Causes and Risk Factors of Breast Cancer, What Do We Know for Sure? An Evidence Synthesis of Systematic Reviews and Meta-Analyses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Search Strategy
2.3. Data Extraction
2.4. Quality Appraisal of the Studies
2.5. Analyses
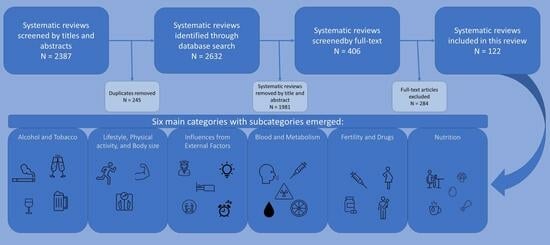
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Cancer Observatory. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/20-breast-fact-sheet.pdf (accessed on 25 March 2024).
- WHO. Breast-Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 25 March 2024).
- Globocan. Breast Cancer Incidence Rates Breast Female, Fact Sheets. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf (accessed on 25 March 2024).
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Cumber, S.N.; Nchanji, K.N.; Tsoka-Gwegweni, J.M. Breast cancer among women in sub-Saharan Africa: Prevalence and a situational analysis. South. Afr. J. Gynaecol. Oncol. 2017, 9, 35–37. [Google Scholar] [CrossRef]
- Francies, F.Z.; Hull, R.; Khanyile, R.; Dlamini, Z. Breast cancer in low-middle income countries: Abnormality in splicing and lack of targeted treatment options. Am. J. Cancer Res. 2020, 10, 1568–1591. [Google Scholar] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Deshmukh, R. Breast cancer: An insight into its inflammatory, molecular, pathological and targeted facets with update on investigational drugs. Crit. Rev. Oncol. Hematol. 2020, 154, 103070. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.L.; Ezzati, M. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Rasanayagam, S.; Engel, C.; Rizzo, J. State of the evidence 2017: An update on the connection between breast cancer and the environment. Environ. Health 2017, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Golubnitschaja, O.; Debald, M.; Yeghiazaryan, K.; Kuhn, W.; Pešta, M.; Costigliola, V.; Grech, G. Breast cancer epidemic in the early twenty-first century: Evaluation of risk factors, cumulative questionnaires and recommendations for preventive measures. Tumour Biol. 2016, 37, 12941–12957. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef]
- Marmot, M.; Allen, J.; Bell, R.; Bloomer, E.; Goldblatt, P. WHO European review of social determinants of health and the health divide. Lancet 2012, 380, 1011–1029. [Google Scholar] [CrossRef]
- Carlsen, K.; Høybye, M.T.; Dalton, S.O.; Tjønneland, A. Social inequality and incidence of and survival from breast cancer in a population-based study in Denmark, 1994-2003. Eur. J. Cancer 2008, 44, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res. Treat. 2019, 177, 537–548. [Google Scholar] [CrossRef]
- Elstad, J.I.; Torstensrud, R.; Lyngstad, T.H.; Kravdal, Ø. Trends in educational inequalities in mortality, seven types of cancers, Norway 1971–2002. Eur. J. Public Health 2011, 22, 771–776. [Google Scholar] [CrossRef]
- Lundqvist, A.; Andersson, E.; Ahlberg, I.; Nilbert, M.; Gerdtham, U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe—A systematic review and meta-analysis. Eur. J. Public Health 2016, 26, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Menvielle, G.; Kunst, A.E.; van Gils, C.H.; Peeters, P.H.; Boshuizen, H.; Overvad, K.; Olsen, A.; Tjonneland, A.; Hermann, S.; Kaaks, R.; et al. The contribution of risk factors to the higher incidence of invasive and in situ breast cancers in women with higher levels of education in the European prospective investigation into cancer and nutrition. Am. J. Epidemiol. 2011, 173, 26–37. [Google Scholar] [CrossRef]
- Trewin, C.B.; Hjerkind, K.V.; Johansson, A.L.V.; Strand, B.H.; Kiserud, C.E.; Ursin, G. Socioeconomic inequalities in stage-specific breast cancer incidence: A nationwide registry study of 1.1 million young women in Norway, 2000–2015. Acta Oncol. 2020, 59, 1284–1290. [Google Scholar] [CrossRef]
- Abel, M.H.; Totland, T.H. Diet and Self-Reported Weight and Weight-Change Based on Data from the National Public Health Survey 2020; Norwegian Institute of Publich Health: Oslo, Norway, 2021; pp. 1–97. Available online: https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2021/rapport-nhus-2020.pdf (accessed on 28 March 2024).
- Dumalaon-Canaria, J.A.; Hutchinson, A.D.; Prichard, I.; Wilson, C. What causes breast cancer? A systematic review of causal attributions among breast cancer survivors and how these compare to expert-endorsed risk factors. Cancer Causes Control 2014, 25, 771–785. [Google Scholar] [CrossRef]
- LoConte, N.K.; Else-Quest, N.M.; Eickhoff, J.; Hyde, J.; Schiller, J.H. Assessment of guilt and shame in patients with non-small-cell lung cancer compared with patients with breast and prostate cancer. Clin. Lung Cancer 2008, 9, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Polit, D.F.; Beck, C.T. Nursing Research: Generating and Assessing Evidence for Nursing Practice, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2017. [Google Scholar]
- Brurberg, K. Meta-Analysis. Available online: https://www.helsebiblioteket.no/kunnskapsbasert-praksis/kritisk-vurdering/metaanalyse (accessed on 25 March 2024).
