Hepatocellular Carcinoma: Current Drug Therapeutic Status, Advances and Challenges
Abstract
:Simple Summary
Abstract
1. Introduction
2. Genetic Alterations and Signaling Pathways in HCC
2.1. Telomerase Reverse Transcriptase
2.2. TP53
2.3. CTNNB1 (WNT/β-Catenin)
2.4. PI3K/AKT/mTOR and RAS/RAF/MAPK
2.5. FGF19/CNND1
2.6. VEGFA
3. Current Treatment Strategies for HCC
3.1. Atezolizumab–Bevacizumab/Sintilimab–IBI305
3.2. Tremelimumab–Durvalumab
3.3. Sorafenib, Lenvatinib, Donafenib, and Rivoceranib
3.4. Other Systemic Drugs
4. The Challenges of Systemic Treatment for HCC
4.1. The Lack of Effective Druggable Targets with a High Mutation Rate
4.2. Lack of Effective Biomarkers
4.3. How Do We Select Optimal Treatment Regimens Efficiently?
5. Potential Future Treatments
5.1. Exploration Surrounding Immunotherapy
5.1.1. Dual-Immunotherapy Combination Therapy
5.1.2. Combination of ICIs and Anti-VEGF/VEGFR
5.1.3. Combinations of ICIs and KIs
5.1.4. Other Immunotherapy
5.2. Precision Therapy Guided by Molecular Mechanisms
5.3. Conversion Therapy Aimed at Improving the R0 Resection Rate
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kato, Y.; Ozawa, Y.; Kodama, K.; Ito, J.; Ichikawa, K.; Yamada, K.; Hori, Y.; Tabata, K.; Takase, K.; et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018, 109, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Data Source: Globocan 2020 Graph Production: Global Cancer Observatory. Available online: http://gco.iarc.fr (accessed on 10 March 2023).
- Liu, Y.; Zheng, J.; Hao, J.; Wang, R.R.; Liu, X.; Gu, P.; Yu, H.; Yu, Y.; Wu, C.; Ou, B.; et al. Global burden of primary liver cancer by five etiologies and global prediction by 2035 based on global burden of disease study 2019. Cancer Med. 2022, 11, 1310–1323. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, L.; Ruan, B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int. J. Infect. Dis. 2012, 16, e82–e88. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 796–829. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Zhai, W.; Lai, H.; Kaya, N.A.; Chen, J.; Yang, H.; Lu, B.; Lim, J.Q.; Ma, S.; Chew, S.C.; Chua, K.P.; et al. Dynamic phenotypic heterogeneity and the evolution of multiple RNA subtypes in hepatocellular carcinoma: The PLANET study. Natl. Sci. Rev. 2021, 9, nwab192. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chen, M.; Cheng, A.L.; Kaseb, A.O.; Kudo, M.; Lee, H.C.; Yopp, A.C.; Zhou, J.; Wang, L.; Wen, X.; et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Pressiani, T.; Merle, P. Systemic Treatment Options in Hepatocellular Carcinoma. Liver Cancer 2019, 8, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Rimassa, L.; Finn, R.S. Molecular therapies for HCC: Looking outside the box. J. Hepatol. 2020, 72, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239.e4. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, R.; Xu, X. Synthetic lethality: A promising therapeutic strategy for hepatocellular carcinoma. Cancer Lett. 2020; 476, 120–128. [Google Scholar] [CrossRef]
- Schulze, K.; Imbeaud, S.; Letouzé, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef]
- Guichard, C.; Amaddeo, G.; Imbeaud, S.; Ladeiro, Y.; Pelletier, L.; Maad, I.B.; Calderaro, J.; Bioulac-Sage, P.; Letexier, M.; Degos, F.; et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, L.; Tian, H.; Zeng, Z.; Chen, J.; Huang, D.; Sun, J.; Guo, J.; Cui, H.; Li, Y. Risk Factors and Prevention of Viral Hepatitis-Related Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 686962. [Google Scholar] [CrossRef]
