Recent Advancement in Diagnosis of Biliary Tract Cancer through Pathological and Molecular Classifications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathologic Classification
2.1. Pathologic Classification of Cholangiocarcinoma
2.2. Pathologic Classification of Gallbladder Cancer
3. Molecular Classification
3.1. Molecular Classification of Cholangiocarcinoma
3.2. Molecular Classification of Gallbladder Cancer
4. Clinical Presentation
5. Diagnostic Tool
5.1. Ultrasonography
5.2. CT and MRI
5.2.1. Radiologic Findings of Mass-Forming Cholangiocarcinoma
5.2.2. Radiologic Findings of Periductal-Infiltrating Cholangiocarcinoma
5.2.3. Radiologic Findings of Intraductal-Growing Cholangiocarcinoma
5.2.4. Radiologic Findings of Gallbladder Cancer
5.3. PET-CT
5.4. EUS
5.5. ERCP or Percutaneous Transhepatic Cholangiography (PTC)
5.5.1. Intraductal Ultrasound (IDUS)
5.5.2. Peroral Cholangioscopy (POC)
5.5.3. Tissue Biopsy
5.6. Liquid Biopsy Based on Bile Samples
5.7. Liquid Biopsy Based on Blood Samples
6. Clinical Aspects for Pathologic and Molecular Diagnosis
6.1. Pathologic Diagnosis
6.2. Molecular Diagnosis
7. Approach to the Patient
7.1. Suspected iCCA
7.2. Suspected pCCA
7.3. Suspected dCCA
7.4. Patients with PSC
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Harada, K.; Sasaki, M.; Sato, Y. Proposal of a new disease concept “biliary diseases with pancreatic counterparts”. Anatomical and pathological bases. Histol. Histopathol. 2014, 29, 1–10. [Google Scholar] [PubMed]
- Jain, A.; Kwong, L.N.; Javle, M. Genomic profiling of biliary tract cancers and implications for clinical practice. Curr. Treat. Options Oncol. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Sato, Y.; Harada, K.; Sasaki, M.; Xu, J.; Ikeda, H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J. Hepatol. 2010, 2, 419–427. [Google Scholar] [CrossRef]
- Dodson, R.M.; Weiss, M.J.; Cosgrove, D.; Herman, J.M.; Kamel, I.; Anders, R.; Geschwind, J.F.; Pawlik, T.M. Intrahepatic cholangiocarcinoma: Management options and emerging therapies. J. Am. Coll. Surg. 2013, 217, 736–750.e734. [Google Scholar] [CrossRef]
- Aishima, S.; Oda, Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: Different characters of perihilar large duct type versus peripheral small duct type. J. Hepatobiliary Pancreat. Sci. 2015, 22, 94–100. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Kakuda, Y. Pathologic classification of cholangiocarcinoma: New concepts. Best. Pr. Res. Clin. Gastroenterol. 2015, 29, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Sigel, C.S.; Drill, E.; Zhou, Y.; Basturk, O.; Askan, G.; Pak, L.M.; Vakiani, E.; Wang, T.; Boerner, T.; Do, R.K.G.; et al. Intrahepatic cholangiocarcinomas have histologically and immunophenotypically distinct small and large duct patterns. Am. J. Surg. Pathol. 2018, 42, 1334–1345. [Google Scholar] [CrossRef]
- Hayashi, A.; Misumi, K.; Shibahara, J.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N.; Fukayama, M. Distinct clinicopathologic and genetic features of 2 histologic subtypes of intrahepatic cholangiocarcinoma. Am. J. Surg. Pathol. 2016, 40, 1021–1030. [Google Scholar] [CrossRef]
- Chung, T.; Rhee, H.; Nahm, J.H.; Jeon, Y.; Yoo, J.E.; Kim, Y.J.; Han, D.H.; Park, Y.N. Clinicopathological characteristics of intrahepatic cholangiocarcinoma according to gross morphologic type: Cholangiolocellular differentiation traits and inflammation- and proliferation-phenotypes. HPB 2020, 22, 864–873. [Google Scholar] [CrossRef]
- Cardinale, V.; Wang, Y.; Carpino, G.; Reid, L.M.; Gaudio, E.; Alvaro, D. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology 2012, 55, 2041–2042. [Google Scholar] [CrossRef]
- Patil, P.A.; Taddei, T.; Jain, D.; Zhang, X.C. Hnf-1β is a more sensitive and specific marker than c-reactive protein for identifying biliary differentiation in primary hepatic carcinomas. Arch. Pathol. Lab. Med. 2022, 146, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Katabi, N.; Torres, J.; Klimstra, D.S. Intraductal tubular neoplasms of the bile ducts. Am. J. Surg. Pathol. 2012, 36, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Jang, K.T.; Fukushima, N.; Furukawa, T.; Hong, S.M.; Kim, H.; Lee, K.B.; Zen, Y.; Jang, J.Y.; Kubota, K. A statement by the japan-korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage. J. Hepatobiliary Pancreat. Sci. 2018, 25, 181–187. [Google Scholar] [CrossRef]
- Henson, D.E.; Albores-Saavedra, J.; Corle, D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer 1992, 70, 1493–1497. [Google Scholar] [CrossRef]
- Adsay, V.; Jang, K.T.; Roa, J.C.; Dursun, N.; Ohike, N.; Bagci, P.; Basturk, O.; Bandyopadhyay, S.; Cheng, J.D.; Sarmiento, J.M.; et al. Intracholecystic papillary-tubular neoplasms (icpn) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are >/=1.0 cm): Clinicopathologic and immunohistochemical analysis of 123 cases. Am. J. Surg. Pathol. 2012, 36, 1279–1301. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer, J.; Tabak, B.; Peix, J.; Sole, M.; Tovar, V.; Alsinet, C.; et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef]
- Andersen, J.B.; Spee, B.; Blechacz, B.R.; Avital, I.; Komuta, M.; Barbour, A.; Conner, E.A.; Gillen, M.C.; Roskams, T.; Roberts, L.R.; et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012, 142, 1021–1031.e1015. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Borger, D.R.; Tanabe, K.K.; Fan, K.C.; Lopez, H.U.; Fantin, V.R.; Straley, K.S.; Schenkein, D.P.; Hezel, A.F.; Ancukiewicz, M.; Liebman, H.M.; et al. Frequent mutation of isocitrate dehydrogenase and in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012, 17, 72–79. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase ii study of bgj398 in patients with fgfr-altered advanced cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Shi, L.; Liu, L.Y.; Fece de la Cruz, F.; Lennerz, J.K.; Raghavan, S.; Leschiner, I.; Elagina, L.; Siravegna, G.; Ng, R.W.S.; et al. Tas-120 overcomes resistance to atp-competitive fgfr inhibitors in patients with fgfr2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 2019, 9, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in idh1-mutant, chemotherapy-refractory cholangiocarcinoma (claridhy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.; Lassen, U.; Elez, E.; Italiano, A.; Curigliano, G.; DeBraud, F.; Prager, G.; Greil, R.; Stein, A.; Angelica, F.; et al. Efficacy and safety of dabrafenib plus trametinib in patients with braf v600e-mutated biliary tract cancer and adenocarcinoma of the small intestine. Eur. J. Cancer 2018, 103, E3–E4. [Google Scholar]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for her2-positive, metastatic biliary tract cancer (mypathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Ueno, M.; Kobayashi, S.; Kawamoto, Y.; Komatsu, Y.; Ikeda, M.; Sasaki, M.; Okano, N.; Furuse, J.; et al. Multicenter phase ii trial of trastuzumab deruxtecan for her2-positive unresectable or recurrent biliary tract cancer: Herb trial. Future Oncol. 2022, 18, 2351–2360. [Google Scholar] [CrossRef]