- Smedslund, G. Metaanalyse. Nor. Epidemiol. 2013, 23, 147–149. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- He, C.; Anand, S.T.; Ebell, M.H.; Vena, J.E.; Robb, S.W. Circadian disrupting exposures and breast cancer risk: A meta-analysis. Int. Arch. Occup. Environ. Health 2015, 88, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, B.B.; Tergas, A.I.; Mateen, F.J.; Bhayani, N.H.; Oh, J. Night-shift work and risk of breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 138, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yeung, K.L.; Chan, W.C.; Kwok, C.C.H.; Leung, S.L.; Wu, C.; Chan, E.Y.Y.; Yu, I.T.S.; Yang, X.R.; Tse, L.A. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann. Oncol. 2013, 24, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Lu, Y.; Wu, K.; Lin, Q.; Shen, W.; Zhu, M.; Huang, S.; Chen, J. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013, 37, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhou, Y.; Zhang, X.; Wei, X.; He, J. Sleep duration and breast cancer risk: A meta-analysis of observational studies. Int. J. Cancer. 2014, 134, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Sun, H.; Huang, J.; Yin, S.; Hou, W.; Zhang, J.; Wang, Y.; Xu, Y.; Xu, H. Long-Term Sleep Duration as a Risk Factor for Breast Cancer: Evidence from a Systematic Review and Dose-Response Meta-Analysis. BioMed Res. Int. 2017, 2017, 4845059. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Deng, Q.; Fan, W.Y.; Wang, W.Y.; Wang, X. Light exposure at night, sleep duration, melatonin, and breast cancer: A dose-response analysis of observational studies. Eur. J. Cancer Prev. 2014, 23, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, X.; Zhong, M.; Yu, Z. Extremely low-frequency electromagnetic fields exposure and female breast cancer risk: A meta-analysis based on 24,338 cases and 60,628 controls. Breast Cancer Res. Treat. 2010, 123, 569–576. [Google Scholar] [CrossRef]
- Zhao, G.; Lin, X.; Zhou, M.; Zhao, J. Relationship between exposure to extremely low-frequency electromagnetic fields and breast cancer risk: A meta-analysis. Eur. J. Gynaecol. Oncol. 2014, 35, 264–269. [Google Scholar]
- Zhang, J.; Huang, Y.; Wang, X.; Lin, K.; Wu, K. Environmental Polychlorinated Biphenyl Exposure and Breast Cancer Risk: A Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0142513. [Google Scholar] [CrossRef]
- Park, J.H.; Cha, E.S.; Ko, Y.; Hwang, M.S.; Hong, J.H.; Lee, W.J. Exposure to dichlorodiphenyltrichloroethane and the risk of breast cancer: A systematic review and meta-analysis. Osong Public Health Res. Perspect. 2014, 5, 77–84. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, C.; Zhong, Y.; Huang, X.; Peng, L.; Shan, G.; Wang, K.; Sun, Q. Striking life events associated with primary breast cancer susceptibility in women: A meta-analysis study. J. Exp. Clin. Cancer Res. CR 2013, 32, 53. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.L.; Horta, B.L.; Amaral, J.J.F.d.; Fernandes, P.F.C.B.C.; Galvao, C.M.; Fernandes, A.F.C. Association between stress and breast cancer in women: A meta-analysis. Cad. De Saude Publica 2009, 25 (Suppl. 3), S453–S463. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, V.F.; Baban, A.; Dumitrascu, D.L. Psychological stress and breast cancer incidence: A systematic review. Clujul Med. 2018, 91, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bahri, N.; Fathi Najafi, T.; Homaei Shandiz, F.; Tohidinik, H.R.; Khajavi, A. The relation between stressful life events and breast cancer: A systematic review and meta-analysis of cohort studies. Breast Cancer Res. Treat. 2019, 176, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Verbeek, J.; Seidler, A.; Lindbohm, M.-L.; Ojajarvi, A.; Orsini, N.; Costa, G.; Neuvonen, K. Night-shift work and breast cancer—A systematic review and meta-analysis. Scand. J. Work. Environ. Health 2013, 39, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, C.; Liu, C. The incidence of breast cancer among female flight attendants: An updated meta-analysis. J. Travel. Med. 2016, 23, taw055. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Ji, Y.; Ren, M.; Pang, B.; Chu, M.; Wei, L. Inconclusive role of human papillomavirus infection in breast cancer. Infect. Agent. Cancer 2015, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, D.; McGlynn, K.A.; Garcia-Closas, M.; Kim, Y.; Yoo, K.Y.; Brinton, L.A. Intrauterine environments and breast cancer risk: Meta-analysis and systematic review. Breast Cancer Res. BCR 2008, 10, R8. [Google Scholar] [CrossRef]