- Miura, S.; Mitsuhashi, N.; Shimizu, H.; Kimura, F.; Yoshidome, H.; Otsuka, M.; Kato, A.; Shida, T.; Okamura, D.; Miyazaki, M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 2012, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Y.; Hu, Z.; Wu, C.; Qian, J.; Jia, W.; Ma, F.; Huang, W.; Yu, L.; Yue, W.; et al. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat. Genet. 2010, 42, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, C.; Xue, R.; Liu, M.; Bai, J.; Bao, J.; Wang, Y.; Jiang, N.; Li, Z.; Wang, W.; et al. Deep whole-genome analysis of 494 hepatocellular carcinomas. Nature 2024, 627, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Nijman, S.M.; Kobayashi, M.; Chan, J.A.; Brunet, J.P.; Chiang, D.Y.; Villanueva, A.; Newell, P.; Ikeda, K.; Hashimoto, M.; et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009, 69, 7385–7392. [Google Scholar] [CrossRef] [PubMed]
- Rebouissou, S.; Nault, J.C. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J. Hepatol. 2020, 72, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, J.; Ziol, M.; Paradis, V.; Zucman-Rossi, J. Molecular and histological correlations in liver cancer. J. Hepatol. 2019, 71, 616–630. [Google Scholar] [CrossRef]
- Lee, J.S.; Heo, J.; Libbrecht, L.; Chu, I.S.; Kaposi-Novak, P.; Calvisi, D.F.; Mikaelyan, A.; Roberts, L.R.; Demetris, A.J.; Sun, Z.; et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat. Med. 2006, 12, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Perugorria, M.J.; Olaizola, P.; Labiano, I.; Esparza-Baquer, A.; Marzioni, M.; Marin, J.J.G.; Bujanda, L.; Banales, J.M. Wnt-β-catenin signalling in liver development, health and disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e23. [Google Scholar] [CrossRef]
- Boyault, S.; Rickman, D.S.; de Reyniès, A.; Balabaud, C.; Rebouissou, S.; Jeannot, E.; Hérault, A.; Saric, J.; Belghiti, J.; Franco, D.; et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007, 45, 42–52. [Google Scholar] [CrossRef]
- International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: A report of the international consensus group for hepatocellular neoplasia. Hepatology 2009, 49, 658–664, Erratum in Hepatology 2009, 49, 1058. [Google Scholar] [CrossRef] [PubMed]
- Boas, F.E.; Kamaya, A.; Do, B.; Desser, T.S.; Beaulieu, C.F.; Vasanawala, S.S.; Hwang, G.L.; Sze, D.Y. Classification of hypervascular liver lesions based on hepatic artery and portal vein blood supply coefficients calculated from triphasic CT scans. J. Digit. Imaging 2015, 28, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Ningarhari, M.; Rebouissou, S.; Zucman-Rossi, J. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Nascimento, E.M.; Gajera, C.R.; Chen, L.; Neuhöfer, P.; Garbuzov, A.; Wang, S.; Artandi, S.E. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature 2018, 556, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218, Erratum in Nat. Commun. 2013, 4, 2577. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Calderaro, J.; Di Tommaso, L.; Balabaud, C.; Zafrani, E.S.; Bioulac-Sage, P.; Roncalli, M.; Zucman-Rossi, J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology 2014, 60, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Chen, L.; Zhang, C.; Fujita, M.; Li, R.; Yan, S.M.; Ong, C.K.; Liao, X.; Gao, Q.; Sasagawa, S. Genomic and Transcriptomic Profiling of Combined Hepatocellular and Intrahepatic Cholangiocarcinoma Reveals Distinct Molecular Subtypes. Cancer Cell 2019, 35, 932–947.e8. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, A.; Calderaro, J.; Paradis, V. Combined hepatocellular-cholangiocarcinoma: An update. J. Hepatol. 2021, 74, 1212–1224. [Google Scholar] [CrossRef]
- Chittmittrapap, S.; Chieochansin, T.; Chaiteerakij, R.; Treeprasertsuk, S.; Klaikaew, N.; Tangkijvanich, P.; Komolmit, P.; Poovorawan, Y. Prevalence of aflatoxin induced p53 mutation at codon 249 (R249s) in hepatocellular carcinoma patients with and without hepatitis B surface antigen (HBsAg). Asian Pac. J. Cancer Prev. 2013, 14, 7675–7679. [Google Scholar] [CrossRef]
