Alteration of the Exhaled Volatile Organic Compound Pattern in Colorectal Cancer Patients after Intentional Curative Surgery—A Prospective Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Study Procedures
2.3. AeonoseTM Technology and Model Development with Machine Learning
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Study Population and Baseline Characteristics
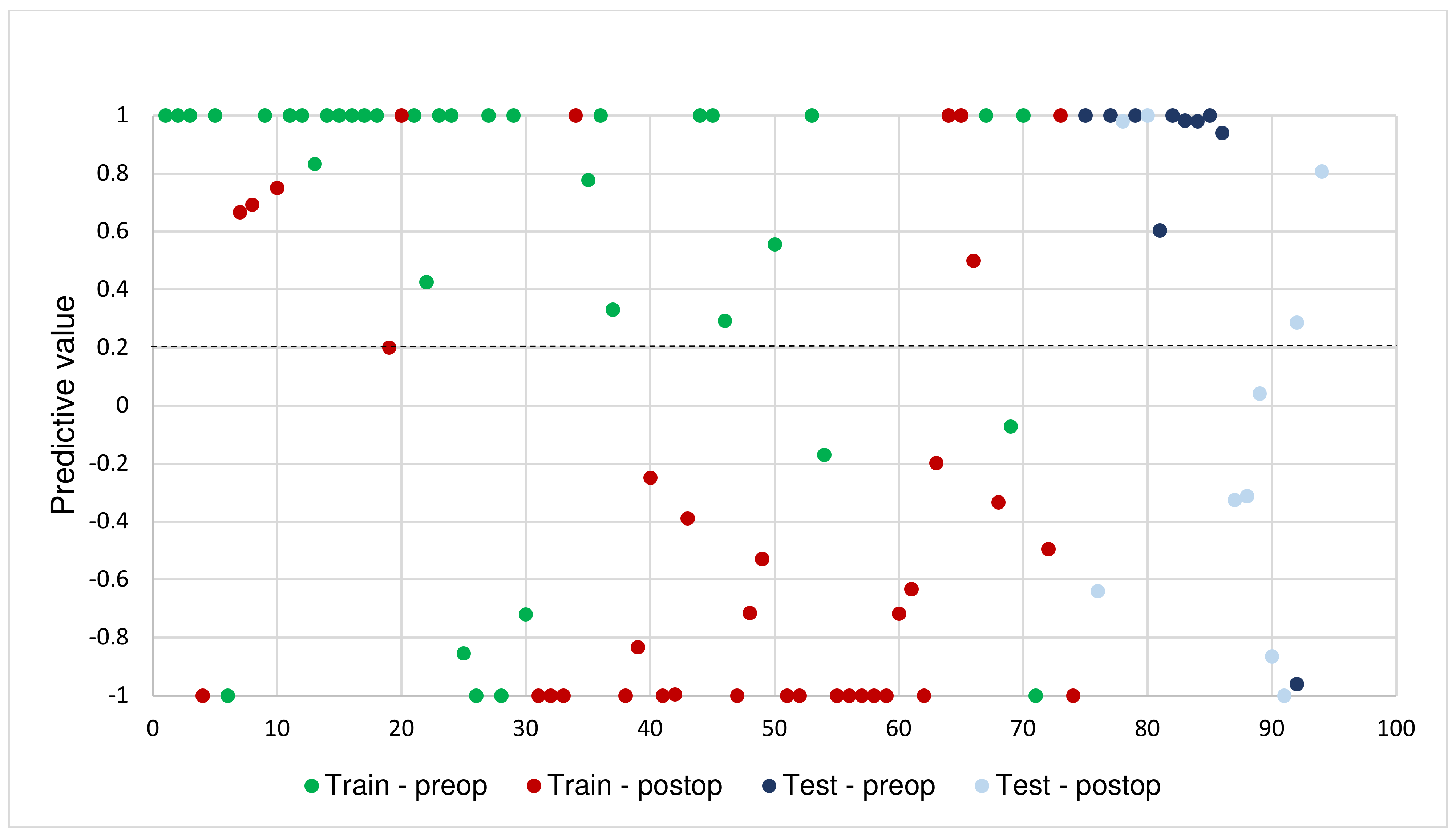
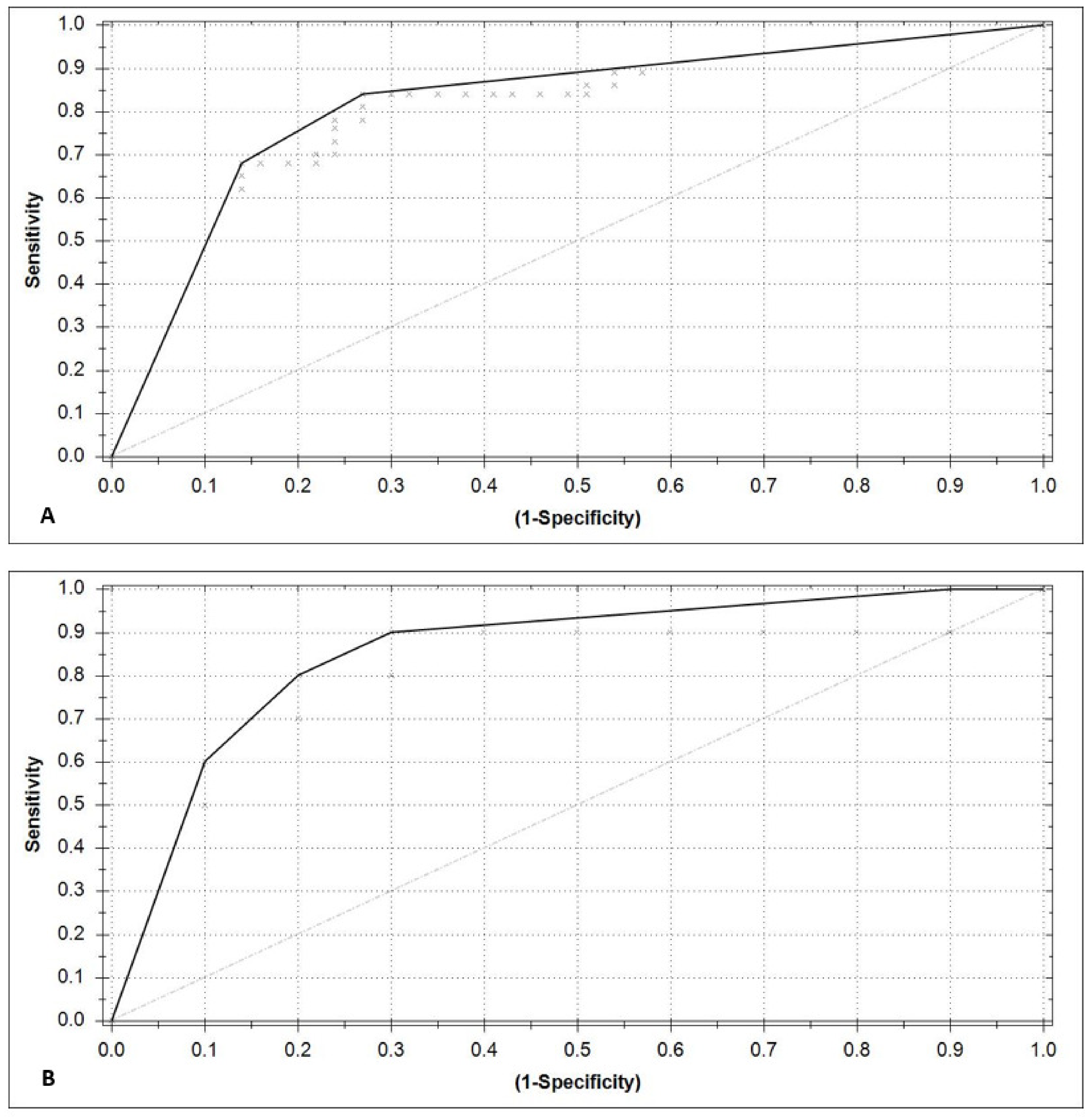
3.2. Model Performance
3.3. Pre- and Post-Operative Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Stok, E.P.; Spaander, M.C.W.; Grünhagen, D.J.; Verhoef, C.; Kuipers, E.J. Surveillance After Curative Treatment for Colorectal Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, R.H. Early Detection and Prevention of Colorectal Cancer (Review). Oncol. Rep. 1999, 6, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Elferink, M.A.G.; de Jong, K.P.; Klaase, J.M.; Siemerink, E.J.; de Wilt, J.H.W. Metachronous Metastases from Colorectal Cancer: A Population-Based Study in North-East Netherlands. Int. J. Colorectal Dis. 2015, 30, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Bastiaenen, V.P.; Hovdenak Jakobsen, I.; Labianca, R.; Martling, A.; Morton, D.G.; Primrose, J.N.; Tanis, P.J.; Laurberg, S. Consensus and Controversies regarding Follow-Up After Treatment with Curative Intent of Nonmetastatic Colorectal Cancer: A Synopsis of Guidelines used in Countries Represented in the European Society of Coloproctology. Colorectal. Dis. 2019, 21, 392–416. [Google Scholar] [CrossRef] [PubMed]
- Kievit, J. Follow-Up of Patients with Colorectal Cancer: Numbers Needed to Test and Treat. Eur. J. Cancer 2002, 38, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- de Boer, N.K.H.; de Meij, T.G.J.; Oort, F.A.; Ben Larbi, I.; Mulder, C.J.J.; van Bodegraven, A.A.; van der Schee, M.P. The Scent of Colorectal Cancer: Detection by Volatile Organic Compound Analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 1085–1089. [Google Scholar] [CrossRef]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; de Gennaro, G. Exhaled Volatile Organic Compounds Identify Patients with Colorectal Cancer. Br. J. Surg. 2013, 100, 144–150. [Google Scholar] [CrossRef]