- Xu, X.; Dailey, A.B.; Peoples-Sheps, M.; Talbott, E.O.; Li, N.; Roth, J. Birth weight as a risk factor for breast cancer: A meta-analysis of 18 epidemiological studies. J. Womens Health 2009, 18, 1169–1178. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Y.; Yang, L.; Xie, Z.; Song, S.; Yin, J.; Kuang, L.; Qin, W. Association between abortion and breast cancer: An updated systematic review and meta-analysis based on prospective studies. Cancer Causes Control CCC 2015, 26, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kang, E.J.; Woo, O.H.; Park, K.H.; Woo, S.U.; Yang, D.S.; Kim, A.-R.; Lee, J.-B.; Kim, Y.H.; Kim, J.S.; et al. The relationship between preeclampsia, pregnancy-induced hypertension and maternal risk of breast cancer: A meta-analysis. Acta Oncol. 2013, 52, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Fan, Y.; Hou, Y.; Fan, Y. Preeclampsia and maternal risk of breast cancer: A meta-analysis of cohort studies. J. Matern. Fetal Neonatal Med. 2018, 31, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Unar-Munguia, M.; Torres-Mejia, G.; Colchero, M.A.; Gonzalez de Cosio, T. Breastfeeding Mode and Risk of Breast Cancer: A Dose-Response Meta-Analysis. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2017, 33, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Hutcheon, J.A.; Julien, S.G.; Tremblay, M.L.; Fuhrer, R. Insufficient milk supply and breast cancer risk: A systematic review. PLoS ONE 2009, 4, e8237. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Costa, M.; Puntoni, M.; Paleari, L.; De Censi, A.; Sormani, M.P.; Provinciali, N.; Bruzzi, P. Breast cancer incidence after hormonal treatments for infertility: Systematic review and meta-analysis of population-based studies. Breast Cancer Res. Treat. 2015, 150, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Zreik, T.G.; Mazloom, A.; Chen, Y.; Vannucci, M.; Pinnix, C.C.; Fulton, S.; Hadziahmetovic, M.; Asmar, N.; Munkarah, A.R.; Ayoub, C.M.; et al. Fertility drugs and the risk of breast cancer: A meta-analysis and review. Breast Cancer Res. Treat. 2010, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Diamantaras, A.A.; Perlepe, C.; Kanavidis, P.; Skalkidou, A.; Petridou, E.T. IVF and breast cancer: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Kim, E.H. Hormone replacement therapy and risk of breast cancer in Korean women: A quantitative systematic review. J. Prev. Med. Public Health 2015, 48, 225–230. [Google Scholar] [CrossRef]
- Kim, S.; Ko, Y.; Lee, H.J.; Lim, J.-e. Menopausal hormone therapy and the risk of breast cancer by histological type and race: A meta-analysis of randomized controlled trials and cohort studies. Breast Cancer Res. Treat. 2018, 170, 667–675. [Google Scholar] [CrossRef]
- Samson, M.; Porter, N.; Orekoya, O.; Hebert, J.R.; Adams, S.A.; Bennett, C.L.; Steck, S.E. Progestin and breast cancer risk: A systematic review. Breast Cancer Res. Treat. 2016, 155, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Asi, N.; Mohammed, K.; Haydour, Q.; Gionfriddo, M.R.; Vargas, O.L.; Prokop, L.J.; Faubion, S.S.; Murad, M.H. Progesterone vs. synthetic progestins and the risk of breast cancer: A systematic review and meta-analysis. Syst. Rev. 2016, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Stute, P.; Wildt, L.; Neulen, J. The impact of micronized progesterone on breast cancer risk: A systematic review. Climacteric 2018, 21, 111–122. [Google Scholar] [CrossRef]
- Walker, K.; Bratton, D.J.; Frost, C. Premenopausal endogenous oestrogen levels and breast cancer risk: A meta-analysis. Br. J. Cancer 2011, 105, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lei, X.; Feng, J.; Wang, Y. Oral contraceptive use and risk of breast cancer: A meta-analysis of prospective cohort studies. Eur. J. Contracept. Reprod. Health Care Off. J. Eur. Soc. Contracept. 2012, 17, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Tio, M.; Andrici, J.; Eslick, G.D. Folate intake and the risk of breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2014, 145, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Shi, W.W.; Gao, H.F.; Zhou, L.; Hou, A.J.; Zhou, Y.H. Folate intake and the risk of breast cancer: A dose-response meta-analysis of prospective studies. PLoS ONE 2014, 9, e100044. [Google Scholar] [CrossRef] [PubMed]
- Takkouche, B.; Regueira-Mendez, C.; Etminan, M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: A meta-analysis. J. Natl. Cancer Inst. 2008, 100, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Zagouri, F.; Zografos, G.C. Is antibiotic use a risk factor for breast cancer? A meta-analysis. Pharmacoepidemiol. Drug Saf. 2010, 19, 1101–1107. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, X.; Qin, A.; Liu, G.; Zhai, Z.; Hao, Y.; Li, H.; Zhu, Z.; Dai, K. Bone mineral density and risk of breast cancer in postmenopausal women. Breast Cancer Res. Treat. 2013, 138, 261–271. [Google Scholar] [CrossRef]