- Ghufran, S.M.; Sharma, S.; Ghose, S.; Biswas, S. Context dependent role of p53 during the interaction of hepatocellular carcinoma and endothelial cells. Microvasc. Res. 2022, 142, 104374. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Martin, Y.; Caruso, S.; Hirsch, T.Z.; Bayard, Q.; Calderaro, J.; Charpy, C.; Copie-Bergman, C.; Ziol, M.; Bioulac-Sage, P.; et al. Clinical Impact of Genomic Diversity from Early to Advanced Hepatocellular Carcinoma. Hepatology 2020, 71, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhu, H.; Dong, L.; Shi, W.; Chen, R.; Song, Z.; Huang, C.; Li, J.; Dong, X.; Zhou, Y.; et al. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell 2019, 179, 561–577.e22, Erratum in Cell 2019, 179, 1240. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, Z.; Zhang, Y.; Evert, M.; Calvisi, D.F.; Chen, X. β-Catenin signaling in hepatocellular carcinoma. J. Clin. Investig. 2022, 132, e154515. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lezana, T.; Lopez-Canovas, J.L.; Villanueva, A. Signaling pathways in hepatocellular carcinoma. Adv. Cancer Res. 2021, 149, 63–101. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef] [PubMed]
- Couri, T.; Pillai, A. Goals and targets for personalized therapy for HCC. Hepatol. Int. 2019, 13, 125–137. [Google Scholar] [CrossRef]
- Sawey, E.T.; Chanrion, M.; Cai, C.; Wu, G.; Zhang, J.; Zender, L.; Zhao, A.; Busuttil, R.W.; Yee, H.; Stein, L.; et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell 2011, 19, 347–358. [Google Scholar] [CrossRef]
- Chiang, D.Y.; Villanueva, A.; Hoshida, Y.; Peix, J.; Newell, P.; Minguez, B.; LeBlanc, A.C.; Donovan, D.J.; Thung, S.N.; Solé, M.; et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008, 68, 6779–6788. [Google Scholar] [CrossRef]
- Llovet, J.M.; Peña, C.E.; Lathia, C.D.; Shan, M.; Meinhardt, G.; Bruix, J.; SHARP Investigators Study Group. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2012, 18, 2290–2300. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, H.; Zhang, L.; Zhu, A.X.; Bernards, R.; Qin, W.; Wang, C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ryoo, B.-Y.; Hsu, C.-H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Randomised efficacy and safety results for Atezolizumab(Atezo) + Bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Ann. Oncol. 2019, 30 (Suppl. S5), v851–v934. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990, Erratum in Lancet Oncol. 2021, 22, e347. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Tremelimumab: First Approval. Drugs 2023, 83, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V.; De Toni, E.N.; et al. Phase 3 randomized, open-label, multicenter study of Tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 2022, 40 (Suppl. S4), 379. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564, Erratum in JAMA Oncol. 2021, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2022, 11, 592–596. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Su, G.L.; Altayar, O.; O’Shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef]
- Li, W.; Wu, H.; Xu, X.; Zhang, Y. Comprehensive analysis of genomic and immunological profiles in Chinese and Western hepatocellular carcinoma populations. Aging 2021, 13, 11564–11594. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, M.A.; Chapresto-Garzón, R.; Cadenas, M.; Navarro-Villarán, E.; Negrete, M.; Gómez-Bravo, M.A.; Victor, V.M.; Padillo, F.J.; Muntané, J. Differential effectiveness of tyrosine kinase inhibitors in 2D/3D culture according to cell differentiation, p53 status and mitochondrial respiration in liver cancer cells. Cell Death Dis. 2020, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.R.; Kim, J.Y.; Hong, J.H.; Hur, M.H.; Cho, H.; Park, M.K.; Kim, J.; Lee, Y.B.; Cho, E.J.; Lee, J.H.; et al. Comparison of the outcomes between sorafenib and lenvatinib as the first-line systemic treatment for HBV-associated hepatocellular carcinoma: A propensity score matching analysis. BMC Gastroenterol. 2022, 22, 135. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Tartaglia, N.; Villani, R.; Serviddio, G.; Ramai, D.; Mohan, B.P.; Chandan, S.; Abd El Aziz, M.A.; Evangelista, J.; Cotsoglou, C.; et al. Lenvatinib versus sorafenib as first-line therapy of advanced hepatocellular carcinoma: A systematic review and meta-analysis. Am. J. Transl. Res. 2021, 13, 2379–2387. [Google Scholar] [PubMed]