- Sun, X.; Shao, K.; Wang, T. Detection of Volatile Organic Compounds (VOCs) from Exhaled Breath as Noninvasive Methods for Cancer Diagnosis. Anal. Bioanal. Chem. 2016, 408, 2759–2780. [Google Scholar] [CrossRef]
- Kort, S.; Brusse-Keizer, M.; Gerritsen, J.; van der Palen, J. Data Analysis of Electronic Nose Technology in Lung Cancer: Generating Prediction Models by Means of Aethena. J. Breath Res. 2017, 11, 026006. [Google Scholar] [CrossRef] [PubMed]
- van Keulen, K.E.; Jansen, M.E.; Schrauwen, R.W.M.; Kolkman, J.J.; Siersema, P.D. Volatile Organic Compounds in Breath can Serve as a Non-Invasive Diagnostic Biomarker for the Detection of Advanced Adenomas and Colorectal Cancer. Aliment. Pharmacol. Ther. 2020, 51, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath Testing as Potential Colorectal Cancer Screening Tool. Int. J. Cancer 2016, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.R.; van Vorstenbosch, R.W.R.; Pachen, D.M.; Meulen, L.W.T.; Straathof, J.W.A.; Dallinga, J.W.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Schooten, F.V.; Mujagic, Z.; et al. Detecting Colorectal Adenomas and Cancer using Volatile Organic Compounds in Exhaled Breath: A Proof-of-Principle Study to Improve Screening. Clin. Transl. Gastroenterol. 2022, 13, e00518. [Google Scholar] [CrossRef] [PubMed]
- Steenhuis, E.G.M.; Schoenaker, I.J.H.; de Groot, J.W.B.; Fiebrich, H.B.; de Graaf, J.C.; Brohet, R.M.; van Dijk, J.D.; van Westreenen, H.L.; Siersema, P.D.; de Vos Tot Nederveen Cappel, W.H. Feasibility of Volatile Organic Compound in Breath Analysis in the Follow-Up of Colorectal Cancer: A Pilot Study. Eur. J. Surg. Oncol. 2020, 46, 2068–2073. [Google Scholar] [CrossRef] [PubMed]
- Chandrapalan, S.; Bosch, S.; Cubiella, J.; Guardiola, J.; Kimani, P.; Mulder, C.; Persaud, K.; de Meij, T.G.J.; Altomare, D.F.; Brenner, H.; et al. Systematic Review with Meta-Analysis: Volatile Organic Compound Analysis to Improve Faecal Immunochemical Testing in the Detection of Colorectal Cancer. Aliment. Pharmacol. Ther. 2021, 54, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Markar, S.; Chin, S.; Romano, A.; Wiggins, T.; Antonowicz, S.; Paraskeva, P.; Ziprin, P.; Darzi, A.; Hanna, G. Breath Volatile Organic Compound Profiling of Colorectal Cancer using Selected Ion Flow-Tube Mass Spectrometry. Ann. Surg. 2019, 269, 903–910. [Google Scholar] [CrossRef]