- Chen, J.H.; Yuan, Q.; Ma, Y.N.; Zhen, S.H.; Wen, D.L. Relationship between bone mineral density and the risk of breast cancer: A systematic review and dose-response meta-analysis of ten cohort studies. Cancer Manag. Res. 2019, 11, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, G.; Ndetan, H.; Das, D.N.; Gupta, G.; Suryavanshi, M.; Mehta, A.; Singh, K.P. Reproductive factors and breast cancer risk: A meta-analysis of case-control studies in Indian women. South Asian J. Cancer 2019, 8, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.W.; Jing, C.X.; Zhuang, S.L.; Pan, W.C.; Hu, X.P. Effect of age at first use of oral contraceptives on breast cancer risk: An updated meta-analysis. Medicine 2019, 98, e15719. [Google Scholar] [CrossRef] [PubMed]
- Macacu, A.; Autier, P.; Boniol, M.; Boyle, P. Active and passive smoking and risk of breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 154, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, Y.-B.; Liu, X.-O.; Gao, Y.; Dai, H.-J.; Song, F.-J.; Li, W.-Q.; Wang, J.; Yan, Y.; Wang, P.-S.; et al. Active and passive smoking with breast cancer risk for Chinese females: A systematic review and meta-analysis. Chin. J. Cancer 2014, 33, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Sadri, G.; Mahjub, H. Passive or active smoking, which is more relevant to breast cancer. Saudi Med. J. 2007, 28, 254–258. [Google Scholar] [PubMed]
- Miller, M.D.; Marty, M.A.; Broadwin, R.; Johnson, K.C.; Salmon, A.G.; Winder, B.; Steinmaus, C.; California Environmental Protection Agency. The association between exposure to environmental tobacco smoke and breast cancer: A review by the California Environmental Protection Agency. Prev. Med. 2007, 44, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shao, J.; Gao, X.; Li, X. Effect of passive smoking on female breast cancer in China: A meta-analysis. Asia-Pac. J. Public Health/Asia-Pac. Acad. Consort. Public Health 2015, 27, NP58–NP64. [Google Scholar] [CrossRef] [PubMed]
- DeRoo, L.A.; Cummings, P.; Mueller, B.A. Smoking before the first pregnancy and the risk of breast cancer: A meta-analysis. Am. J. Epidemiol. 2011, 174, 390–402. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhu, H.C.; Guo, Q.; Shu, Z.; Bao, X.H.; Sun, F.; Qin, Q.; Yang, X.; Zhang, C.; Cheng, H.Y.; et al. Dose-Dependent Associations between Wine Drinking and Breast Cancer Risk—Meta-Analysis Findings. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 1221–1233. [Google Scholar] [CrossRef]
- Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Wakai, K.; Inoue, M.; Tsugane, S.; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Alcohol drinking and breast cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2007, 37, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, H.; MacInnis, R.J.; Room, R.; English, D.R. Long-Term Alcohol Consumption and Breast, Upper Aero-Digestive Tract and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis. Alcohol Alcohol. 2016, 51, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, F.; Skrip, L.; Wang, Y.; Liu, S. Lack of an Association between Passive Smoking and Incidence of Female Breast Cancer in Non-Smokers: Evidence from 10 Prospective Cohort Studies. PLoS ONE 2013, 8, e77029. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, D.; Kang, S. Physical activity and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2013, 137, 869–882. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Zhang, Y.; Xie, Q.; Tan, X. Physical Activity and Risk of Breast Cancer: A Meta-Analysis of 38 Cohort Studies in 45 Study Reports. Value Health 2019, 22, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Neilson, H.K.; Farris, M.S.; Stone, C.R.; Vaska, M.M.; Brenner, D.R.; Friedenreich, C.M. Moderate-vigorous recreational physical activity and breast cancer risk, stratified by menopause status: A systematic review and meta-analysis. Menopause 2017, 24, 322–344. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, H.; Peng, C. Association of sedentary behavior with the risk of breast cancer in women: Update meta-analysis of observational studies. Ann. Epidemiol. 2015, 25, 687–697. [Google Scholar] [CrossRef]
- Cheraghi, Z.; Poorolajal, J.; Hashem, T.; Esmailnasab, N.; Doosti Irani, A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: A meta-analysis. PLoS ONE 2012, 7, e51446. [Google Scholar] [CrossRef]
- Xia, X.; Chen, W.; Li, J.; Chen, X.; Rui, R.; Liu, C.; Sun, Y.; Liu, L.; Gong, J.; Yuan, P. Body mass index and risk of breast cancer: A nonlinear dose-response meta-analysis of prospective studies. Sci. Rep. 2014, 4, 7480. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Zhou, Q.; Imam, M.U.; Cai, J.; Wang, Y.; Qi, M.; Sun, P.; Ping, Z.; Fu, X. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: A dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health 2017, 17, 936. [Google Scholar] [CrossRef]