- Lee, C.K.; Chon, H.J.; Cheon, J.; Lee, M.A.; Im, H.S.; Jang, J.S.; Kim, M.H.; Park, S.; Kang, B.; Hong, M.; et al. Trastuzumab plus folfox for her2-positive biliary tract cancer refractory to gemcitabine and cisplatin: A multi-institutional phase 2 trial of the korean cancer study group (kcsg-hb19-14). Lancet Gastroenterol. Hepatol. 2023, 8, 56–65. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. Pd-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Oh, D.Y.; Ruth He, A.; Qin, S.; Chen, L.T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Slater, S.; Cunningham, D. Pembrolizumab plus chemotherapy as first-line treatment for advanced biliary tract cancer. Lancet 2023, 401, 1826–1827. [Google Scholar] [CrossRef]
- Farshidfar, F.; Zheng, S.; Gingras, M.C.; Newton, Y.; Shih, J.; Robertson, A.G.; Hinoue, T.; Hoadley, K.A.; Gibb, E.A.; Roszik, J.; et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct idh-mutant molecular profiles. Cell Rep. 2017, 18, 2780–2794. [Google Scholar] [CrossRef]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef]
- Dong, L.; Lu, D.; Chen, R.; Lin, Y.; Zhu, H.; Zhang, Z.; Cai, S.; Cui, P.; Song, G.; Rao, D.; et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell 2022, 40, 70–87.e15. [Google Scholar] [CrossRef]
- Martin-Serrano, M.A.; Kepecs, B.; Torres-Martin, M.; Bramel, E.R.; Haber, P.K.; Merritt, E.; Rialdi, A.; Param, N.J.; Maeda, M.; Lindblad, K.E.; et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut 2023, 72, 736–748. [Google Scholar] [CrossRef]
- Cho, S.Y.; Hwang, H.; Kim, Y.H.; Yoo, B.C.; Han, N.; Kong, S.Y.; Baek, M.J.; Kim, K.H.; Lee, M.R.; Park, J.G.; et al. Refining classification of cholangiocarcinoma subtypes via proteogenomic integration reveals new therapeutic prospects. Gastroenterology 2023, 164, 1293–1309. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Li, X.; Ye, J.; Wu, X.; Tan, Z.; Liu, C.; Shen, B.; Wang, X.A.; Wu, W.; et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the erbb pathway. Nat. Genet. 2014, 46, 872–876. [Google Scholar] [CrossRef]
- Nepal, C.; Zhu, B.; O’Rourke, C.J.; Bhatt, D.K.; Lee, D.; Song, L.; Wang, D.F.; Van Dyke, A.L.; Choo-Wosoba, H.; Liu, Z.W.; et al. Integrative molecular characterisation of gallbladder cancer reveals micro-environment-associated subtypes. J. Hepatol. 2021, 74, 1132–1144. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Drill, E.; Satravada, B.A.; Dika, I.E.; Brannon, A.R.; Dermawan, J.; Mohanty, A.; Ozcan, K.; Chakravarty, D.; Benayed, R.; et al. Comprehensive molecular characterization of gallbladder carcinoma and potential targets for intervention. Clin. Cancer Res. 2022, 28, 5359–5367. [Google Scholar] [CrossRef]
- Alvaro, D.; Bragazzi, M.C.; Benedetti, A.; Fabris, L.; Fava, G.; Invernizzi, P.; Marzioni, M.; Nuzzo, G.; Strazzabosco, M.; Stroffolini, T.; et al. Cholangiocarcinoma in italy: A national survey on clinical characteristics, diagnostic modalities and treatment. Results from the “cholangiocarcinoma” committee of the italian association for the study of liver disease. Dig. Liver Dis. 2011, 43, 60–65. [Google Scholar] [CrossRef]
- Strom, B.L.; Maislin, G.; West, S.L.; Atkinson, B.; Herlyn, M.; Saul, S.; Rodriguez-Martinez, H.A.; Rios-Dalenz, J.; Iliopoulos, D.; Soloway, R.D. Serum cea and ca 19-9: Potential future diagnostic or screening tests for gallbladder cancer? Int. J. Cancer 1990, 45, 821–824. [Google Scholar] [CrossRef]
- Ritts, R.E., Jr.; Nagorney, D.M.; Jacobsen, D.J.; Talbot, R.W.; Zurawski, V.R., Jr. Comparison of preoperative serum ca19-9 levels with results of diagnostic imaging modalities in patients undergoing laparotomy for suspected pancreatic or gallbladder disease. Pancreas 1994, 9, 707–716. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, M.H.; Myung, S.J.; Lim, B.C.; Park, E.T.; Yoo, K.S.; Seo, D.W.; Lee, S.K.; Min, Y.I. A new strategy for the application of ca19-9 in the differentiation of pancreaticobiliary cancer: Analysis using a receiver operating characteristic curve. Am. J. Gastroenterol. 1999, 94, 1941–1946. [Google Scholar] [CrossRef]
- Joo, I.; Lee, J.M.; Yoon, J.H. Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: Recent advances and challenges. Radiology 2018, 288, 7–13. [Google Scholar] [CrossRef]
- Jhaveri, K.S.; Hosseini-Nik, H. Mri of cholangiocarcinoma. J. Magn. Reson. Imaging 2015, 42, 1165–1179. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, C.H.; Kim, B.H.; Kim, W.B.; Yeom, S.K.; Kim, K.A.; Park, C.M. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J. Comput. Assist. Tomogr. 2012, 36, 704–709. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.K.; Park, M.J.; Lee, W.J. Small intrahepatic mass-forming cholangiocarcinoma: Target sign on diffusion-weighted imaging for differentiation from hepatocellular carcinoma. Abdom. Imaging 2013, 38, 793–801. [Google Scholar] [CrossRef]
- Somoracz, A.; Tatrai, P.; Horvath, G.; Kiss, A.; Kupcsulik, P.; Kovalszky, I.; Schaff, Z. Agrin immunohistochemistry facilitates the determination of primary versus metastatic origin of liver carcinomas. Hum. Pathol. 2010, 41, 1310–1319. [Google Scholar] [CrossRef]
- Manfredi, R.; Masselli, G.; Maresca, G.; Brizi, M.G.; Vecchioli, A.; Marano, P. Mr imaging and mrcp of hilar cholangiocarcinoma. Abdom. Imaging 2003, 28, 319–325. [Google Scholar] [CrossRef]
- Vanderveen, K.A.; Hussain, H.K. Magnetic resonance imaging of cholangiocarcinoma. Cancer Imaging 2004, 4, 104–115. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, J.H.; Kim, S.Y.; Lee, S.S.; Kim, H.J.; Kim, M.H.; Lee, M.G. Sclerosing cholangitis with autoimmune pancreatitis versus primary sclerosing cholangitis: Comparison on endoscopic retrograde cholangiography, mr cholangiography, ct, and mri. Acta Radiol. 2013, 54, 601–607. [Google Scholar] [CrossRef]