- Bosch, S.; Lemmen, J.P.; Menezes, R.; van der Hulst, R.; Kuijvenhoven, J.; Stokkers, P.C.; de Meij, T.G.; de Boer, N.K. The Influence of Lifestyle Factors on Fecal Volatile Organic Compound Composition as Measured by an Electronic Nose. J. Breath Res. 2019, 13, 046001. [Google Scholar] [CrossRef]
- Kort, S.; Brusse-Keizer, M.; Gerritsen, J.W.; Schouwink, H.; Citgez, E.; de Jongh, F.; van der Maten, J.; Samii, S.; van den Bogart, M.; van der Palen, J. Improving Lung Cancer Diagnosis by Combining Exhaled-Breath Data and Clinical Parameters. ERJ Open Res. 2020, 6, 00221-2019. [Google Scholar] [CrossRef]
- Kort, S.; Brusse-Keizer, M.; Schouwink, H.; Citgez, E.; de Jongh, F.; van Putten, J.; van den Borne, B.; Kastelijn, L.; Stolz, D.; Schuurbiers, M.; et al. Diagnosing Non-Small Cell Lung Cancer by Exhaled-Breath Profiling using an Electronic Nose: A Multicenter Validation Study. Chest 2022, 163, 697–706. [Google Scholar] [CrossRef]
- Bruins, M.; Gerritsen, J.W.; van de Sande, W.W.J.; van Belkum, A.; Bos, A. Enabling a Transferable Calibration Model for Metal-Oxide Type Electronic Noses. Sens. Actuators B Chem. 2013, 188, 1187–1195. [Google Scholar] [CrossRef]
- Waltman, C.G.; Marcelissen, T.A.T.; van Roermund, J.G.H. Exhaled-Breath Testing for Prostate Cancer Based on Volatile Organic Compound Profiling using an Electronic Nose Device (Aeonose™): A Preliminary Report. Eur. Urol. Focus 2020, 6, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- van de Goor, R.; Leunis, N.; Hooren, M.; Francisca, E.; Masclee, A.; Kremer, B.; Kross, K. Feasibility of Electronic Nose Technology for Discriminating between Head and Neck, Bladder, and Colon Carcinomas. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.; Rahim, Z.; Bos, A.; van de Sande, W.W.J.; Endtz, H.P.; van Belkum, A. Diagnosis of Active Tuberculosis by E-Nose Analysis of Exhaled Air. Tuberculosis 2013, 93, 232–238. [Google Scholar] [CrossRef] [PubMed]
