Endometrial Serous Carcinoma: Its Molecular Characteristics and Histology-Specific Treatment Strategies
Abstract
:1. Introduction
2. Clinical Characteristics of Endometrial Serous Carcinoma (ESC)
3. Pathological and Molecular Features of ESC
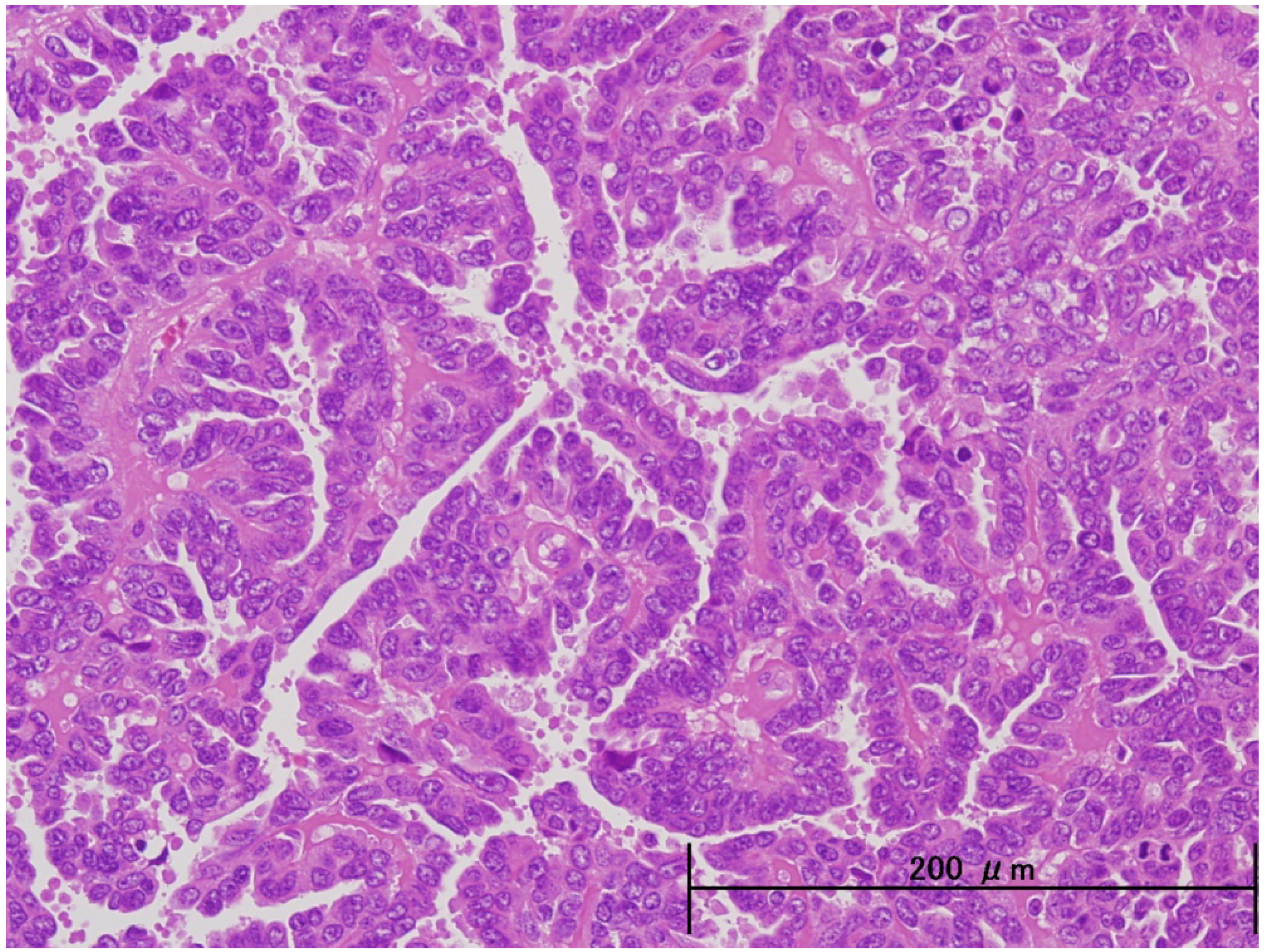
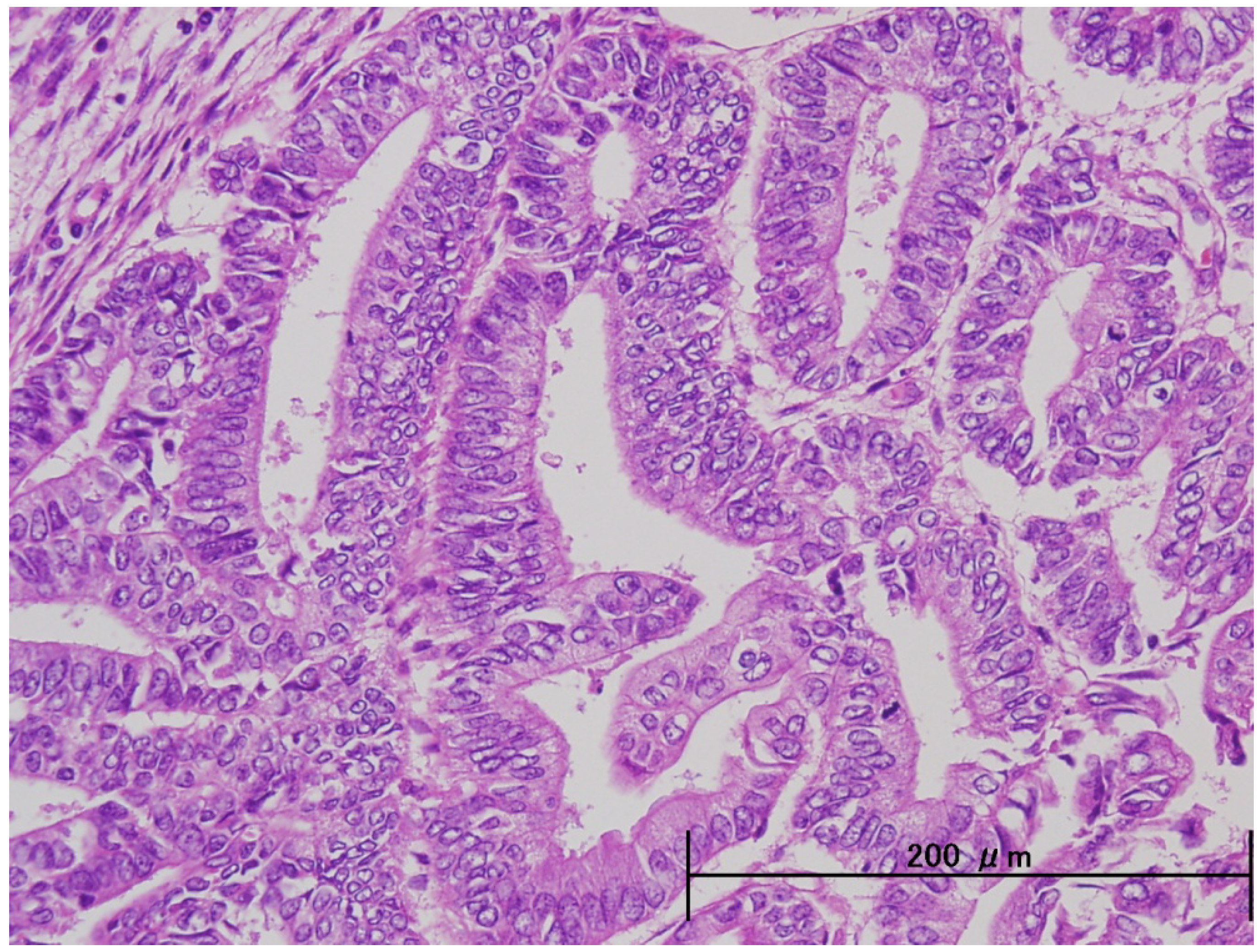
| Endometrioid | Serous | |
|---|---|---|
| Precursor lesion | Atypical hyperplasis | Endometrial intraepithelial carcinoma(EIC) |
| Estrogen | Dependent | Independent |
| Age | 50–60 years | Over 60 years |
| Menopause | Peri menopause | Post menopausse |
| Obesity | Positive | Negative |
| History of Gravidity and Parity | Non | Multi |
| Grade | Low | High |
| Myometrial invasion | Slight | Deep |
| Pattern of recurrence | Local | Distant |
| Stage of presentation | I (73%) | I (54%) |
| II (11%) | II (8%) | |
| III (13%) | III (22%) | |
| IV (3%) | IV (16%) | |
| Survival by stage | I (85–90%) | I (50–80%) |
| II (70%) | II (50%) | |
| III (40–50%) | III (20%) | |