- Choi, B.W.; Kim, M.J.; Chung, J.J.; Chung, J.B.; Yoo, H.S.; Lee, J.T. Radiologic findings of mirizzi syndrome with emphasis on mri. Yonsei Med. J. 2000, 41, 144–146. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Nundy, S. Portal biliopathy. World J. Gastroenterol. 2012, 18, 6177–6182. [Google Scholar] [CrossRef]
- Madhusudhan, K.S.; Das, P.; Gunjan, D.; Srivastava, D.N.; Garg, P.K. Igg4-related sclerosing cholangitis: A clinical and imaging review. AJR Am. J. Roentgenol. 2019, 213, 1221–1231. [Google Scholar] [CrossRef]
- Krasinskas, A.M.; Raina, A.; Khalid, A.; Tublin, M.; Yadav, D. Autoimmune pancreatitis. Gastroenterol. Clin. North. Am. 2007, 36, 239–257. [Google Scholar] [CrossRef]
- Finkelberg, D.L.; Sahani, D.; Deshpande, V.; Brugge, W.R. Autoimmune pancreatitis. N. Engl. J. Med. 2006, 355, 2670–2676. [Google Scholar] [CrossRef]
- Lim, J.H.; Yi, C.A.; Lim, H.K.; Lee, W.J.; Lee, S.J.; Kim, S.H. Radiological spectrum of intraductal papillary tumors of the bile ducts. Korean J. Radiol. 2002, 3, 57–63. [Google Scholar] [CrossRef]
- Kumar, A.; Aggarwal, S. Carcinoma of the gallbladder: Ct findings in 50 cases. Abdom. Imaging 1994, 19, 304–308. [Google Scholar] [CrossRef]
- Liang, J.L.; Chen, M.C.; Huang, H.Y.; Ng, S.H.; Sheen-Chen, S.M.; Liu, P.P.; Kung, C.T.; Ko, S.F. Gallbladder carcinoma manifesting as acute cholecystitis: Clinical and computed tomographic features. Surgery 2009, 146, 861–868. [Google Scholar] [CrossRef]
- Lamarca, A.; Barriuso, J.; Chander, A.; McNamara, M.G.; Hubner, R.A.; ÓReilly, D.; Manoharan, P.; Valle, J.W. (18)f-fluorodeoxyglucose positron emission tomography ((18)fdg-pet) for patients with biliary tract cancer: Systematic review and meta-analysis. J. Hepatol. 2019, 71, 115–129. [Google Scholar] [CrossRef]
- Abu-Hamda, E.M.; Baron, T.H. Endoscopic management of cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 165–175. [Google Scholar] [CrossRef]
- Mohamadnejad, M.; DeWitt, J.M.; Sherman, S.; LeBlanc, J.K.; Pitt, H.A.; House, M.G.; Jones, K.J.; Fogel, E.L.; McHenry, L.; Watkins, J.L.; et al. Role of eus for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest. Endosc. 2011, 73, 71–78. [Google Scholar] [CrossRef]
- Fujita, N.; Noda, Y.; Kobayashi, G.; Kimura, K.; Yago, A. Diagnosis of the depth of invasion of gallbladder carcinoma by eus. Gastrointest. Endosc. 1999, 50, 659–663. [Google Scholar] [CrossRef]
- Sadamoto, Y.; Kubo, H.; Harada, N.; Tanaka, M.; Eguchi, T.; Nawata, H. Preoperative diagnosis and staging of gallbladder carcinoma by eus. Gastrointest. Endosc. 2003, 58, 536–541. [Google Scholar] [CrossRef]
- Wu, L.M.; Jiang, X.X.; Gu, H.Y.; Xu, X.; Zhang, W.; Lin, L.H.; Deng, X.; Yin, Y.; Xu, J.R. Endoscopic ultrasound-guided fine-needle aspiration biopsy in the evaluation of bile duct strictures and gallbladder masses: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2011, 23, 113–120. [Google Scholar] [CrossRef]
- Kang, H.; Kim, S.J.; Young Do, M.; Kim, E.J.; Kim, Y.S.; Jang, S.I.; Bang, S.; Cho, J.H. Endoscopic ultrasound-guided fine needle aspiration and biopsy for cytohistological diagnosis of gallbladder cancer: A multicenter retrospective study. Gastrointest. Endosc. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, M.; Tsukamoto, Y.; Naitoh, Y.; Hirooka, Y.; Furukawa, T.; Katou, T. New technique using intraductal ultrasonography for the diagnosis of bile duct cancer. J. Ultrasound Med. 1994, 13, 189–195. [Google Scholar] [CrossRef]
- Tamada, K.; Ueno, N.; Ichiyama, M.; Tomiyama, T.; Nishizono, T.; Wada, S.; Oohashi, A.; Tano, S.; Aizawa, T.; Ido, K.; et al. Assessment of pancreatic parenchymal invasion by bile duct cancer using intraductal ultrasonography. Endoscopy 1996, 28, 492–496. [Google Scholar] [CrossRef]
- Choi, E.R.; Chung, Y.H.; Lee, J.K.; Lee, K.T.; Lee, K.H.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Jang, K.T.; Park, S.M.; et al. Preoperative evaluation of the longitudinal extent of borderline resectable hilar cholangiocarcinoma by intraductal ultrasonography. J. Gastroenterol. Hepatol. 2011, 26, 1804–1810. [Google Scholar] [CrossRef]
- Shah, R.J.; Langer, D.A.; Antillon, M.R.; Chen, Y.K. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin. Gastroenterol. Hepatol. 2006, 4, 219–225. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tsuyuguchi, T.; Sakai, Y.; Tsuchiya, S.; Saisyo, H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest. Endosc. 2005, 62, 374–382. [Google Scholar] [CrossRef]
- Iqbal, S.; Stevens, P.D. Cholangiopancreatoscopy for targeted biopsies of the bile and pancreatic ducts. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 567–577. [Google Scholar] [CrossRef]
- Wen, L.J.; Chen, J.H.; Xu, H.J.; Yu, Q.; Liu, K. Efficacy and safety of digital single-operator cholangioscopy in the diagnosis of indeterminate biliary strictures by targeted biopsies: A systematic review and meta-analysis. Diagnostic 2020, 10, 666. [Google Scholar] [CrossRef]
- Seo, D.W.; Lee, S.K.; Yoo, K.S.; Kang, G.H.; Kim, M.H.; Suh, D.J.; Min, Y.I. Cholangioscopic findings in bile duct tumors. Gastrointest. Endosc. 2000, 52, 630–634. [Google Scholar] [CrossRef]
- Pereira, P.; Santos, S.; Morais, R.; Gaspar, R.; Rodrigues-Pinto, E.; Vilas-Boas, F.; Macedo, G. Role of peroral cholangioscopy for diagnosis and staging of biliary tumors. Dig. Dis. 2020, 38, 431–440. [Google Scholar] [CrossRef]
- Trikudanathan, G.; Navaneethan, U.; Njei, B.; Vargo, J.J.; Parsi, M.A. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: A systematic review and meta-analysis. Gastrointest. Endosc. 2014, 79, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Atomi, Y.; Wada, N.; Kuroda, A.; Muto, T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: A prospective comparative study with bile and brush cytology. Am. J. Gastroenterol. 1996, 91, 465–467. [Google Scholar] [PubMed]
- Rabinovitz, M.; Zajko, A.B.; Hassanein, T.; Shetty, B.; Bron, K.M.; Schade, R.R.; Gavaler, J.S.; Block, G.; Van Thiel, D.H.; Dekker, A. Diagnostic value of brush cytology in the diagnosis of bile duct carcinoma: A study in 65 patients with bile duct strictures. Hepatology 1990, 12, 747–752. [Google Scholar] [CrossRef]
- Ponchon, T.; Gagnon, P.; Berger, F.; Labadie, M.; Liaras, A.; Chavaillon, A.; Bory, R. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: Results of a prospective study. Gastrointest. Endosc. 1995, 42, 565–572. [Google Scholar] [CrossRef]