- van Hooren, M.R.A.; Leunis, N.; Brandsma, D.S.; Dingemans, A.C.; Kremer, B.; Kross, K.W. Differentiating Head and Neck Carcinoma from Lung Carcinoma with an Electronic Nose: A Proof of Concept Study. Eur. Arch. Otorhinolaryngol. 2016, 273, 3897–3903. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.K.; Zakko, L.; Visrodia, K.H.; Leggett, C.L.; Lutzke, L.S.; Clemens, M.A.; Allen, J.D.; Anderson, M.A.; Wang, K.K. Breath Testing for Barrett’s Esophagus using Exhaled Volatile Organic Compound Profiling with an Electronic Nose Device. Gastroenterology 2017, 152, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Goldoni, M.; Caglieri, A.; Ceresa, G.; Acampa, O.; Carbognani, P.; Rusca, M.; Corradi, M. Breath Analysis in Non Small Cell Lung Cancer Patients After Surgical Tumour Resection. Acta Biomed. 2008, 79 (Suppl. 1), 64–72. [Google Scholar] [PubMed]
- Broza, Y.Y.; Kremer, R.; Tisch, U.; Gevorkyan, A.; Shiban, A.; Best, L.A.; Haick, H. A Nanomaterial-Based Breath Test for Short-Term Follow-Up After Lung Tumor Resection. Nanomedicine 2013, 9, 15–21. [Google Scholar] [CrossRef]
- Hartwig, S.; Raguse, J.; Pfitzner, D.; Preissner, R.; Paris, S.; Preissner, S. Volatile Organic Compounds in the Breath of Oral Squamous Cell Carcinoma Patients: A Pilot Study. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2017, 157, 981–987. [Google Scholar] [CrossRef]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Travaglio, E.; Longobardi, F.; Tutino, M.; Depalma, N.; Tedesco, G.; Sardaro, A.; Memeo, R.; et al. Effects of Curative Colorectal Cancer Surgery on Exhaled Volatile Organic Compounds and Potential Implications in Clinical Follow-Up. Ann. Surg. 2015, 262, 862–867. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton Pump Inhibitors Affect the Gut Microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton Pump Inhibitors Alter the Composition of the Gut Microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]


| Training n = 37 | Validation n = 10 | Total n = 47 | |
|---|---|---|---|
| Patient characteristics | |||
| Age, mean ± SD | 64 ± 11.2 | 68 ± 7.4 | 65 ± 10.5 |
| Gender, male, n (%) | 23 (62.2) | 7 (70.0) | 30 (63.8) |
| BMI, kg/m2, mean ± SD | 25.7 ± 4.1 | 25.7 ± 2.8 | 25.7 ± 3.8 |
| ASA, n (%) | |||
| 1 | 7 (18.9) | 2 (20) | 9 (19.1) |
| 2 | 25 (67.6) | 7 (70) | 32 (68.1) |
| 3 | 5 (13.5) | 1 (10) | 6 (12.8) |
| Comorbidity, n (%) | |||
| Hypertension | 11 (29.7) | 1 (10) | 16 (34.0) |
| Cardiovascular comorbidity | 5 (13.5) | 1 (10) | 6 (12.8) |
| Lung comorbidity | 6 (16.2) | 1 (10) | 7 (14.9) |
| Thyroid disease | 1 (2.7) | - | 1 (2.1) |