- Sexton, K.R.; Franzini, L.; Day, R.S.; Brewster, A.; Vernon, S.W.; Bondy, M.L. A review of body size and breast cancer risk in Hispanic and African American women. Cancer 2011, 117, 5271–5281. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Yang, C.M.; Shi, B.M. Body fatness at a young age, body fatness gain and risk of breast cancer: Systematic review and meta-analysis of cohort studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Zahmatkesh, B.H.; Alavi, N.; Keramat, A.; Khosravi, A.; Chaman, R. Body Mass Index and Risk of Breast Cancer: A Systematic Review and Meta-Analysis in Iran. Int. J. Cancer Manag. 2017, 10, e5921. [Google Scholar] [CrossRef]
- Jansen, L.A.; Backstein, R.M.; Brown, M.H. Breast size and breast cancer: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 1615–1623. [Google Scholar] [CrossRef]
- Bae, J.M.; Kim, E.H. Breast Density and Risk of Breast Cancer in Asian Women: A Meta-analysis of Observational Studies. J. Prev. Med. Public Health 2016, 49, 367–375. [Google Scholar] [CrossRef]
- Nelson, H.D.; Zakher, B.; Cantor, A.; Fu, R.; Griffin, J.; O’Meara, E.S.; Buist, D.S.; Kerlikowske, K.; van Ravesteyn, N.T.; Trentham-Dietz, A.; et al. Risk factors for breast cancer for women aged 40 to 49 years: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Namiranian, N.; Moradi-Lakeh, M.; Razavi-Ratki, S.K.; Doayie, M.; Nojomi, M. Risk factors of breast cancer in the Eastern Mediterranean Region: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 9535–9541. [Google Scholar] [CrossRef] [PubMed]
- Namazi, N.; Irandoost, P.; Heshmati, J.; Larijani, B.; Azadbakht, L. The association between fat mass and the risk of breast cancer: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 1496–1503. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 2019, 30, 1183–1200. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Chen, S.J.; Zhang, R.; Hidayat, K.; Qin, J.B.; Zhang, Y.S.; Qin, L.Q. Central obesity and risks of pre- and postmenopausal breast cancer: A dose-response meta-analysis of prospective studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016, 17, 1167–1177. [Google Scholar] [CrossRef]
- Li, J.; Zou, L.; Chen, W.; Zhu, B.; Shen, N.; Ke, J.; Lou, J.; Song, R.; Zhong, R.; Miao, X. Dietary mushroom intake may reduce the risk of breast cancer: Evidence from a meta-analysis of observational studies. PLoS ONE 2014, 9, e93437. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Seely, D.; McGowan, J.; Skidmore, B.; Fernandes, R.; Kennedy, D.A.; Cooley, K.; Wong, R.; Sagar, S.; Balneaves, L.G.; et al. Black cohosh and breast cancer: A systematic review. Integr. Cancer Ther. 2014, 13, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-C.; Dong, D.-S.; Bai, Y.; Xia, P. Meta-analysis of black tea consumption and breast cancer risk: Update 2013. Nutr. Cancer 2014, 66, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Qu, K.; Jiang, Z.; Yang, X.; Gao, P. Egg consumption and breast cancer risk: A meta-analysis. Breast Cancer 2014, 21, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhou, B.; Wang, B.; Yu, R. Coffee consumption and risk of breast cancer: A metaanalysis. Am. J. Obstet. Gynecol. 2009, 200, 290. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wei, W.; Zhan, L. Red and processed meat intake and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2015, 151, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Morimoto, L.M.; Mink, P.J.; Cushing, C.A. A review and meta-analysis of red and processed meat consumption and breast cancer. Nutr. Res. Rev. 2010, 23, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.H.; Misra, M.; Mukherjee, S.D. Is red meat intake a risk factor for breast cancer among premenopausal women? Breast Cancer Res. Treat. 2009, 117, 1–8. [Google Scholar] [CrossRef]
- Anderson, J.J.; Darwis, N.D.M.; Mackay, D.F.; Celis-Morales, C.A.; Lyall, D.M.; Sattar, N.; Gill, J.M.R.; Pell, J.P. Red and processed meat consumption and breast cancer: UK Biobank cohort study and meta-analysis. Eur. J. Cancer 2018, 90, 73–82. [Google Scholar] [CrossRef]
- Rezaianzadeh, A.; Ghorbani, M.; Rezaeian, S.; Kassani, A. Red Meat Consumption and Breast Cancer Risk in Premenopausal Women: A Systematic Review and Meta-Analysis. Middle East J. Cancer 2018, 9, 5–12. [Google Scholar]
- Farvid, M.S.; Stern, M.C.; Norat, T.; Sasazuki, S.; Vineis, P.; Weijenberg, M.P.; Wolk, A.; Wu, K.; Stewart, B.W.; Cho, E. Consumption of red and processed meat and breast cancer incidence: A systematic review and meta-analysis of prospective studies. Int. J. Cancer 2018, 143, 2787–2799. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.L.F.; Leung, H.W.C.; Wang, S.-F. Multivitamin supplement use and risk of breast cancer: A meta-analysis. Ann. Pharmacother. 2011, 45, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Chen, G.C.; Zhang, R.; Du, X.; Zou, S.Y.; Shi, B.M.; Qin, L.Q. Calcium intake and breast cancer risk: Meta-analysis of prospective cohort studies. Br. J. Nutr. 2016, 116, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Chen, M.-L.; Qin, Y.; Zhang, Q.-Y.; Xu, H.-X.; Zhou, Y.; Mi, M.-T.; Zhu, J.-D. Isoflavone consumption and risk of breast cancer: A dose-response meta-analysis of observational studies. Asia Pac. J. Clin. Nutr. 2013, 22, 118–127. [Google Scholar] [PubMed]