- Gonda, T.A.; Viterbo, D.; Gausman, V.; Kipp, C.; Sethi, A.; Poneros, J.M.; Gress, F.; Park, T.; Khan, A.; Jackson, S.A.; et al. Mutation profile and fluorescence in situ hybridization analyses increase detection of malignancies in biliary strictures. Clin. Gastroenterol. Hepatol. 2017, 15, 913–919.e911. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Njei, B.; Venkatesh, P.G.; Vargo, J.J.; Parsi, M.A. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: A systematic review and meta-analysis. Gastrointest. Endosc. 2014, 79, 943–950.e943. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, C.; Hoshikawa, M.; Imura, J.; Ueno, T.; Koike, J. Bile cytology: A new scoring system for improving diagnostic accuracy. Diagn. Cytopathol. 2019, 47, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.K.; Kim, K.H.; Lee, Y.M.; Lee, B.S.; Choi, S.Y. The usefulness of adding p53 immunocytochemistry to bile drainage cytology for the diagnosis of malignant biliary strictures. Diagn. Cytopathol. 2017, 45, 592–597. [Google Scholar] [CrossRef]
- Liu, F.; Hao, X.; Liu, B.; Liu, S.; Yuan, Y. Bile liquid biopsy in biliary tract cancer. Clin. Chim. Acta 2023, 551, 117593. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Leng, K.; Yao, Y.; Kang, P.; Liao, G.; Han, Y.; Shi, G.; Ji, D.; Huang, P.; Zheng, W.; et al. A circular rna, cholangiocarcinoma-associated circular rna 1, contributes to cholangiocarcinoma progression, induces angiogenesis, and disrupts vascular endothelial barriers. Hepatology 2021, 73, 1419–1435. [Google Scholar] [CrossRef]
- Kubicka, S.; Kuhnel, F.; Flemming, P.; Hain, B.; Kezmic, N.; Rudolph, K.L.; Manns, M.; Meier, P.N. K-ras mutations in the bile of patients with primary sclerosing cholangitis. Gut 2001, 48, 403–408. [Google Scholar] [CrossRef]
- Andresen, K.; Boberg, K.M.; Vedeld, H.M.; Honne, H.; Jebsen, P.; Hektoen, M.; Wadsworth, C.A.; Clausen, O.P.; Lundin, K.E.; Paulsen, V.; et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology 2015, 61, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Vedeld, H.M.; Grimsrud, M.M.; Andresen, K.; Pharo, H.D.; von Seth, E.; Karlsen, T.H.; Honne, H.; Paulsen, V.; Farkkila, M.A.; Bergquist, A.; et al. Early and accurate detection of cholangiocarcinoma in patients with primary sclerosing cholangitis by methylation markers in bile. Hepatology 2022, 75, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, C.; Bai, L.; Yao, F.; Shi, S.; Zhang, Y. Value of bile soluble b7h3 for the diagnosis of malignant biliary strictures: Results of a retrospective study. Surg. Oncol. 2019, 28, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Yadav, M.; Tripathi, G.; Mathew, B.; Bindal, V.; Falari, S.; Pamecha, V.; Maras, J.S. Bile multi-omics analysis classifies lipid species and microbial peptides predictive of carcinoma of gallbladder. Hepatology 2022, 76, 920–935. [Google Scholar] [CrossRef]
- Li, L.; Masica, D.; Ishida, M.; Tomuleasa, C.; Umegaki, S.; Kalloo, A.N.; Georgiades, C.; Singh, V.K.; Khashab, M.; Amateau, S.; et al. Human bile contains microrna-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 2014, 60, 2135. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Tang, L.; Wang, Y.; Wang, N.; Zhou, J.; Deng, X.; Zhong, Y.; Li, Q.; Wang, F.; Jiang, G.; et al. The diagnostic value of exosomal mirnas in human bile of malignant biliary obstructions. Dig. Liver Dis. 2021, 53, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shao, S.J.; Sun, H.; Zhu, H.F.; Fang, H.X. Bile-derived exosome noncoding rnas as potential diagnostic and prognostic biomarkers for cholangiocarcinoma. Front. Oncol. 2022, 12, 985089. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wang, Y.; Nie, J.; Li, Q.; Tang, L.; Deng, X.; Wang, F.; Xu, B.; Wu, X.; Zhang, X.; et al. The diagnostic/prognostic potential and molecular functions of long non-coding rnas in the exosomes derived from the bile of human cholangiocarcinoma. Oncotarget 2017, 8, 69995–70005. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, C.; Haga, H.; Makino, N.; Inuzuka, T.; Kurimoto, A.; Ueda, T.; Matsuda, A.; Kakizaki, Y.; Ishizawa, T.; Kobayashi, T.; et al. Utility of claudin-3 in extracellular vesicles from human bile as biomarkers of cholangiocarcinoma. Sci. Rep. 2021, 11, 1195. [Google Scholar] [CrossRef]
- Lee, J.G.; Leung, J.W.; Cotton, P.B.; Layfield, L.J.; Mannon, P.J. Diagnostic utility of k-ras mutational analysis on bile obtained by endoscopic retrograde cholangiopancreatography. Gastrointest. Endosc. 1995, 42, 317–320. [Google Scholar] [CrossRef]
- Saurin, J.C.; Joly-Pharaboz, M.O.; Pernas, P.; Henry, L.; Ponchon, T.; Madjar, J.J. Detection of ki-ras gene point mutations in bile specimens for the differential diagnosis of malignant and benign biliary strictures. Gut 2000, 47, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Ahn, K.S.; Kim, T.S.; Kim, Y.H.; Cho, K.B.; Shin, D.W.; Baek, W.K.; Suh, S.I.; Jang, B.C.; Kang, K.J. Liquid biopsy from bile-circulating tumor DNA in patients with biliary tract cancer. Cancers 2021, 13, 4581. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Takei, K.; Shinohara, Y.; Takeda, K.; Nakamura, K.; Horibe, T.; Sanada, A.; Ohno, H.; Matsubayashi, H.; Saito, T.; et al. K-ras codon 12 and p53 mutations in biopsy specimens and bile from biliary tract cancers. Pathol. Int. 1999, 49, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Ostwald, C.; Püschel, K.; Brinkmann, B.; Plath, F.; Kröger, J.; Barten, M.; Nizze, H.; Schareck, W.D.; Hauenstein, K.; et al. Low frequency of p53 and ras mutations in bile of patients with hepato-biliary disease:: A prospective study in more than 100 patients. Eur. J. Clin. Invest. 2001, 31, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yamaguchi, Y.; Watanabe, H.; Ohtsubo, K.; Wakabayashi, T.; Sawabu, N. Usefulness of p53 gene mutations in the supernatant of bile for diagnosis of biliary tract carcinoma: Comparison with k- ras mutation. J. Gastroenterol. 2002, 37, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, H.; Nouso, K.; Ako, S.; Dohi, C.; Matsushita, H.; Matsumoto, K.; Kato, H.; Okada, H. Liquid biopsy of bile for the molecular diagnosis of gallbladder cancer. Cancer Biol. Ther. 2018, 19, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Klump, B.; Hsieh, C.J.; Dette, S.; Holzmann, K.; Kiesslich, R.; Jung, M.; Sinn, U. Promoter methylation of ink4a/arf as detected in bile-significance for the differential diagnosis in biliary disease. Clin Cancer Res 2003, 9, 2877. [Google Scholar]