| Diabetes | 3 (8.1) | 1 (10) | 4 (8.5) |
| Hypercholesterolemia | 3 (8.1) | 1 (10) | 4 (8.5) |
| Autoimmune disease | |||
| IBD | 1 (2.7) | - | 1 (2.1) |
| Rheumatoid arthritis | 2 (2.7) | - | 2 (4.3) |
| Other | |||
| Reflux esophagitis | 3 (8.1) | - | 3 (6.4) |
| OSAS | - | 1 (10) | 1 (2.1) |
| Gout | - | 1 (10) | 1 (2.1) |
| Depression | 1 (2.7) | - | 1 (2.1) |
| Epileptic Disorder | 1 (2.7) | - | 1 (2.1) |
| Tumor characteristics, n (%) | |||
| Primary tumor location | |||
| Right colon | 14 (37.8) | 9 (90) | 23 (48.9) |
| Left colon | 22 (59.5) | 1 (10) | 24 (48.9) |
| Rectum | 3 (8.1) | - | 3 (6.4) |
| Synchronous double tumor | 2 (5.4) | - | 2 (4.3) |
| MMR-status, n (%) | |||
| MSS | 23 (62.2) | 5 (50) | 28 (59.6) |
| MSI | 1 (2.7) | - | 1 (2.1) |
| Undetermined | 12 (32.4) | 4 (40) | 18 (38.3) |
| AJCC (8th edition) stage, n (%) | |||
| Stage I (T1-2N0M0) | 7 (18.9) | 6 (60) | 13 (27.7) |
| Stage II (T3-T4N0M0) | 12 (32.4) | 1 (10) | 13 (27.7) |
| Stage III (T1-4N1-2M0) | 18 (48.6) | 3 (30) | 21 (44.7) |
| Operation, n (%) | |||
| (Extended) Right hemicolectomy | 14 (37.8) | 8 (80) | 22 (46.1) |
| Left hemicolectomy | 5 (13.5) | - | 5 (10.6) |
| Sigmoid colectomy | 12 (35.1) | 1 (10) | 14 (29.8) |
| Low-anterior resection | 4 (8.5) | - | 4 (8.5) |
| Local resection (TEM & CAL-WR) | 1 (2.7) | 1 (10) | 2 (4.3) |
| Proctocolectomy | 1 (2.1) | - | 1 (2.1) |
| Time between operation and 2nd test (days), median [range] | 20 (10–113) | 15.5 (11–76) | 18 (10–113) |
| Pre-Operative | Post-Operative | |||||
|---|---|---|---|---|---|---|
| Correctly Predicted n = 38 | Incorrectly Predicted n = 9 | p-Value | Correctly Predicted n = 33 | Incorrectly Predicted n = 14 | p-Value | |
| Age, mean ± SD | 64 ± 10.7 | 69 ± 9.5 | 0.22 | 65 ± 11.5 | 64.5 ± 8.6 | 0.80 |
| Gender, male, n (%) | 25 (65.8) | 5 (55.6) | 0.56 | 21 (63.6) | 9 (64.3) | 0.97 |
| BMI, kg/m2 ± SD | 25.8 ± 4.0 | 25.5 ± 2.8 | 0.85 | 26.1 ± 3.7 | 24.3 ± 3.5 | 0.13 |
| Current smoker, n (%) | 9 (23.7) | 1 (11) | 0.41 | 4 (12.1) | 1 (7.1) | 0.61 |
| Alcohol use < 24 h, n (%) | 22 (57.9) | 3 (33.3) | 0.18 | 8 (24.2) | 4 (28.6) | 0.76 |
| Use of corticosteroids, n (%) | 4 (10.5) | 1 (11.1) | - | 3 (9.1) | 2 (14.3) | - |
| Use of antibiotics a, n (%) | 2 (5.3) | 1 (11.1) | - | 3 (9.1) | 1 (7.1) | - |