- Qin, S.; Bi, F.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu, Y.; Meng, Z.; Pan, H.; et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J. Clin. Oncol. 2021, 39, 3002–3011. [Google Scholar] [CrossRef]
- Qin, S.; Li, Q.; Gu, S.; Chen, X.; Lin, L.; Wang, Z.; Xu, A.; Chen, X.; Zhou, C.; Ren, Z.; et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 559–568. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef]
- Ningarhari, M.; Caruso, S.; Hirsch, T.Z.; Bayard, Q.; Franconi, A.; Védie, A.L.; Noblet, B.; Blanc, J.F.; Amaddeo, G.; Ganne, N.; et al. Telomere length is key to hepatocellular carcinoma diversity and telomerase addiction is an actionable therapeutic target. J. Hepatol. 2021, 74, 1155–1166, Erratum in J. Hepatol. 2022, 76, 1242–1243. [Google Scholar] [CrossRef] [PubMed]
- Khemlina, G.; Ikeda, S.; Kurzrock, R. The biology of Hepatocellular carcinoma: Implications for genomic and immune therapies. Mol. Cancer 2017, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Khan, R.; Afzal, M.; Kazmi, I. Oxyphenbutazone promotes cytotoxicity in rats and Hep3B cellsvia suppression of PGE2 and deactivation of Wnt/β-catenin signaling pathway. Mol. Cell Biochem. 2018, 444, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Croy, H.E.; Fuller, C.N.; Giannotti, J.; Robinson, P.; Foley, A.V.A.; Yamulla, R.J.; Cosgriff, S.; Greaves, B.D.; von Kleeck, R.A.; An, H.H.; et al. The Poly(ADP-ribose) Polymerase Enzyme Tankyrase Antagonizes Activity of the β-Catenin Destruction Complex through ADP-ribosylation of Axin and APC2. J. Biol. Chem. 2016, 291, 12747–12760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Jochemsen, A.G. Reactivation of p53 as therapeutic intervention for malignant melanoma. Curr. Opin. Oncol. 2014, 26, 114–119. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.L.; Liang, Y.; Tang, Y.G.; Song, H.X.; Wu, L.L.; Xing, Y.F.; Yan, N.; Li, Y.T.; Wang, Z.Y.; et al. Arsenic Trioxide Rescues Structural p53 Mutations through a Cryptic Allosteric Site. Cancer Cell 2021, 39, 225–239.e8. [Google Scholar] [CrossRef]
- Xie, D.; Shi, J.; Zhou, J.; Fan, J.; Gao, Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: A Chinese perspective. Clin. Mol. Hepatol. 2023, 29, 206–216. [Google Scholar] [CrossRef]
- Pinato, D.J.; Mauri, F.A.; Spina, P.; Cain, O.; Siddique, A.; Goldin, R.; Victor, S.; Pizio, C.; Akarca, A.U.; Boldorini, R.L.; et al. Clinical implications of heterogeneity in PD-L1 immunohistochemical detection in hepatocellular carcinoma: The Blueprint-HCC study. Br. J. Cancer 2019, 120, 1033–1036. [Google Scholar] [CrossRef]
- Hsu, P.C.; Jablons, D.M.; Yang, C.T.; You, L. Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated Protein (YAP) and the Regulation of Programmed Death-Ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC). Int. J. Mol. Sci. 2019, 20, 3821. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952, Erratum in Lancet Oncol. 2018, 19, e440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, Y.; Qu, Q.; Zhu, J.; Liu, Z.; Ning, W.; Zeng, H.; Zhang, N.; Du, W.; Chen, C.; et al. PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int. J. Clin. Oncol. 2017, 22, 1026–1033. [Google Scholar] [CrossRef]
- Nguyen, C.D.K.; Yi, C. YAP/TAZ Signaling and Resistance to Cancer Therapy. Trends Cancer 2019, 5, 283–296. [Google Scholar] [CrossRef]