- Zhang, Y.; Yang, B.; Du, Z.; Gao, Y.T.; Wang, Y.J.; Jing, X.; Bai, T. Identification and validation of specific methylation profile in bile for differential diagnosis of malignant biliary stricture. Clin. Biochem. 2010, 43, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, K.; Kim, B.H.; Cho, N.Y.; Jang, J.Y.; Kim, Y.T.; Kim, D.; Jang, J.J.; Kang, G.H. Bile-based detection of extrahepatic cholangiocarcinoma with quantitative DNA methylation markers and its high sensitivity. J. Mol. Diagn. 2012, 14, 256–263. [Google Scholar] [CrossRef]
- He, S.; Zeng, F.; Yin, H.; Wang, P.; Bai, Y.; Song, Q.; Chu, J.; Huang, Z.; Liu, Y.; Liu, H.; et al. Molecular diagnosis of pancreatobiliary tract cancer by detecting mutations and methylation changes in bile samples. EClinicalMedicine 2023, 55, 101736. [Google Scholar] [CrossRef]
- Shen, N.J.; Zhang, D.D.; Yin, L.; Qiu, Y.H.; Liu, J.; Yu, W.L.; Fu, X.H.; Zhu, B.; Xu, X.Y.; Duan, A.Q.; et al. Bile cell-free DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol. Rep. 2019, 42, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Gou, Q.; Zhang, C.Z.; Sun, Z.H.; Wu, L.G.; Chen, Y.; Mo, Z.Q.; Mai, Q.C.; He, J.; Zhou, Z.X.; Shi, F.; et al. Cell-free DNA from bile outperformed plasma as a potential alternative to tissue biopsy in biliary tract cancer. Esmo Open 2021, 6, 100275. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Tsutsui, K.; Ezaki, T.; Fukuma, H.; Kobara, H.; Kamata, H.; Aritomo, Y.; Masaki, T.; Watanabe, S.; Kobayashi, S.; et al. Combination of assay of human telomerase reverse transcriptase mrna and cytology using bile obtained by endoscopic transpapillary catheterization into the gallbladder for diagnosis of gallbladder carcinoma. Am. J. Gastroenterol. 2003, 98, 2415–2419. [Google Scholar] [CrossRef] [PubMed]
- Shigehara, K.; Yokomuro, S.; Ishibashi, O.; Mizuguchi, Y.; Arima, Y.; Kawahigashi, Y.; Kanda, T.; Akagi, I.; Tajiri, T.; Yoshida, H.; et al. Real-time pcr-based analysis of the human bile micrornaome identifies as a potential diagnostic biomarker for biliary tract cancer. PLoS ONE 2011, 6, e23584. [Google Scholar] [CrossRef] [PubMed]
- Baraniskin, A.; Nopel-Dunnebacke, S.; Schumacher, B.; Gerges, C.; Bracht, T.; Sitek, B.; Meyer, H.E.; Gerken, G.; Dechene, A.; Schlaak, J.F.; et al. Analysis of u2 small nuclear rna fragments in the bile differentiates cholangiocarcinoma from primary sclerosing cholangitis and other benign biliary disorders. Dig. Dis. Sci. 2014, 59, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Voigtlander, T.; Gupta, S.K.; Thum, S.; Fendrich, J.; Manns, M.P.; Lankisch, T.O.; Thum, T. Micrornas in serum and bile of patients with primary sclerosing cholangitis and/or cholangiocarcinoma. PLoS ONE 2015, 10, e0139305. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Kim, M.J.; Han, J.H.; Yun, J.; Kim, H.K.; Yang, Y.; Kim, K.B.; Park, S.M. Bile-derived circulating extracellular mir-30d-5p and mir-92a-3p as potential biomarkers for cholangiocarcinoma. Hepatob Pancreat. Dis. 2020, 19, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.B.; Correa-Gallego, C.; Li, Y.; Nelson, J.; Alseidi, A.; Helton, W.S.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; Fong, Y.M.; et al. The role of biliary carcinoembryonic antigen-related cellular adhesion molecule 6 (ceacam6) as a biomarker in cholangiocarcinoma. PLoS ONE 2016, 11, e0150195. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Dumonceau, J.M.; Antinori, P.; Annessi-Ramseyer, I.; Frossard, J.L.; Hochstrasser, D.F.; Delhaye, M.; Lescuyer, P. Bile carcinoembryonic cell adhesion molecule 6 (ceam6) as a biomarker of malignant biliary stenoses. Bba-Proteins Proteom. 2014, 1844, 1018–1025. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Zhang, Q.; Jin, B.; Zhu, M.; Zhang, Z. Identification of bile survivin and carbohydrate antigen 199 in distinguishing cholangiocarcinoma from benign obstructive jaundice. Biomark. Med. 2017, 11, 11–18. [Google Scholar] [CrossRef]
- Matsuda, A.; Kuno, A.; Kawamoto, T.; Matsuzaki, H.; Irimura, T.; Ikehara, Y.; Zen, Y.; Nakanuma, Y.; Yamamoto, M.; Ohkohchi, N.; et al. Agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology 2010, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Matull, W.R.; Andreola, F.; Loh, A.; Adiguzel, Z.; Deheragoda, M.; Qureshi, U.; Batra, S.K.; Swallow, D.M.; Pereira, S.P. Muc4 and muc5ac are highly specific tumour-associated mucins in biliary tract cancer. Br. J. Cancer 2008, 98, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Ruzzenente, O.; Ruzzenente, A.; Iacono, C.; Bertuzzo, F.; Gelati, M.; Conci, S.; Bendinelli, S.; Bonizzato, G.; Guglielmi, A.; et al. Assessment of bile and serum mucin5ac in cholangiocarcinoma: Diagnostic performance and biologic significance. Surgery 2014, 156, 1218–1224. [Google Scholar] [CrossRef]
- Koopmann, J.; Thuluvath, P.J.; Zahurak, M.L.; Kristiansen, T.Z.; Pandey, A.; Schulick, R.; Argani, P.; Hidalgo, M.; Iacobelli, S.; Goggins, M.; et al. Mac-2-binding protein is a diagnostic marker for biliary tract carcinoma. Cancer 2004, 101, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Gutierrez, N.G.; Jegadeesan, R.; Venkatesh, P.G.; Poptic, E.; Liu, X.; Sanaka, M.R.; Jang, S.; Vargo, J.J.; Parsi, M.A. Vascular endothelial growth factor levels in bile distinguishes pancreatic cancer from other etiologies of biliary stricture: A pilot study. Dig. Dis. Sci. 2013, 58, 2986–2992. [Google Scholar] [CrossRef]
- Ayaru, L.; Stoeber, K.; Webster, G.J.; Hatfield, A.R.; Wollenschlaeger, A.; Okoturo, O.; Rashid, M.; Williams, G.; Pereira, S.P. Diagnosis of pancreaticobiliary malignancy by detection of minichromosome maintenance protein 5 in bile aspirates. Br. J. Cancer 2008, 98, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Harada, K.; Sasaki, M.; Yasaka, T.; Nakanuma, Y. Heat shock proteins 27 and 70 are potential biliary markers for the detection of cholangiocarcinoma. Am. J. Pathol. 2012, 180, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, W.Z.; Wu, J.D.; Feng, B.; Chen, W.; Wang, M.; Tang, J.C.; Wang, F.Q.; Cheng, F.; Pu, L.Y.; et al. Comparative proteomic profiling of human bile reveals ssp411 as a novel biomarker of cholangiocarcinoma. PLoS ONE 2012, 7, e47476. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, A.; Nowakowska-Dulawa, E.; Marek, T.; Boldys, H.; Nowak, A.; Hartleb, M. Differentiation of pancreatobiliary cancer from benign biliary strictures using neutrophil gelatinase-associated lipocalin. J. Physiol. Pharmacol. 2013, 64, 109–114. [Google Scholar]