| PPI use, n (%) | 8 (21.1) | 5 (55.5) | 0.04 | 12 (36.4) | 2 (14.3) | 0.13 |
| Last meal < 3 h, n (%) | 17 (44.7) | 5 (55.5) | 0.56 | 23 (69.7) | 10 (71.4) | 0.91 |
| Depth of tumor invasion, n (%) | ||||||
| pT1-2 | 14 (36.8) | 3 (33.3) | 0.84 | |||
| pT3-4 | 24 (63.2) | 6 (66.7) | ||||
| Postoperative complications, n (%) | 6 (18.8) | 4 (26.7) | 0.54 | |||
| Minor complication (grade I–II b) | 5 | 3 | - | |||
| Major complication (grade ≥ III b) | 1 | 1 | - | |||
| Polyps in place during second test, n (%) | 13 (39.4) | 3 (21.4) | 0.24 | |||
| Time between operation and 2nd test (days), median, [range] | 20 (10–113) | 17 (11–76) | 0.23 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanevelt, J.; Schoenaker, I.J.H.; Brohet, R.M.; Schrauwen, R.W.M.; Baas, F.J.N.; Tanis, P.J.; van Westreenen, H.L.; de Vos tot Nederveen Cappel, W.H. Alteration of the Exhaled Volatile Organic Compound Pattern in Colorectal Cancer Patients after Intentional Curative Surgery—A Prospective Pilot Study. Cancers 2023, 15, 4785. https://doi.org/10.3390/cancers15194785
Hanevelt J, Schoenaker IJH, Brohet RM, Schrauwen RWM, Baas FJN, Tanis PJ, van Westreenen HL, de Vos tot Nederveen Cappel WH. Alteration of the Exhaled Volatile Organic Compound Pattern in Colorectal Cancer Patients after Intentional Curative Surgery—A Prospective Pilot Study. Cancers. 2023; 15(19):4785. https://doi.org/10.3390/cancers15194785
Chicago/Turabian StyleHanevelt, Julia, Ivonne J. H. Schoenaker, Richard M. Brohet, Ruud W. M. Schrauwen, Frederique J. N. Baas, Pieter J. Tanis, Henderik L. van Westreenen, and Wouter H. de Vos tot Nederveen Cappel. 2023. "Alteration of the Exhaled Volatile Organic Compound Pattern in Colorectal Cancer Patients after Intentional Curative Surgery—A Prospective Pilot Study" Cancers 15, no. 19: 4785. https://doi.org/10.3390/cancers15194785
APA StyleHanevelt, J., Schoenaker, I. J. H., Brohet, R. M., Schrauwen, R. W. M., Baas, F. J. N., Tanis, P. J., van Westreenen, H. L., & de Vos tot Nederveen Cappel, W. H. (2023). Alteration of the Exhaled Volatile Organic Compound Pattern in Colorectal Cancer Patients after Intentional Curative Surgery—A Prospective Pilot Study. Cancers, 15(19), 4785. https://doi.org/10.3390/cancers15194785