- Mourouti, N.; Kontogianni, M.D.; Papavagelis, C.; Panagiotakos, D.B. Diet and breast cancer: A systematic review. Int. J. Food Sci. Nutr. 2015, 66, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.C.; Baltar, V.T.; Marchioni, D.M. Breast cancer and dietary patterns: A systematic review. Nutr. Rev. 2014, 72, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, L.; Zhang, D.; Jiang, W. Systematic review and meta-analysis suggest that dietary cholesterol intake increases risk of breast cancer. Nutr. Res. 2016, 36, 627–635. [Google Scholar] [CrossRef]
- Cao, Y.; Hou, L.; Wang, W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer 2016, 138, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz Mohammadi, R.; Bagheri, M.; Kolahdouz Mohammadi, M.; Shidfar, F. Ruminant trans-fatty acids and risk of breast cancer: A systematic review and meta-analysis of observational studies. Minerva Endocrinol. 2017, 42, 385–396. [Google Scholar] [CrossRef]
- Yu, L.; Tan, Y.; Zhu, L. Dietary vitamin B2 intake and breast cancer risk: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2017, 295, 721–729. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, R.; Huang, J.; Li, X.; Zhang, J.; Ho, J.C.; Zheng, Y. Dietary Protein Sources and Incidence of Breast Cancer: A Dose-Response Meta-Analysis of Prospective Studies. Nutrients 2016, 8, 730. [Google Scholar] [CrossRef]
- Xiao, Y.; Xia, J.; Li, L.; Ke, Y.; Cheng, J.; Xie, Y.; Chu, W.; Cheung, P.; Kim, J.H.; Colditz, G.A.; et al. Associations between dietary patterns and the risk of breast cancer: A systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, H.; Djalalinia, S.; Sadeghi, O.; Asayesh, H.; Noroozi, M.; Gorabi, A.M.; Mohammadi, R.; Qorbani, M. Dietary Inflammatory Potential Score and Risk of Breast Cancer: Systematic Review and Meta-analysis. Clin. Breast Cancer 2018, 18, e561–e570. [Google Scholar] [CrossRef]
- Mulholland, H.G.; Murray, L.J.; Cardwell, C.R.; Cantwell, M.M. Dietary glycaemic index, glycaemic load and breast cancer risk: A systematic review and meta-analysis. Br. J. Cancer 2008, 99, 1170–1175. [Google Scholar] [CrossRef]
- Schlesinger, S.; Chan, D.S.M.; Vingeliene, S.; Vieira, A.R.; Abar, L.; Polemiti, E.; Stevens, C.A.T.; Greenwood, D.C.; Aune, D.; Norat, T. Carbohydrates, glycemic index, glycemic load, and breast cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Nutr. Rev. 2017, 75, 420–441. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Lombaert, N.; Lison, D. Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: A systematic review and meta-analysis. Environ. Int. 2016, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, X.; Xie, M. Association between dietary cadmium exposure and breast cancer risk: An updated meta-analysis of observational studies. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 769–775. [Google Scholar]
- Lin, J.; Zhang, F.; Lei, Y. Dietary intake and urinary level of cadmium and breast cancer risk: A meta-analysis. Cancer Epidemiol. 2016, 42, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Urinary cadmium concentration and risk of breast cancer: A systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2015, 182, 375–380. [Google Scholar] [CrossRef]
- Hou, R.; Wei, J.; Hu, Y.; Zhang, X.; Sun, X.; Chandrasekar, E.K.; Voruganti, V.S. Healthy dietary patterns and risk and survival of breast cancer: A meta-analysis of cohort studies. Cancer Causes Control 2019, 30, 835–846. [Google Scholar] [CrossRef]
- Chang, V.C.; Cotterchio, M.; Khoo, E. Iron intake, body iron status, and risk of breast cancer: A systematic review and meta-analysis. BMC Cancer 2019, 19, 543. [Google Scholar] [CrossRef] [PubMed]
- Macis, D.; Guerrieri-Gonzaga, A.; Gandini, S. Circulating adiponectin and breast cancer risk: A systematic review and meta-analysis. Int. J. Epidemiol. 2014, 43, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tang, S.; Ma, H.; Duan, H.; Zeng, Y. Association of serum adiponectin with breast cancer: A meta-analysis of 27 case-control studies. Medicine 2019, 98, e14359. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jia, J.; Dong, S.; Zhang, C.; Yu, S.; Li, L.; Mao, C.; Wang, D.; Chen, J.; Yuan, G. Circulating adiponectin levels and the risk of breast cancer: A meta-analysis. Eur. J. Cancer Prev. 2014, 23, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Cao, C.; Fu, J.; Li, Q.; Li, D.H.; Chen, M.Y. Serum adiponectin in breast cancer: A meta-analysis. Medicine 2018, 97, e11433. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Deng, L.L.; Cui, J.Q.; Shi, L.; Yang, Y.C.; Luo, J.H.; Qin, D.; Wang, L. Association between serum leptin levels and breast cancer risk: An updated systematic review and meta-analysis. Medicine 2018, 97, e11345. [Google Scholar] [CrossRef] [PubMed]