- Zabron, A.A.; Horneffer-van der Sluis, V.M.; Wadsworth, C.A.; Laird, F.; Gierula, M.; Thillainayagam, A.V.; Vlavianos, P.; Westaby, D.; Taylor-Robinson, S.D.; Edwards, R.J.; et al. Elevated levels of neutrophil gelatinase-associated lipocalin in bile from patients with malignant pancreatobiliary disease. Am. J. Gastroenterol. 2011, 106, 1711–1717. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yeh, T.S.; Wu, R.C.; Pang, J.H.S.; Cheng, C.T.; Wang, S.Y.; Juang, H.H.; Yeh, C.N. Lipocalin 2 (lcn2) is a promising target for cholangiocarcinoma treatment and bile lcn2 level is a potential cholangiocarcinoma diagnostic marker. Sci. Rep. 2016, 6, 36138. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Harada, K.; Sasaki, M.; Nakanuma, Y. Clinicopathological significance of s100 protein expression in cholangiocarcinoma. J. Gastroenterol. Hepatol. 2013, 28, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Laohaviroj, M.; Potriquet, J.; Jia, X.; Suttiprapa, S.; Chamgramol, Y.; Pairojkul, C.; Sithithaworn, P.; Mulvenna, J.; Sripa, B. A comparative proteomic analysis of bile for biomarkers of cholangiocarcinoma. Tumour Biol. 2017, 39, 1010428317705764. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lin, X.Z.; Wu, H.C.; Shiesh, S.C. The value of biliary amylase and hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein i (hip/pap-i) in diagnosing biliary malignancies. Clin. Biochem. 2005, 38, 520–525. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tsai, W.L.; Wu, H.C.; Syu, M.J.; Wu, C.C.; Shiesh, S.C. Diagnostic role of biliary pancreatic elastase for cholangiocarcinoma in patients with cholestasis. Clin. Chim. Acta 2008, 390, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.K.; Damink, S.W.M.O.; Brindley, J.H.; Godfrey, A.; Chapman, M.H.; Sandanayake, N.S.; Andreola, F.; Mazurek, S.; Hasan, T.; Malago, M.; et al. Pyruvate kinase m2 is a novel diagnostic marker and predicts tumor progression in human biliary tract cancer. Cancer 2013, 119, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Ahn, K.S.; Baek, W.K.; Suh, S.I.; Kim, Y.H.; Kim, T.S.; Kang, K.J. Usefulness of bile as a biomarker via ferroptosis and cysteine prenylation in cholangiocarcinoma; role of diagnosis and differentiation from benign biliary disease. Surg. Oncol. Oxf. 2020, 34, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Shotton, R.; Lamarca, A.; Valle, J.; McNamara, M.G. Potential utility of liquid biopsies in the management of patients with biliary tract cancers: A review. World J. Gastrointest. Oncol. 2021, 13, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.F.; Jakobsen, A. Screening for circulating ras/raf mutations by multiplex digital pcr. Clin. Chim. Acta 2016, 458, 138–143. [Google Scholar] [CrossRef]
- Kumari, S.; Tewari, S.; Husain, N.; Agarwal, A.; Pandey, A.; Singhal, A.; Lohani, M. Quantification of circulating free DNA as a diagnostic marker in gall bladder cancer. Pathol. Oncol. Res. 2017, 23, 91–97. [Google Scholar] [CrossRef]
- Wasenang, W.; Chaiyarit, P.; Proungvitaya, S.; Limpaiboon, T. Serum cell-free DNA methylation of opcml and hoxd9 as a biomarker that may aid in differential diagnosis between cholangiocarcinoma and other biliary diseases. Clin. Epigenetics 2019, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zill, O.A.; Greene, C.; Sebisanovic, D.; Siew, L.M.; Leng, J.; Vu, M.; Hendifar, A.E.; Wang, Z.; Atreya, C.E.; Kelley, R.K.; et al. Cell-free DNA next-generation sequencing in pancreatobiliary carcinomas. Cancer Discov. 2015, 5, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Ross, P.; Wasan, H.S.; Hubner, R.A.; McNamara, M.G.; Lopes, A.; Manoharan, P.; Palmer, D.; Bridgewater, J.; Valle, J.W. Advanced intrahepatic cholangiocarcinoma: Post hoc analysis of the abc-01, -02, and -03 clinical trials. J. Natl. Cancer Inst. 2020, 112, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, T.; Yang, M.; Song, J.; Huang, M.; Bai, Y.; Su, H. Genomic profiling of blood-derived circulating tumor DNA from patients with advanced biliary tract cancer. Pathol. Oncol. Res. 2021, 27, 1609879. [Google Scholar] [CrossRef] [PubMed]
- Okamura, R.; Kurzrock, R.; Mallory, R.J.; Fanta, P.T.; Burgoyne, A.M.; Clary, B.M.; Kato, S.; Sicklick, J.K. Comprehensive genomic landscape and precision therapeutic approach in biliary tract cancers. Int. J. Cancer 2021, 148, 702–712. [Google Scholar] [CrossRef]
- Mody, K.; Kasi, P.M.; Yang, J.; Surapaneni, P.K.; Bekaii-Saab, T.; Ahn, D.H.; Mahipal, A.; Sonbol, M.B.; Starr, J.S.; Roberts, A.; et al. Circulating tumor DNA profiling of advanced biliary tract cancers. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Csoma, S.L.; Bedekovics, J.; Veres, G.; Arokszallasi, A.; Andras, C.; Mehes, G.; Mokanszki, A. Circulating cell-free DNA-based comprehensive molecular analysis of biliary tract cancers using next-generation sequencing. Cancers 2022, 14, 233. [Google Scholar] [CrossRef]
- Astier, C.; Ngo, C.; Colmet-Daage, L.; Marty, V.; Bawa, O.; Nicotra, C.; Ngo-Camus, M.; Italiano, A.; Massard, C.; Scoazec, J.Y.; et al. Molecular profiling of biliary tract cancers reveals distinct genomic landscapes between circulating and tissue tumor DNA. Exp. Hematol. Oncol. 2024, 13, 2. [Google Scholar] [CrossRef]
- Aguado, E.; Abou-Alfa, G.K.; Zhu, A.X.; Macarulla, T.; Fan, B.; Nejad, P.; Choe, S.; Jiang, L.W.; Gliser, C.; Pandya, S.S.; et al. Idh1 mutation detection in plasma circulating tumor DNA (ctdna) and association with clinical response in patients with advanced intrahepatic cholangiocarcinoma (ihc) from the phase iii claridhy study. J. Clin. Oncol. 2020, 38, 4576. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Mussolin, B.; Reyes, S.; et al. Polyclonal secondary fgfr2 mutations drive acquired resistance to fgfr inhibition in fgfr2 fusion-positive cholangiocarcinoma patients. Cancer Res. 2017, 77, 252–263. [Google Scholar] [CrossRef]