- Qian, J.; Wang, C.; Wang, B.; Yang, J.; Wang, Y.; Luo, F.; Xu, J.; Zhao, C.; Liu, R.; Chu, Y. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: Hints for glioma anti-PD-1/PD-L1 therapy. J. Neuroinflamm. 2018, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362, eaar3593, Erratum in Science 2019, 363, eaax1384. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Sharma, A.; Seow, J.J.W.; Dutertre, C.A.; Pai, R.; Blériot, C.; Mishra, A.; Wong, R.M.M.; Singh, G.S.N.; Sudhagar, S.; Khalilnezhad, S.; et al. Onco-fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell 2020, 183, 377–394.e21. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Duan, J.; Cui, L.; Zhao, X.; Bai, H.; Cai, S.; Wang, G.; Zhao, Z.; Zhao, J.; Chen, S.; Song, J.; et al. Use of Immunotherapy with Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients with Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Piñero, F.; Dirchwolf, M.; Pessôa, M.G. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells 2020, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Golan, T.; Dahan, L.; Fu, S.; Moreno, V.; Park, K.; Geva, R.; De Braud, F.; Wainberg, Z.A.; Reck, M.; et al. Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: An open-label, phase Ia/b study (JVDJ). Eur. J. Cancer 2020, 137, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P. Ramucirumab: Boon or bane. J. Egypt. Natl. Canc Inst. 2016, 28, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, R.C.; Pestana, R.C.; Miyamura, B.V.; Kaseb, A.O. Hepatocellular Carcinoma Immunotherapy. Annu. Rev. Med. 2022, 73, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.H.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Updated efficacy and safety of KEYNOTE-224: A phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur. J. Cancer 2022, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.Y.; Ren, Z.; et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1399–1410. [Google Scholar] [CrossRef]
- Yang, X.; Chen, B.; Wang, Y.; Wang, Y.; Long, J.; Zhang, N.; Xue, J.; Xun, Z.; Zhang, L.; Cheng, J.; et al. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol. Int. 2023, 17, 709–719. [Google Scholar] [CrossRef]
- Dai, H.; Tong, C.; Shi, D.; Chen, M.; Guo, Y.; Chen, D.; Han, X.; Wang, H.; Wang, Y.; Shen, P. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: A single-arm, open-label, phase II trial. Oncoimmunology 2020, 9, 1846926. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Do, R.K.; Yaqubie, A.; Cleverly, A.; Zhao, Y.; Gueorguieva, I.; Lahn, M.; Benhadji, K.A.; Kelley, R.K.; Abou-Alfa, G.K. Phase 1b study of galunisertib and ramucirumab in patients with advanced hepatocellular carcinoma. Cancer Med. 2021, 10, 3059–3067. [Google Scholar] [CrossRef]
- Thura, M.; Al-Aidaroos, A.Q.; Gupta, A.; Chee, C.E.; Lee, S.C.; Hui, K.M.; Li, J.; Guan, Y.K.; Yong, W.P.; So, J.; et al. PRL3-zumab as an immunotherapy to inhibit tumors expressing PRL3 oncoprotein. Nat. Commun. 2019, 10, 2484, Erratum in Nat. Commun. 2021, 12, 6431. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Cheu, J.W.; Wong, C.C. Mechanistic Rationales Guiding Combination Hepatocellular Carcinoma Therapies Involving Immune Checkpoint Inhibitors. Hepatology 2021, 74, 2264–2276. [Google Scholar] [CrossRef]
- Paik, J. Nivolumab Plus Relatlimab: First Approval. Drugs 2022, 82, 925–931. [Google Scholar] [CrossRef]
- Moroishi, T.; Hayashi, T.; Pan, W.W.; Fujita, Y.; Holt, M.V.; Qin, J.; Carson, D.A.; Guan, K.L. The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 2016, 167, 1525–1539.e17. [Google Scholar] [CrossRef]
- Cho, K.; Ro, S.W.; Lee, H.W.; Moon, H.; Han, S.; Kim, H.R.; Ahn, S.H.; Park, J.Y.; Kim, D.Y. YAP/TAZ Suppress Drug Penetration into Hepatocellular Carcinoma Through Stromal Activation. Hepatology 2021, 74, 2605–2621. [Google Scholar] [CrossRef]