- Shekarriz-Foumani, R.; Khodaie, F. The Correlation of Plasma 25-Hydroxyvitamin D Deficiency with Risk of Breast Neoplasms: A Systematic Review. Iran. J. Cancer Prev. 2016, 9, e4469. [Google Scholar] [CrossRef]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U.; Arndt, V.; Brenner, H. Meta-analysis: Serum vitamin D and breast cancer risk. Eur. J. Cancer 2010, 46, 2196–2205. [Google Scholar] [CrossRef]
- Tommie, J.L.; Pinney, S.M.; Nommsen-Rivers, L.A. Serum Vitamin D Status and Breast Cancer Risk by Receptor Status: A Systematic Review. Nutr. Cancer 2018, 70, 804–820. [Google Scholar] [CrossRef]
- Hu, F.; Wu, Z.; Li, G.; Teng, C.; Liu, Y.; Wang, F.; Zhao, Y.; Pang, D. The plasma level of retinol, vitamins A, C and alpha-tocopherol could reduce breast cancer risk? A meta-analysis and meta-regression. J. Cancer Res. Clin. Oncol. 2015, 141, 601–614. [Google Scholar] [CrossRef]
- Wulaningsih, W.; Sagoo, H.K.; Hamza, M.; Melvin, J.; Holmberg, L.; Garmo, H.; Malmstrom, H.; Lambe, M.; Hammar, N.; Walldius, G.; et al. Serum Calcium and the Risk of Breast Cancer: Findings from the Swedish AMORIS Study and a Meta-Analysis of Prospective Studies. Int. J. Mol. Sci. 2016, 17, 1487. [Google Scholar] [CrossRef]
- Esposito, K.; Capuano, A.; Giugliano, D. Metabolic syndrome and cancer: Holistic or reductionist? Endocrine 2014, 45, 362–364. [Google Scholar] [CrossRef]
- Bhandari, R.; Kelley, G.A.; Hartley, T.A.; Rockett, I.R.H. Metabolic syndrome is associated with increased breast cancer risk: A systematic review with meta-analysis. Int. J. Breast Cancer 2014, 2014, 189384. [Google Scholar] [CrossRef]
- Guo, L.; Liu, S.; Zhang, S.; Chen, Q.; Zhang, M.; Quan, P.; Lu, J.; Sun, X. C-reactive protein and risk of breast cancer: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 10508. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Bandera, E.V.; Greenwood, D.C.; Norat, T. Circulating C-reactive protein and breast cancer risk-systematic literature review and meta-analysis of prospective cohort studies. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1439–1449. [Google Scholar] [CrossRef]
- Touvier, M.; Fassier, P.; His, M.; Norat, T.; Chan, D.S.M.; Blacher, J.; Hercberg, S.; Galan, P.; Druesne-Pecollo, N.; Latino-Martel, P. Cholesterol and breast cancer risk: A systematic review and meta-analysis of prospective studies. Br. J. Nutr. 2015, 114, 347–357. [Google Scholar] [CrossRef]
- Ni, H.; Liu, H.; Gao, R. Serum Lipids and Breast Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0142669. [Google Scholar] [CrossRef]
- Miao, S.-Y.; Zhou, W.; Chen, L.; Wang, S.; Liu, X.-A. Influence of ABO blood group and Rhesus factor on breast cancer risk: A meta-analysis of 9665 breast cancer patients and 244,768 controls. Asia-Pac. J. Clin. Oncol. 2014, 10, 101–108. [Google Scholar] [CrossRef]
- Lee, J.D.; Cai, Q.; Shu, X.O.; Nechuta, S.J. The Role of Biomarkers of Oxidative Stress in Breast Cancer Risk and Prognosis: A Systematic Review of the Epidemiologic Literature. J. Womens Health 2017, 26, 467–482. [Google Scholar] [CrossRef]
- Jouybari, L.; Saei Ghare Naz, M.; Sanagoo, A.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Hasanpour Dehkordi, A. Toxic elements as biomarkers for breast cancer: A meta-analysis study. Cancer Manag. Res. 2018, 10, 69–79. [Google Scholar] [CrossRef]
- He, X.Y.; Liao, Y.D.; Yu, S.; Zhang, Y.; Wang, R. Sex hormone binding globulin and risk of breast cancer in postmenopausal women: A meta-analysis of prospective studies. Horm. Metab. Res. 2015, 47, 485–490. [Google Scholar] [CrossRef]
- Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D exposure and Risk of Breast Cancer: A meta-analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q. 2016, 94, 485–514. [Google Scholar] [CrossRef]
- Siontis, K.C.; Hernandez-Boussard, T.; Ioannidis, J.P. Overlapping meta-analyses on the same topic: Survey of published studies. BMJ 2013, 347, f4501. [Google Scholar] [CrossRef]
- Druyts, E.; Thorlund, K.; Humphreys, S.; Lion, M.; Cooper, C.L.; Mills, E.J. Interpreting discordant indirect and multiple treatment comparison meta-analyses: An evaluation of direct acting antivirals for chronic hepatitis C infection. Clin. Epidemiol. 2013, 5, 173–183. [Google Scholar] [CrossRef]
- Lebel, S.; Devins, G.M. Stigma in cancer patients whose behavior may have contributed to their disease. Future Oncol. 2008, 4, 717–733. [Google Scholar] [CrossRef]
- Morstad, H.O. Mom. In The Pink Book: Stories about Breast Cancer; Hegdal, Å., Ed.; Cappelen Damm: Oslo, Norway, 2013. [Google Scholar]