- Yang, X.; Hu, Y.; Yang, K.Y.; Wang, D.X.; Lin, J.Z.; Long, J.Y.; Xie, F.C.; Mao, J.Z.; Bian, J.; Guan, M.; et al. Cell-free DNA copy number variations predict efficacy of immune checkpoint inhibitor-based therapy in hepatobiliary cancers. J. Immunother. Cancer 2021, 9, e001942. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, W.; Liang, P.; Hu, W.; Zhang, K.; Shen, S.; Chen, J.; Zhang, Z.; Chen, B.; Han, Y.; et al. Serum cyfra 21-1 in biliary tract cancers: A reliable biomarker for gallbladder carcinoma and intrahepatic cholangiocarcinoma. Dig. Dis. Sci. 2015, 60, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, T.; Yamazaki, O.; Tanaka, H.; Takemura, S.; Yamamoto, T.; Tanaka, S.; Nishiguchi, S.; Kubo, S. Serum cytokeratin 19 fragment (cyfra21-1) as a prognostic factor in intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2008, 15, 583–589. [Google Scholar] [CrossRef]
- Leelawat, K.; Sakchinabut, S.; Narong, S.; Wannaprasert, J. Detection of serum mmp-7 and mmp-9 in cholangiocarcinoma patients: Evaluation of diagnostic accuracy. BMC Gastroenterol. 2009, 9, 30. [Google Scholar] [CrossRef]
- Leelawat, K.; Narong, S.; Wannaprasert, J.; Ratanashu-Ek, T. Prospective study of mmp7 serum levels in the diagnosis of cholangiocarcinoma. World J. Gastroentero 2010, 16, 4697–4703. [Google Scholar] [CrossRef]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Pombeiro, I.; Leyh, C.; Benz, F.; Vucur, M.; Longerich, T.; Koch, A.; Braunschweig, T.; et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J. Hepatol. 2017, 67, 749–757. [Google Scholar] [CrossRef]
- Thuwajit, C.; Thuwajit, P.; Jamjantra, P.; Pairojkul, C.; Wongkham, S.; Bhudhisawasdi, V.; Ono, J.; Ohta, S.; Fujimoto, K.; Izuhara, K. Clustering of patients with intrahepatic cholangiocarcinoma based on serum periostin may be predictive of prognosis. Oncol. Lett. 2017, 14, 623–634. [Google Scholar] [CrossRef]
- Kobayashi, S.; Werneburg, N.W.; Bronk, S.F.; Kaufmann, S.H.; Gores, G.J. Interleukin-6 contributes to mcl-1 up-regulation and trail resistance via an akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology 2005, 128, 2054–2065. [Google Scholar] [CrossRef] [PubMed]
- Arbelaiz, A.; Azkargorta, M.; Krawczyk, M.; Santos-Laso, A.; Lapitz, A.; Perugorria, M.J.; Erice, O.; Gonzalez, E.; Jimenez-Aguero, R.; Lacasta, A.; et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017, 66, 1125–1143. [Google Scholar] [CrossRef]
- Banales, J.M.; Inarrairaegui, M.; Arbelaiz, A.; Milkiewicz, P.; Muntane, J.; Munoz-Bellvis, L.; La Casta, A.; Gonzalez, L.M.; Arretxe, E.; Alonso, C.; et al. Serum metabolites as diagnostic biomarkers for cholangiocarcinoma, hepatocellular carcinoma, and primary sclerosing cholangitis. Hepatology 2019, 70, 547–562. [Google Scholar] [CrossRef]
- Razumilava, N.; Gleeson, F.C.; Gores, G.J. Awareness of tract seeding with endoscopic ultrasound tissue acquisition in perihilar cholangiocarcinoma. Am. J. Gastroenterol. 2015, 110, 200. [Google Scholar] [CrossRef] [PubMed]
- Pelsang, R.E.; Johlin, F.C. A percutaneous biopsy technique for patients with suspected biliary or pancreatic cancer without a radiographic mass. Abdom. Imaging 1997, 22, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klumpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary tract cancer: Esmo clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Komuta, M.; Govaere, O.; Vandecaveye, V.; Akiba, J.; Van Steenbergen, W.; Verslype, C.; Laleman, W.; Pirenne, J.; Aerts, R.; Yano, H.; et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology 2012, 55, 1876–1888. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, Y.K.; Park, M.J.; Lee, M.H.; Kim, S.H.; Lee, W.J.; Rhim, H.C. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced mri. J. Magn. Reson. Imaging 2012, 36, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.S.; Kim, Y.K.; Lee, M.W.; Kim, S.H.; Lee, W.J.; Rhim, H.C.; Lee, S.J. Differentiating mass-forming intrahepatic cholangiocarcinoma from atypical hepatocellular carcinoma using gadoxetic acid-enhanced mri. Clin. Radiol. 2012, 67, 766–773. [Google Scholar] [CrossRef]
- Weber, S.M.; Ribero, D.; O’Reilly, E.M.; Kokudo, N.; Miyazaki, M.; Pawlik, T.M. Intrahepatic cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 669–680. [Google Scholar] [CrossRef]
- Claessen, M.M.; Vleggaar, F.P.; Tytgat, K.M.; Siersema, P.D.; van Buuren, H.R. High lifetime risk of cancer in primary sclerosing cholangitis. J. Hepatol. 2009, 50, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J.; American Association for the Study of Liver, D. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef]
- Tischendorf, J.J.; Hecker, H.; Kruger, M.; Manns, M.P.; Meier, P.N. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am. J. Gastroenterol. 2007, 102, 107–114. [Google Scholar] [CrossRef]
- Levy, C.; Lymp, J.; Angulo, P.; Gores, G.J.; Larusso, N.; Lindor, K.D. The value of serum ca 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig. Dis. Sci. 2005, 50, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Moreno Luna, L.E.; Kipp, B.; Halling, K.C.; Sebo, T.J.; Kremers, W.K.; Roberts, L.R.; Barr Fritcher, E.G.; Levy, M.J.; Gores, G.J. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 2006, 131, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Boberg, K.M.; Jebsen, P.; Clausen, O.P.; Foss, A.; Aabakken, L.; Schrumpf, E. Diagnostic benefit of biliary brush cytology in cholangiocarcinoma in primary sclerosing cholangitis. J. Hepatol. 2006, 45, 568–574. [Google Scholar] [CrossRef] [PubMed]
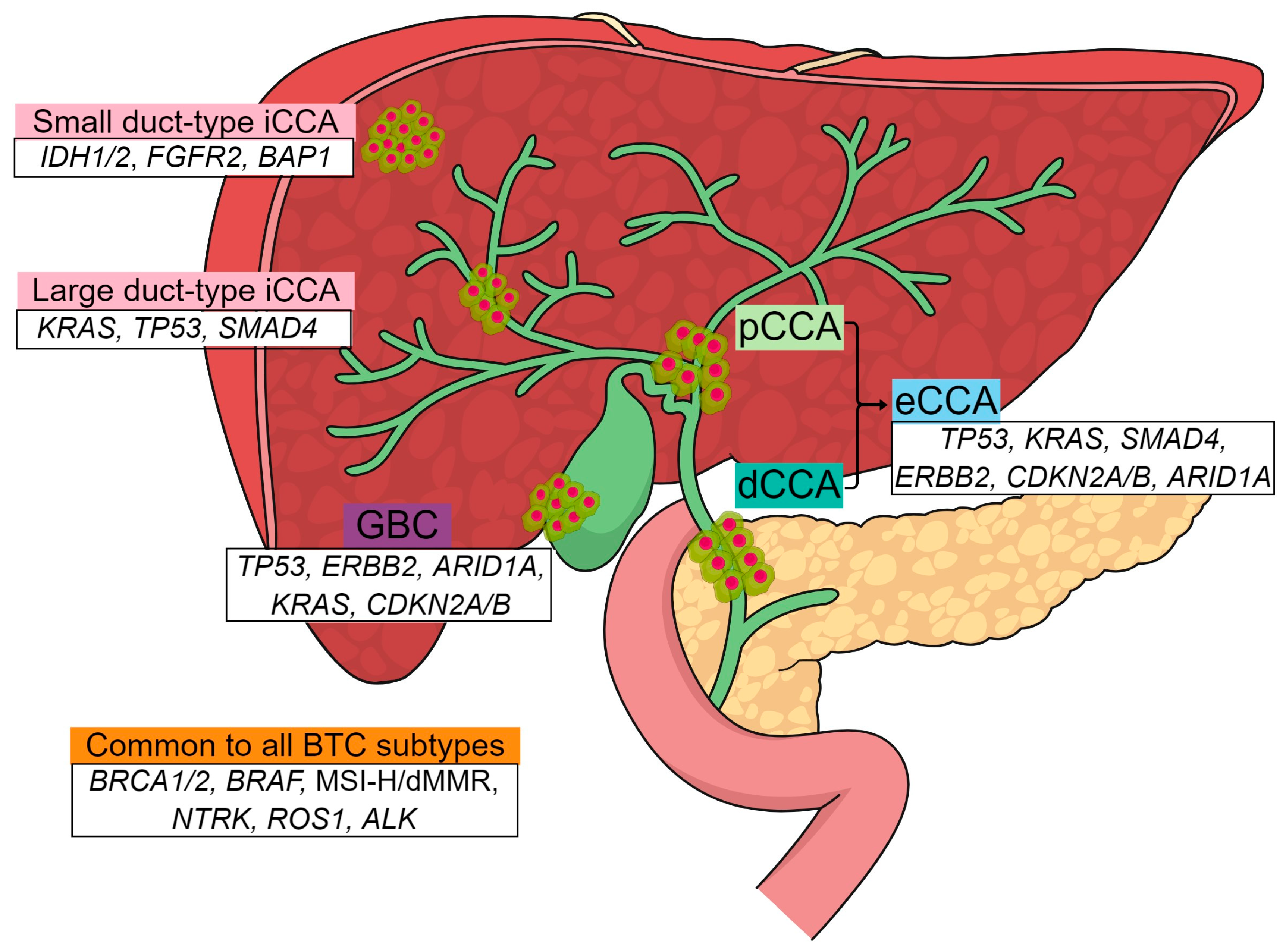
| Cholangiocarcinoma Type | Growth Pattern | Precancerous Lesion | Main Etiology |
|---|---|---|---|
| iCCA—small-duct type | Mass forming | None | Chronic hepatitis Cirrhosis |
| iCCA—large-duct type | Periductal infiltrating | BilIN | Hepatolithiasis Liver flukes PSC |
| Intraductal growing | IPNB, MCN, and ITNB | ||
| pCCA—dCCA | Flat or nodular sclerosing | BilIN | |
| Intraductal papillary | IPNB, MCN, and ITNB |
| Reference | Tumor Type | n | Classification | Molecular Characteristics and Prognosis |
|---|---|---|---|---|
| Sia et al. [17] | iCCA | 149 | Inflammation class | Activation of inflammatory signaling pathways Overexpression of IL-4 and IL-10 (Th2 marker) Favorable prognosis |
| Proliferation class | Activation of oncogenic signaling pathways Overexpression of EGF, RAS, AKT, and MET Worse prognosis | |||
| Andersen et al. [18] | Cholangiocarcinoma | 104 | Cluster 1 | No KRAS mutation Absence or weak expression of HER2 and MET Good prognosis |
| Cluster 2 | Enriched VEGF/ERBB, CTNNB1/MYC, and KRAS mutations Poor prognosis | |||
| Farshidfar et al. [36] | Cholangiocarcinoma | 32 | IDH-mutant cluster * | IDH1/2 mutation Elevated mitochondrial gene expression Loss of function of ARID1A and PBRM1 |
| CCND1 amplification cluster * | Highly hypermethylated | |||