- Yu, M.; Peng, Z.; Qin, M.; Liu, Y.; Wang, J.; Zhang, C.; Lin, J.; Dong, T.; Wang, L.; Li, S.; et al. Interferon-γ induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Mol. Cell 2021, 81, 1216–1230.e9. [Google Scholar] [CrossRef]
- Ni, X.; Tao, J.; Barbi, J.; Chen, Q.; Park, B.V.; Li, Z.; Zhang, N.; Lebid, A.; Ramaswamy, A.; Wei, P.; et al. YAP Is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2018, 8, 1026–1043. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Shi, Y.; Lv, Y.; Yuan, S.; Ramirez, C.F.A.; Lieftink, C.; Wang, L.; Wang, S.; Wang, C.; Dias, M.H.; et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature 2021, 595, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Rosmorduc, O.; Evans, T.R.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Kudo, M.; Erinjeri, J.; Qin, S.; Ren, Z.; Chan, S.; Arai, Y.; Heo, J.; Mai, A.; Escobar, J.; et al. EMERALD-1: A phase 3, randomized, placebo-controlled study of transarterial chemoembolization combined with durvalumab with or without bevacizumab in participants with unresectable hepatocellular carcinoma eligible for embolization. J. Clin. Oncol. 2024, 42 (Suppl. S3), LBA432. [Google Scholar] [CrossRef]
- Sun, H.C.; Zhou, J.; Wang, Z.; Liu, X.; Xie, Q.; Jia, W.; Zhao, M.; Bi, X.; Li, G.; Bai, X.; et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg. Nutr. 2022, 11, 227–252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Song, T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci. Trends 2021, 15, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Huang, C.; Shen, Y.H.; Ji, Y.; Ge, N.L.; Qu, X.D.; Chen, L.; Shi, W.K.; Li, M.L.; Zhu, J.J.; et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer 2021, 10, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Changing the Treatment Paradigm for Hepatocellular Carcinoma Using Atezolizumab plus Bevacizumab Combination Therapy. Cancers 2021, 13, 5475. [Google Scholar] [CrossRef]
- Vitale, A.; Cabibbo, G.; Iavarone, M.; Viganò, L.; Pinato, D.J.; Ponziani, F.R.; Lai, Q.; Casadei-Gardini, A.; Celsa, C.; Galati, G.; et al. Personalised management of patients with hepatocellular carcinoma: A multiparametric therapeutic hierarchy concept. Lancet Oncol. 2023, 24, e312–e322. [Google Scholar] [CrossRef]
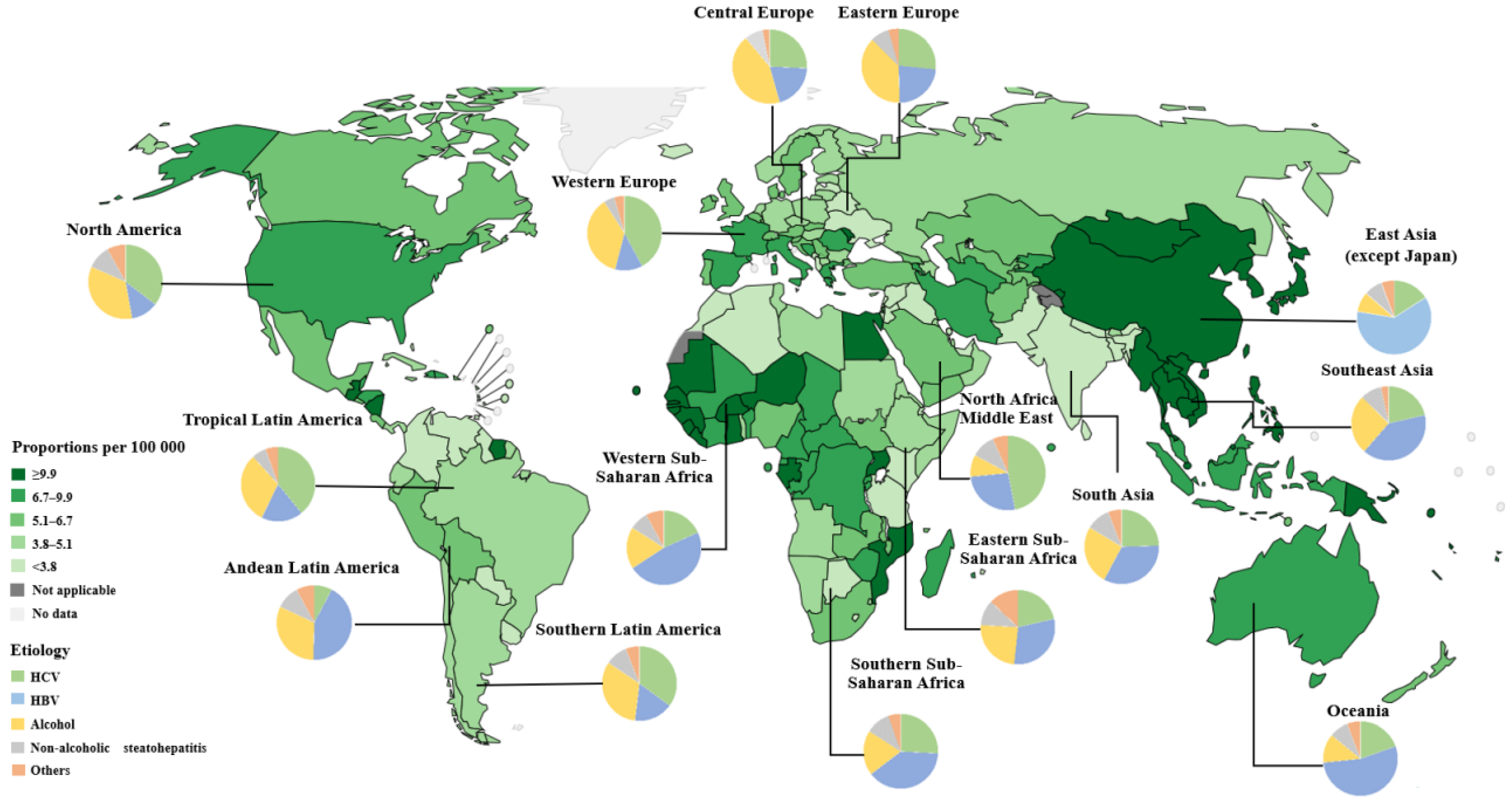
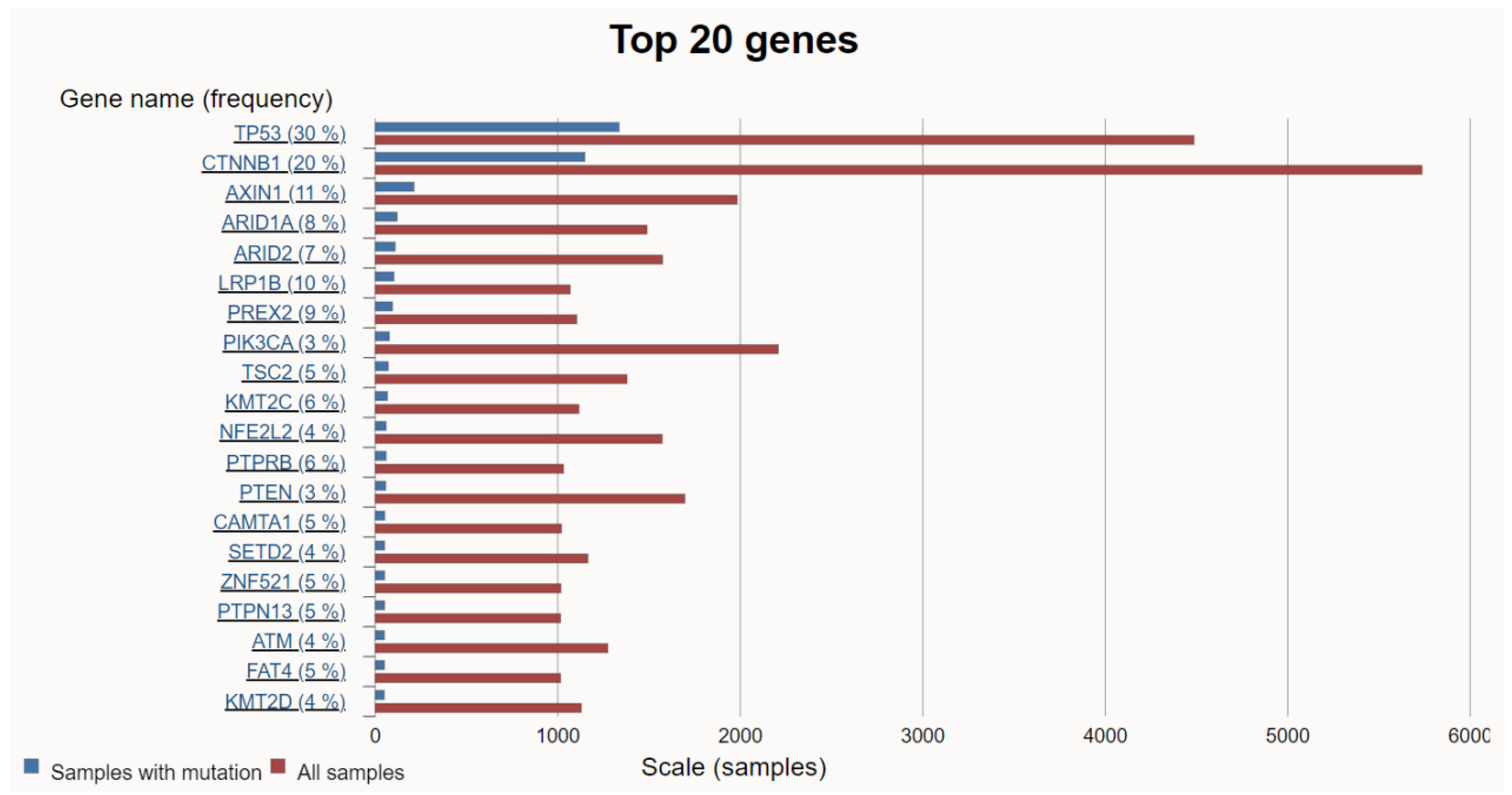
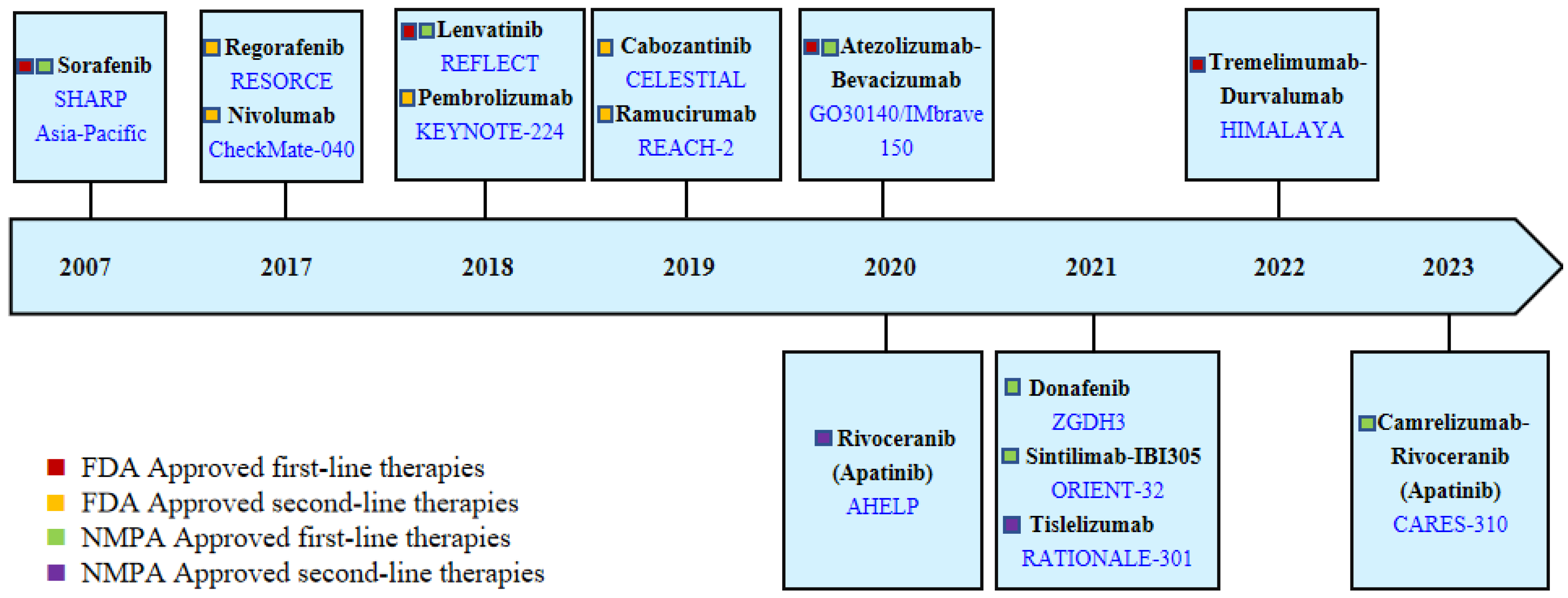
| Treatments (Trial) | Dosing Regimen | Line Setting/ Patients Number | Outcome | TRAE | Remark | ||||
|---|---|---|---|---|---|---|---|---|---|
| mOS (Months) (HR, p-Value) | mPFS (Months) (HR, p-Value) | ORR (%) | CR (%) | ≥Grade 3 (%) | Death/Grade 5 (%) | ||||
| Sorafenib vs. Placebo (SHARP) | KI monotherapy | 1L/602 | 10.7 vs. 7.9 HR 0.69, p < 0.001 | NA | NA | NA | NA | NA | TTP: 5.5 vs. 2.8 months (HR 0.58, p < 0.001); any-grade TRAEs: 80% vs. 52% |
| Sorafenib vs. Placebo (Asia–Pacific) | KI monotherapy | 1L/271 | 6.5 vs. 4.2 HR 0.68, p = 0.014 | NA | 3.3 vs. 1.3 Based on RECIST v1.0 | NA | NA | NA | TTP: 2.8 vs. 1.4 months (HR 0.57, p = 0.0005) |
| Lenvatinib vs. Sorafenib (REFLECT) | KI monotherapy | 1L/954 | 13.6 vs. 12.3 HR 0.92, noninferiority Based on mRECIST | 7.3 vs. 3.6 HR 0.65, p < 0.0001 | 18.8 vs. 6.5 | 0.4 vs. <0.2 | 56.7 vs. 48.6 | 2.3 vs. 0.8 | The mOS of Lenvatinib is noninferior to Sorafenib in the REFLECT trial. However, Lenvatinib has shown a tendency to be superior to Sorafenib, especially in HBV-related and AFP-elevated HCC subgroups. |
| Regorafenib vs. Placebo (RESORCE) | KI monotherapy | 2L/567 | 10.6 vs. 7.8 HR 0.63, p < 0.0001 | 3.4 vs. 1.5 HR 0.43, p < 0.0001 | 6.6 vs. 2.6 | 0.5 vs. 0 | 50.0 vs. 16.6 | 1.9 vs. 1.0 | |
| Cabozantinib vs. Placebo (CELESTIAL) | KI monotherapy | 2L/707 | 10.2 vs. 8.0 HR 0.76, p = 0.005 | 5.2 vs. 1.9 HR 0.44, p < 0.001 | 3.8 vs. <0.4 | 0 vs. 0 | 67.7 vs. 36.3 | 1.3 vs. 0.4 | |
