| Visible blood in stool/rectal bleeding | • | • | • | • | • | • | • |
| Anemia | • | • | • | • | • | • | |
| Altered bowel habits | • a) | • | • | • | • | • | |
| Finding from rectal examination | • | • | • b) | • | • | ||
| Weight loss | • c) | • | • | • | |||
| Abdominal pain | • | • | |||||
| Radiological finding | • | • | |||||
| FOBT/FIT | • | • | |||||
| Palpable abdominal mass | • | ||||||
| Other | • | • | • | • | • | • | |
| Maximum days until endoscopy | 10 days | 21 days | 10 (14) days | 2 weeks | 10 days | Not specified |
| Study Population 2016–2018 (n = 1271) | Cancer (n = 194) | No Cancer (n = 1077) | p-Value | |
| Age, median (IQR) | 72.5 (13) | 70 (19) | <0.001 * | |
| Male, n (%) | 101 (52.1) | 489 (45.4) | 0.089 ** | |
| Study population 2019–2020 (n = 1268) | Cancer (n = 222) | No cancer (n = 1046) | ||
| Age, median (IQR) | 73 (14) | 70 (17) | <0.001 * | |
| Male, n (%) | 120 (54.1) | 467 (44.6) | 0.011 ** | |
| Study population pooled (n = 2539) | Cancer (n = 416) | No cancer (n = 2123) | ||
| Age, median (IQR) | 73 (13) | 70 (17) | <0.001 * | |
| Male, n (%) | 221 (53.1) | 956 (45.0) | 0.002 ** |
| SCC-CRC Entry Criteria | n (%) | n (%) | PPV (%) | NPV (%) | Specificity (%) | Sensitivity (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|
| SCC-CRC criteria 2016–2018 (n = 1271) | Cancer (n = 194) | No cancer (n = 1077) | ||||||
| Visible blood in stool/rectal bleeding | 33 (17.0) | 227 (21.1) | 12.7 | 84.1 | 78.9 | 17.0 | 0.8 (0.5–1.1) | 0.196 |
| Anemia (a) | 101 (54.9) | 324 (35.7) | 23.8 | 87.5 | 64.3 | 54.9 | 2.2 (1.6–3.0) | <0.001 |
| Abnormal rectal finding (rectoscopy/rectal examination) | 51 (26.3) | 124 (11.5) | 29.1 | 87.0 | 88.5 | 26.3 | 2.8 (1.9–4.0) | <0.001 |
| Abnormal radiology | 52 (26.8) | 126 (11.7) | 29.2 | 87.0 | 88.3 | 26.8 | 2.8 (1.9–4.0) | <0.001 |
| Altered bowel habits (b) | 81 (41.8) | 582 (54.0) | 12.2 | 81.4 | 46.0 | 41.8 | 0.6 (0.4–0.8) | 0.002 |
| SCC-CRC criteria 2019–2020 (n = 1268) | Cancer (n = 222) | No Cancer (n = 1046) | ||||||
| Visible blood in stool/rectal bleeding | 57 (25.7) | 286 (27.3) | 16.6 | 82.2 | 72.7 | 25.7 | 0.9 (0.7–1.3) | 0.612 |
| Anemia (c) | 122 (56.2) | 351 (39.6) | 25.8 | 84.9 | 60.4 | 56.2 | 2.0 (1.5–2.6) | <0.001 |
| Abnormal rectal finding (rectoscopy/rectal examination | 53 (23.9) | 143 (13.7) | 27.0 | 84.2 | 86.3 | 23.9 | 2.0 (1.4–2.8) | <0.001 |
| Abnormal radiology | 65 (29.3) | 140 (13.4) | 31.7 | 85.2 | 86.6 | 29.3 | 2.7 (1.9–3.8) | <0.001 |
| Combination of altered bowel habits and visible blood in stool/rectal bleeding (d) | 36 (16.2) | 138 (13.2) | 20.7 | 83.0 | 86.8 | 16.2 | 1.3 (0.9–1.9) | 0.234 |
| Combination of altered bowel habits and anemia (d) | 50 (22.5) | 127 (12.1) | 28.2 | 84.2 | 87.9 | 22.5 | 2.1 (1.5–3.0) | <0.001 |
| SCC-CRC criteria 2016–2020 (n = 2539) | Cancer (n = 416) | No Cancer (n = 2123) | ||||||
| Visible blood in stool/rectal bleeding | 90 (21.6) | 513 (24.2) | 14.9 | 83.2 | 75.8 | 21.9 | 0.9 (0.7–1.1) | 0.268 |
| Anemia (e) | 223 (55.6) | 675 (37.6) | 24.8 | 86.3 | 62.4 | 55.6 | 2.1 (1.7–2.6) | <0.001 |
| Abnormal rectal finding (rectoscopy/rectal examination | 104 (25.0) | 267 (12.6) | 28.0 | 85.6 | 87.4 | 25.0 | 2.3 (1.8–3.0) | <0.001 |
| Abnormal radiology | 117 (28.1) | 266 (12.5) | 30.5 | 86.1 | 87.5 | 28.1 | 2.7 (2.1–3.5) | <0.001 |
| Altered bowel habits (b) | 187 (45.0) | 1117 (52.6) | 14.3 | 81.5 | 47.4 | 45.0 | 0.7 (0.6–0.9) | 0.004 |
| Combination of altered bowel habits and anemia (d) | 83 (20.0) | 234 (11.0) | 26.2 | 85.0 | 89.0 | 20.0 | 2.0 (1.5–2.7) | <0.001 |