| IV (15–20%) | IV (5–10%) |
| Endometrioid | Serous | |
|---|---|---|
| Microsatellite instability | 30% | 0–5% |
| p53 mutation | 10% | 90% |
| K-ras mutation | 20–40% | 0–5% |
| ER/PR expression | 70–80% | 5% |
| Her-2 amplification/overexpression | 15–20% | 18–45% |
| PTEN mutation | 40–50% | 10% |
| b-catenin mutation | 30% | 0–5% |
| CCNE1 amplification | 0–5% | 30% |
| ARID1A mutation | 57% | 0% |
| PPP2R1A mutation | 5% | 41% |
| PIK3CA mutation | 40% | 15% |
4. Therapeutic Strategies for ESC
4.1. Surgical Treatment
4.2. Chemotherapy
| Regimen | Response rate |
|---|---|
| AP (Adriamycin + Cisplatin) | 42% |
| AT (Adriamycin + Paclitaxel) | 37% |
| CAP (Cyclophsophamide + Adriamycin + Cisplatin) | 18–27% |
| TAP (Paclitaxel + Adriamycin + Cisplatin) | 50% |
| Paclitaxel + Platinum agent | 50–60% |
4.3. Combination of Chemotherapy and Radiation Therapy
5. Conclusions
Conflict of Interest
References
- Bokhaman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- JSOG. Annual report of endometrial cancer, 2008. Acta Obstet. Gynaecol. Jpn. 2010, 62, 853–876.