| Ramucirumab vs. Placebo (REACH-2) | anti-VEGFR monotherapy | 2L/292 | 8.5 vs. 7.3 HR 0.71, p = 0.0199 | 2.8 vs. 1.6 HR: 0.45, p < 0.0001 | 4.6 vs. 1.1 | NA | NA | 1.% vs. 0 | Only recommended for patients with AFP ≥ 400 ng/mL. |
| Nivolumab vs. Sorafenib (CheckMate459) | anti-PD-1 monotherapy | 1L/743 | 16.4 vs. 14.7 HR: 0.85, p = 0.075 | 3.7 vs. 3.8 HR:0.93 p value NA | 15.4 vs. 6.9 | 3.8 vs. 1.3 | 22.3 vs. 49.6 | NA | Based on CheckMate 040, Nivolumab was approved by the FDA as a second-line treatment for advanced HCC. However, the indication of Nivolumab as a monotherapy for second-line treatment of advanced HCC has been withdrawn due to the failure of CheckMate 459. |
| Pembrolizumab vs. Sorafenib (KEYNOTE-394) | anti-PD-1 monotherapy | 2L/453 | 14.6 vs. 13.0 HR 0.79, p = 0.0180 | 2.6 vs. 2.3 HR 0.74, p = 0.0032 | 13.7 vs. 1.3 | 2.0 vs. 0.7 | 14.4 vs. 5.9 | 1.0 vs. 0 | Pembrolizumab was approved by the FDA for advanced HCC second-line treatments based on KEYNOTE-224, while KEYNOTE-394 is the updated support trial. |
| Atezolizumab–Bevacizumab Vs. Sorafenib (IMbrave150) | anti-PD-L1 and anti-VEGF | 1L/501 | 19.2 vs. 13.4 HR 0.66, p < 0.001 | 6.9 vs. 4.3 HR 0.65, p < 0.001 | 29.8 vs. 11.3 | 7.7 vs. 0.6 | 43.5 vs. 46.2 | 1.8 vs. 0.6 | Patients should have adequate endoscopic evaluation and management for esophageal varices before administration. |
| Tremelimumab–Durvalumab vs. Sorafenib (HIMALAYA) | anti-CTLA-4 and anti-PD-L1 | 1L/1171 | 16.4 vs. 13.8 HR: 0.78, p = 0.0035 | 3.8 vs. 4.1 HR:0.90, p value NA | 20.1 vs. 5.1 | 3.1 vs. 0 | 25.8 vs. 36.9 | 2.3 vs. 0.8 | |
| Durvalumab vs. Sorafenib (HIMALAYA) | anti-PD-L1 monotherapy | 1L/1171 | 16.6 vs. 13.8 HR:0.86, noninferiority margin 1.08 | 3.7 vs. 4.1 HR:1.02, p value NA | 17.0 vs. 5.1 | 1.5 vs. 0 | 12.9 vs. 36.9 | 0 vs. 0.8 | |
| Treatments (Trial) | Dosing Regimen | Line Setting/ Patients Number | Outcome | TRAE | Remark | ||||
|---|---|---|---|---|---|---|---|---|---|
| mOS (Months) (HR, p Value) | mPFS (Months) (HR, p Value) | ORR (%) | CR (%) | ≥Grade 3 (%) | Death/Grade 5 (%) | ||||
| Rivoceranib vs. Placebo (AHELP) | anti-VEGFR monotherapy | 2L/400 | 8.7 vs. 6.8 HR 0.785, p = 0·048 | 4.5 vs. 1.9 HR 0·471, p <0·0001; | 10.7 vs. 1.5 | 0 vs. 0 | 77.4 vs. 19.2 | 0 vs. 0 | The study population is limited to China |
| Donafenib vs. Sorafenib (ZGDH3) | KI monotherapy | 1L/668 | 12.1 vs. 10.3 HR 0.83, p = 0.0245 | 3.7 vs. 3.6 HR 0.91, p = 0.057 | 4.6 vs. 2.7 | 0.3 vs. 0 | 37.5 vs. 49.7 | 1.8 vs. 3.6 | The study population is limited to China |
| Tislelizumab vs. Sorafenib (RATIONALE-301) | anti-PD-1 monotherapy | 1L/674 | 15.9 vs. 14.1 HR: 0.85, p = 0.0398 | 2.2 vs. 3.6 HR: 1.11, p value NA | 14.3 vs. 5.4 | 2.9 vs. 0.3 | 22.2 vs. 53.4 | 4.4 vs. 5.2 | |
| Sintilimab + IBI305 vs. Sorafenib (ORIENT-32) | anti-PD-1 and anti-VEGF | 1L/571 | NE vs. 10.4 HR 0.57, p < 0.0001 | 4.6 vs. 2.8 HR 0.56, p < 0.0001 | 20.5 vs. 4.1 | 0 vs. 0 | 33.7 vs. 35.7 | 1.6 vs. 1.0 | The study population is limited to China |
| Camrelizumab–Rivoceranib vs. Sorafenib (CARES-310) | anti-PD-1 and anti-VEGFR | 1L/543 | 22.1 vs. 15.2 HR 0.62, p < 0.0001 | 5.6 vs. 3.7 HR 0.52, p < 0.0001 | 25.4 vs. 5.9 | 1.1 vs. 0.4 | 80.9 vs. 52.4 | 0.4 vs. 0.4 | |
| (HIMALAYA) | (IMbrave150) | (CARES-310) | LEAP 002 | ||
|---|---|---|---|---|---|
| Immunotherapy Combination Strategies | Dual-Immunotherapy (Anti-CTLA-4 and Anti-PD-L1) | ICIs and Anti-VEGF/VEGFR (Anti-PD-L1 and Anti-VEGF) | ICIs and Anti-VEGF/VEGFR (Anti-PD-1 and Anti-VEGFR) | ICIs and KIs (Anti-PD-1 And KI) | |
| Treatments | Tremelimumab–Durvalumab | Atezolizumab–Bevacizumab | Camrelizumab–Rivoceranib | Pembrolizumab–Lenvatinib | |
| Control agent | Sorafenib | Sorafenib | Sorafenib | Lenvatinib | |