| Combination of altered bowel habits and visible blood in stool/rectal bleeding (d) | 53 (12.7) | 224 (10.6) | 19.1 | 84.0 | 89.4 | 12.7 | 1.2 (0.9–1.7) | 0.190 |
| SCC-CRC Criteria 2016–2018 (n = 1271) | OR (95% CI) | p-Value |
| Visible blood in stool/rectal bleeding | 1.6 (1.0–2.5) | 0.066 |
| Anemia | 2.2 (1.5–3.1) | <0.001 |
| Abnormal rectal finding (rectoscopy/rectal examination) | 4.1 (2.6–6.3) | <0.001 |
| Abnormal radiology | 5.0 (3.2–8.0) | <0.001 |
| Change in bowel habits | 1.0 (0.7–1.4) | 0.968 |
| SCC-CRC criteria 2019–2020 (n = 1268) | ||
| Visible blood in stool/rectal bleeding | 0.9 (0.5–1.5) | 0.643 |
| Anemia | 2.3 (1.6–3.4) | <0.001 |
| Abnormal radiology | 4.6 (3.1–6.9) | <0.001 |
| Abnormal rectal finding (rectoscopy/rectal examination) | 3.3 (2.2–5.0) | <0.001 |
| Combination of change in bowel habits and anemia | 1.1 (0.7–1.7) | 0.812 |
| Combination of change in bowel habits and blood in stool | 2.5 (1.3–4.7) | 0.008 |
| SCC-CRC criteria 2016–2020 (n = 2539) | ||
| Visible blood in stool/rectal bleeding | 0.9 (0.6–1.4) | 0.705 |
| Anemia | 2.1 (1.5–3.0) | <0.001 |
| Abnormal radiology | 4.6 (3.4–6.3) | <0.001 |
| Abnormal rectal finding (rectoscopy/rectal examination) | 3.6 (2.7–4.8) | <0.001 |
| Change in bowel habits | 0.9 (0.6–1.3) | 0.479 |
| Combination of change in bowel habits and anemia | 1.1 (0.7–1.8) | 0.669 |
| Combination of change in bowel habits and visible blood in stool | 2.5 (1.4–4.5) | 0.001 |
| Missing Values, CRC/No CRC (%) | PPV (%) | NPV (%) | p-Values | OR (95% CI) | |||
|---|---|---|---|---|---|---|---|
| SCC-CRC criteria 2016–2018 (n = 1271) | Cancer (n = 194) | No cancer (n = 1077) | |||||
| Positive FIT, n (%) | 84 (98.8) | 402 (68.0) | 56.2/45.1 | 17.3 | 99.5 | <0.001 | 39.5 (5.5–285.8) |
| Hb, g/L, median (IQR) - Males - Females | 120 (32) 124 (29) 118 (30) | 130 (26) 136 (27) 128 (23) | 5.2/15.8 8/16.4 2.1/15.3 | N/A | N/A | <0.001 <0.001 <0.001 | N/A |
| SCC-CRC criteria 2019–2020 (n = 1268) | Cancer (n = 222) | No Cancer (n = 1046) | |||||
| Positive FIT, n (%) | 97 (94.2) | 379 (70.8) | 53.6/48.9 | 20.4 | 96.3 | <0.001 | 6.7 (2.9–15.5) |
| Hb, g/L, median (IQR) - Males - Females | 123 (32) 130 (31) 114 (32) | 129 (31) 135 (34) 127 (29) | 2.3/15.3 1.7/15.0 2.9/15.5 | N/A | N/A | 0.005 0.177 <0.001 | N/A |
| SCC-CRC criteria 2016–2020 (n = 2539) | Cancer (n = 416) | No cancer (n = 2123) | |||||
| Positive FIT, n (%) | 181 (96.3) | 781 (69.4) | 54.8/47.0 | 18.8 | 98.0 | <0.001 | 11.4 (5.3–24.6) |
| Hb, g/L, median (IQR) - Males - Females | 122 (32) 128 (31) 116 (30) | 130 (28) 135 (32) 128 (25) | 3.6/15.5 4.5/15.7 2.6/15.4 | N/A | N/A | <0.001 <0.001 <0.001 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uebel, L.; Kromodikoro, I.; Nyhlin, N.; van Nieuwenhoven, M. Colorectal Cancer Fast Tracks: Cancer Yield and the Predictive Value of Entry Criteria. Cancers 2023, 15, 4778. https://doi.org/10.3390/cancers15194778
Uebel L, Kromodikoro I, Nyhlin N, van Nieuwenhoven M. Colorectal Cancer Fast Tracks: Cancer Yield and the Predictive Value of Entry Criteria. Cancers. 2023; 15(19):4778. https://doi.org/10.3390/cancers15194778
Chicago/Turabian StyleUebel, Linnea, Indy Kromodikoro, Nils Nyhlin, and Michiel van Nieuwenhoven. 2023. "Colorectal Cancer Fast Tracks: Cancer Yield and the Predictive Value of Entry Criteria" Cancers 15, no. 19: 4778. https://doi.org/10.3390/cancers15194778
APA StyleUebel, L., Kromodikoro, I., Nyhlin, N., & van Nieuwenhoven, M. (2023). Colorectal Cancer Fast Tracks: Cancer Yield and the Predictive Value of Entry Criteria. Cancers, 15(19), 4778. https://doi.org/10.3390/cancers15194778