| BAP1/FGFR cluster * | BAP1 mutation or FGFR2 fusion | |||
| Jusakul et al. [19] | Cholangiocarcinoma | 69 | Cluster 1 | ARID1A, BRCA1/2, and TP53 mutations ERBB2 amplification CpG island hypermethylation |
| Cluster 2 | Enriched in TP53 mutations High expressions of CTNNB1, WNT5B and AKT1 | |||
| Cluster 3 | High CNA burden Enriched immune-related pathways | |||
| Cluster 4 | BAP1 or IDH1/2 mutation High expression of FGFR family proteins CpG shore hypermethylation Favorable prognosis | |||
| Job et al. [37] | iCCA | 78 | Immune desert subtype | Minimal expression of all TME signatures |
| Immunogenic subtype | High adaptive immune cell presence Strong activation of fibroblasts and inflammatory and immune checkpoint pathways Best prognosis | |||
| Myeloid-rich subtype | Strong monocyte-derived myeloid cell signatures Weak lymphoid signatures | |||
| Mesenchymal subtype | Strong activation of fibroblast signatures Worst prognosis | |||
| Dong et al. [38] | iCCA | 262 | S1 | Enriched KRAS mutations Upregulated inflammatory pathways and immunosuppressive TME signature Worst prognosis |
| S2 | High expression of proteins related to CAFs and ECM (FAP, POSTN, and FLT1) | |||
| S3 | Enriched in TP53 mutations Upregulated pathways of cell cycle and MAPK signaling | |||
| S4 | FGFR2 alterations, and BAP1 and IDH1/2 mutations High expression of adhesion and biliary-specific proteins (ANXA4, KRT18, and EPCAM) Best prognosis | |||
| Martin-Serrano et al. [39] | iCCA | 122 | Immune classical | High infiltration of immune cells (type-1 IFN) Enriched in TP53 mutations alone Elevated metabolic-related pathways |
| Inflammatory stroma ** | Abundance of stromal deposition, TGFβ signaling, and T cell exhaustion Enriched KRAS mutations alone | |||
| Hepatic stem-like | High M2-like macrophage levels in TME FGFR2 alterations, and BAP1 and IDH1/2 mutations Elevated stemness-related pathways (NOTCH and YAP1) | |||
| Tumor classical ** | Enriched in TP53 mutations alone and co-occurrence of TP53 and KRAS mutations High expression of cholangiocyte markers | |||
| Desert-like | Scarce immune infiltration and abundance of Tregs in TME Enriched in TP53 mutations alone Enriched in mitotic spindles and WNT/β-catenin signaling | |||
| Cho et al. [40] | iCCA | 102 | Metabolism | IDH1 and BAP1 mutations Favorable prognosis |
| Stem-like | High expression of ALDH1A1 and ALDH families | |||
| Poorly immunogenic | TP53 and KRAS mutations Poor prognosis |
| Biomarkers | n | ROC-AUC | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|
| Exosomal cargoes | |||||
| MicroRNA (miR-191, miR-486-3p, miR-1274b, miR-16, and miR-484) | 96 | 0.67 | 0.69 | [95] | |
| MicroRNA (miR-483-5p, and miR-126-3p) | 92 | 0.81, 0.74 | 0.811, 0.73 | 0.811, 0.865 | [96] |
| MicroRNA (miR-141-3p, miR-200a-3p, miR-200c-3p, miR-200b-3p, and ENST00000588480.1) | 100 | 0.757~0.869 | 0.63~0.83 | 0.6~0.867 | [97] |
| LncRNA (ENST00000588480.1 and ENST00000517758.1) | 91 | 0.709 | 0.829 | 0.589 | [98] |
| Circle-RNA (circ-CCAC1) | 316 | 0.857 | [89] | ||
| Protein (claudin-3/CLDN3) | 20 | 0.945 | 0.875 | 0.875 | [99] |
| DNA | 20 | 0.667 | 0.33 | 1 | [100] |
| KRAS mutation | 115 | 0.25 | 0.96 | [101] | |
| KRAS mutation | 46 | 0.738 | 0.476 | 1 | [102] |
| KRAS mutation | 43 | 0.742 | 0.526 | 0.958 | [103] |
| KRAS mutation and TP53 mutation | 109 | 0.564/0.508 | 0.279/0.047 | 0.848/0.970 | [104] |
| KRAS mutation and TP53 mutation | 50 | 0.783, 0.750 | 0.567, 0.5 | 1, 1 | [105] |
| KRAS mutation and TP53 mutation | 49 | 0.733 | 0.467 | 1 | [106] |
| TP53, ERBB2, and KRAS | 42 | 0.955 | 0.909 | 1 | [102] |
| KRAS, TP53, CDKN2A, SMAD4, and BRAF | 60 | 0.737/0.715 | 0.536/0.462 | 0.937/0.969 | [107] |
| Promotor methylation INK4a/ARF | 243 | 0.84~0.98 | 0.67~0.96 | 0.93~0.98 | [92] |
| Promotor methylation of COD1, CNRIP1, SEPT9, and VIM | 80 | 0.775 | 0.773 | 0.778 | [108] |
| Methylation of DKK3, p16, SFRP2, DKK2, NPTX2, and ppENK | 125 | 0.71~0.83 | 0.94 | [109] | |
| CCND2, CDH13, GRIN2B, RUNX3, and TWIST1 | 241 | 0.92 | 0.98 | [110] | |
| Gene mutations in KRAS, TP53, SMAD4, and CDNK2A; methylation changes in SOX17, 3-OST-2, NXPH1, SEPT9, and TERT | |||||
| 150 tumor-related genes (widely targeted deep sequencing) | 10 | 0.947 | 0.999 | [111] | |
| 520 tumor-related genes (widely targeted deep sequencing) | 28 | 0.955 | [112] | ||
| RNA | |||||
| Human telomerase reverse transcriptase mRNA | 20 | 0.833 | 1 | [113] | |
| miR-9, miR-145, and miR-944 | 18 | 0.765~0.975 | [114] | ||
| RNU2-1f | 23 | 0.856 | 0.67 | 0.91 | [115] |
| miR-412, miR640, miR-1537, and miR-3189 | 83 | 0.78~0.81 | 0.5~0.67 | 0.89~0.92 | [116] |
| miR-30d-5 and miR-92a-3p | 106 | 0.730, 0.652 | 0.811, 0.657 | 0.605, 0.667 | [117] |
| Protein | |||||
| CEACAM6 | 73 | 0.74 | 87.5 | 69.1 | [118] |
| CEACAM6 | 41 | 0.92 | 83.3 | 93.1 | [119] |
| SVV and CA199 | 102 | 0.78, 0.75 | 67.3, 96.4 | 80.9, 46.7 | [120] |
| MUC1 | 68 | 0.85 | 90.0 | 76.3 | [121] |
| MUC4 | 134 | 27 | 93 | [122] | |
| MUC5AC | 46 | 0.85 | 75 | 76.9 | [123] |
| Mac-2BP | 78 | 0.70 | 69 | 67 | [124] |
| VEGF | 53 | 0.89 | 99.3 | 88.9 | [125] |
| MCM2 and MCM5 | 42 | 0.80 | [126] | ||
| HSP27 and HSP70 | 20 | 0.86, 0.81 | 90, 80 | 90, 80 | [127] |
| SSP411 | 67 | 0.913 * | 90.0 | 83.3 | [128] |
| NGAL | 40 | 0.74 | 77.3 | 77.2 | [129] |
| NGAL | 38 | 0.76 | 94 | 55 | [130] |
| LCN2/NGAL | 144 | 0.81 | 87 | 75 | [131] |
| S100P | 24 | 0.861 | 92.9 | 70 | [132] |
| sB7-H3 | 323 | 0.878 | 81.2 | 81.6 | [93] |
| α-1-antitrypsin | 8 | 0.833 | 80 | 75 | [133] |
| Amylase | 239 | 0.751 | 66 | 74 | [134] |
| PE-3B/amylase | 68 | 0.877 | 81.8 | 89.3 | [135] |
| M2-PK | 167 | 90.3 | 84.3 | [136] | |
| GSH, hydrogen peroxide, GPx, Fe2+, and FNTA | 46 | 0.683~0.852 | 67.9~100 | 52.9~76.5 | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Song, S.Y. Recent Advancement in Diagnosis of Biliary Tract Cancer through Pathological and Molecular Classifications. Cancers 2024, 16, 1761. https://doi.org/10.3390/cancers16091761
Lee S-H, Song SY. Recent Advancement in Diagnosis of Biliary Tract Cancer through Pathological and Molecular Classifications. Cancers. 2024; 16(9):1761. https://doi.org/10.3390/cancers16091761
Chicago/Turabian StyleLee, Sang-Hoon, and Si Young Song. 2024. "Recent Advancement in Diagnosis of Biliary Tract Cancer through Pathological and Molecular Classifications" Cancers 16, no. 9: 1761. https://doi.org/10.3390/cancers16091761