| Endpoints and results (RECIST 1.1) | Primary endpoint: mOS STRIDE arm was superior to Sorafenib; Durvalumab monotherapy was noninferior to Sorafenib. | Primary endpoint: mOS and mPFS Both meet with statistical significance. | Primary endpoint: mOS and mPFS Both meet with statistical significance. | Primary endpoint: mOS and mPFS Neither meets the prespecified statistical significance. | |
| Outcome | mOS (months) (HR, p value) | STRIDE vs. Sorafenib: 16.43 vs. 13.77 HR: 0.78, p = 0.0035 Durvalumab vs. Sorafenib: 16.56 vs. 13.77 HR:0.86, noninferiority margin 1.08 | 19.2 vs. 13.4 HR: 0.66 p = 0.0009 | 22.1 vs. 15.2 HR: 0.62 p < 0.0001 | 21.2 vs. 19.0 HR: 0.840 p = 0.0227 |
| mPFS (months) (HR, p value) | STRIDE vs. Durvalumab vs. Sorafenib: 3.78 vs. 3.65 vs. 4.07 p value NA | 6.9 vs. 4.3 HR: 0.65 p = 0.0001 | 5.6 vs. 3.7 HR: 0.52 p < 0.0001 | 8.2 vs. 8.0 HR: 0.867 p = 0.0446 | |
| ORR (%) | STRIDE vs. Durvalumab vs. Sorafenib: 20.1 vs. 17.0 vs. 5.1 | 29.8 vs. 11.3 | 25.4 vs. 5.9 | 26.1 vs. 17.5 | |
| CR (%) | STRIDE vs. Durvalumab vs. Sorafenib: 3.1 vs. 1.5 vs. 0 | 7.7 vs. 0.6 | 1.1 vs. 0.4 | 1.5 vs. 1.5 | |
| PR (%) | STRIDE vs. Durvalumab vs. Sorafenib: 17.0 vs. 15.4 vs. 5.1 | 22.1 vs. 10.7 | 24.3 vs. 5.5 | 24.6 vs. 16.0 | |
| SD (%) | STRIDE vs. Durvalumab vs. Sorafenib: 39.9 vs. 37.8 vs. 55.5 | 44.2 vs. 43.4 | 52.9 vs. 48.0 | 55.2 vs. 60.9 | |
| DCR (%) | STRIDE vs. Durvalumab vs. Sorafenib 60.1 vs. 54.8 vs. 60.7 | 73.9 vs. 54.7 | 78.3 vs. 53.9 | 81.3 vs. 78.4 | |
| DoR (months) | STRIDE:22.34 STRIDE: long-tail effect; 3-year survival rate was 30.7% | 18.1 | 14.8 | 16.6 | |
| Safety profile | TRAE ≥Grade 3 (%) | STRIDE vs. Durvalumab vs. Sorafenib: 25.8 vs. 12.9 vs. 36.9 | 43.5 vs. 46.2 | 80.9 vs. 52.4 | 61.5 vs. 56.7 |
| Discontinuation (%) | STRIDE vs. Durvalumab vs. Sorafenib: 8.2 vs. 4.1 vs. 11.0 | 15.5 vs. 10.3 | 24.3 vs. 4.5 | 18.0 vs. 10.6 | |
| TRAE Grade 5/Death (%) | STRIDE vs. Durvalumab vs. Sorafenib: 2.3 vs. 0 vs. 0.8 | 1.8 vs. 0.6 | 0.4 vs. 0.4 | 1.0 vs. 0.8 | |
| Remark | The success of the HIMALAYA trial transformed the theoretical advantages of dual-immunotherapy treatment into clinical benefits. STRIDE regimen is a successful paradigm of sequential combination therapy. Dual-immunotherapy treatment shows an excellent DoR, a long-tail effect, and lower toxicity. | The IMbrave150 trial established the anti-PD-L1/PD-1 inhibitor and anti-VEGF/VEGFR combination strategies as the first-line recommendation for advanced HCC. Atezolizumab–Bevacizumab combination therapy significantly improved mOS of patients with portal vein invasion at the main portal branch (Vp4). | The CARES-310 trial reached the longest mOS in clinical trials for advanced HCC systemic treatment agents. Comparison of HR between trials shows anti-PD-1/PD-L1 and TKI/anti-VEGF have better ORR and PD outcomes than dual-immunotherapy regimens. | The LEAP 002 trial is the sole clinical trial with a double-blind design in this table, and it is the sole clinical trial that used Lenvatinib as the control agent in this table. Pembrolizumab–Lenvatinib therapy shows a clear tendency of OS benefit, although the prespecified significance endpoint is not met. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Chan, S.W.; Liu, F.; Liu, J.; Chow, P.K.H.; Toh, H.C.; Hong, W. Hepatocellular Carcinoma: Current Drug Therapeutic Status, Advances and Challenges. Cancers 2024, 16, 1582. https://doi.org/10.3390/cancers16081582
Zheng S, Chan SW, Liu F, Liu J, Chow PKH, Toh HC, Hong W. Hepatocellular Carcinoma: Current Drug Therapeutic Status, Advances and Challenges. Cancers. 2024; 16(8):1582. https://doi.org/10.3390/cancers16081582
Chicago/Turabian StyleZheng, Shunzhen, Siew Wee Chan, Fei Liu, Jun Liu, Pierce Kah Hoe Chow, Han Chong Toh, and Wanjin Hong. 2024. "Hepatocellular Carcinoma: Current Drug Therapeutic Status, Advances and Challenges" Cancers 16, no. 8: 1582. https://doi.org/10.3390/cancers16081582