| Category: | Subcategories: | Number: | Total: |
|---|---|---|---|
| Alcohol and tobacco | Passive and active smoking | 3 | 10 |
| Passive smoking | 3 | ||
| Smoking before pregnancy | 1 | ||
| Alcohol | 1 | ||
| Wine | 1 | ||
| Long-term alcohol intake | 1 | ||
| Lifestyle, physical activity, and body size | Physical activity | 4 | 17 |
| Sedentary behavior | 1 | ||
| BMI | 5 | ||
| Obesity and overweight | 2 | ||
| Abdominal fatness | 1 | ||
| Breast size | 1 | ||
| Breast density | 1 | ||
| Several lifestyle factors | 2 | ||
| Influences from external factors | Night shift | 4 | 17 |
| Circadian | 1 | ||
| Light expose at night | 1 | ||
| Sleep duration | 2 | ||
| Flight attendant | 1 | ||
| Electromagnetic fields | 2 | ||
| Polychlorinated biphenyl | 1 | ||
| DDE | 1 | ||
| Stress | 3 | ||
| Striking life events | 1 | ||
| Blood and metabolism | Adiponectin | 4 | 21 |
| Leptin | 1 | ||
| Vitamin D | 4 | ||
| Calcium | 1 | ||
| Vitamin A | 1 | ||
| Metabolic syndrome | 2 | ||
| CRP | 2 | ||
| Cholesterol | 1 | ||
| Lipid | 1 | ||
| Blood type | 1 | ||
| Oxidative stress | 1 | ||
| Toxic elements | 1 | ||
| Sex-hormone-binding globulin | 1 | ||
| Fertility and drugs | HPV | 1 | 26 |
| Intrauterine environment | 1 | ||
| Abortion | 1 | ||
| Breast feeding | 3 | ||
| Preeclampsia | 2 | ||
| Birth Weight | 1 | ||
| In vitro fertilization | 3 | ||
| Hormone replacement therapy | 2 | ||
| Progesterone | 3 | ||
| Estradiol | 1 | ||
| Oral contraceptives | 2 | ||
| Folate | 2 | ||
| NSAIDs | 1 | ||
| Antibiotics | 1 | ||
| Bone mineral density | 2 | ||
| Nutrition | Red and processed meat | 6 | 31 |
| Mushrooms | 1 | ||
| Black Cohosh | 1 | ||
| Black tea | 1 | ||
| Eggs | 1 | ||
| Coffee | 1 | ||
| Multivitamins | 1 | ||
| Calcium | 1 | ||
| Iron | 1 | ||
| Isoflavone | 1 | ||
| Overall diet | 1 | ||
| Dietary patterns | 3 | ||
| Cholesterol | 1 | ||
| Total and serum fat | 2 | ||
| B2 | 1 | ||
| Protein | 1 | ||
| Inflammatory potential | 1 | ||
| Glycemic index and load | 2 | ||
| Cadmium | 4 | ||
| Total: | 122 | 122 |
| Increased Risk | Reduced Risk | ||||
|---|---|---|---|---|---|
| First Author | Effect | Theme | First Author | Effect | Theme |
| Bae [91] | 3.23 | Breast density | He [147] | 0.64 | SHGB |
| Vishwakarma [68] | 2.29 | Never married | Asi [58] | 0.67 † | Progestin |
| Namiranian [93] | 2.21 * | BMI > 30 | Unar-Munguia [50] | 0.72 * | Breast feeding |
| Qu [66] | 1.82 | Bone mineral density | Nelson [92] | 0.74 | BMI > 30 woman 40–49 years |
| Namiranian [93] | 1.71 * | BMI 25–30 | Chen J.H. [67] | 0.75 | Bone mineral density |
| Qu [66] | 1.62 | Bone mineral density | Chan D.S.M. [95] | 0.79 | Vigorous activity premenopausal |
| Bae [91] | 1.62 | Breast density | Wulaningsih [137] | 0.80 † | Calcium |
| Vishwakarma [68] | 1.57 | Age when having first child | Chan D.S.M. [95] | 0.85 | Adult weight loss premenopausal |
| Liu [43] | 1.40 | Flight attendant | Yu [116] | 0.85 | B2 intake |
| Namiranian [93] | 1.39 * | Meat consumption more the three times a week | Chan D.S.M. [95] | 0.86 | Early adult BMI per 5 kg/m |
| Yang W.S. [33] | 1.17 | Light exposure at night | Chan D.S.M. [95] | 0.86 | Total physical activity postmenopausal |
| Chan D.S.M. [95] | 1.17 | Gain in BMI per 5 kg/m2. postmenopausal | Nelson [92] | 0.86 | BMI 25–30-woman 40–49 years |
| Guo [140] | 1.16 | C-reactive protein | Chen X. [81] | 0.87 | Physical activity |
| Ji [69] | 1.16 * | When started with oral contraceptives | Wu Y. [80] | 0.88 | Different physical activity |
| Chang V.C. [127] | 1.12 | Iron intake | Chan D.S.M. [95] | 0.88 | Recreational activity postmenopausal |
| Chan D.S.M. [95] | 1.11 | Waist circumference pr 10 cm postmenopausal | Chan D.S.M. [95] | 0.90 | Adult weight loss postmenopausal |
| DeRoo [75] | 1.10 * | Smoking before pregnancy | Chan D.S.M. [95] | 0.90 | Vigorous activity postmenopausal |
| Cao [114] | 1.10 | Fat intake | Chan D.S.M. [95] | 0.90 | Occupational activity postmenopausal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Løyland, B.; Sandbekken, I.H.; Grov, E.K.; Utne, I. Causes and Risk Factors of Breast Cancer, What Do We Know for Sure? An Evidence Synthesis of Systematic Reviews and Meta-Analyses. Cancers 2024, 16, 1583. https://doi.org/10.3390/cancers16081583
Løyland B, Sandbekken IH, Grov EK, Utne I. Causes and Risk Factors of Breast Cancer, What Do We Know for Sure? An Evidence Synthesis of Systematic Reviews and Meta-Analyses. Cancers. 2024; 16(8):1583. https://doi.org/10.3390/cancers16081583
Chicago/Turabian StyleLøyland, Borghild, Ida Hellum Sandbekken, Ellen Karine Grov, and Inger Utne. 2024. "Causes and Risk Factors of Breast Cancer, What Do We Know for Sure? An Evidence Synthesis of Systematic Reviews and Meta-Analyses" Cancers 16, no. 8: 1583. https://doi.org/10.3390/cancers16081583