- Hamilton, C.A.; Cheung, M.K.; Osann, K.; Chen, L.; Teng, N.N.; Longacre, T.A.; Powell, M.A.; Hendrickson, M.R.; Kapp, D.S.; Chan, J.K. Uterinepapillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br. J. Cancer 2006, 94, 642–646. [Google Scholar]
- Slomovitz, B.M.; Burke, T.W.; Eifel, P.J.; Ramondetta, L.M.; Silva, E.G.; Jhingran, A.; Oh, J.C.; Atkinson, E.N.; Broaddus, R.R.; Gershenson, D.M.; et al. Uterine papillary serous carcinoma (UPSC): A single institution review of 129 cases. Gynecol. Oncol. 2003, 91, 463–469. [Google Scholar] [CrossRef]
- Chan, J.K.; Loizzi, V.; Youssef, M.; Osann, K.; Rutgers, J.; Vasilev, S.A.; Berman, M.L. Significance of comprehensive surgical staging in noninvasive papillary serous carcinoma of the endometrium. Gynecol. Oncol. 2003, 90, 181–185. [Google Scholar] [CrossRef]
- Gehrig, P.A.; Groben, P.A.; Fowler, W.C., Jr.; Walton, L.A.; van Le, L. Noninvasive papillary serous carcinoma of the endometrium. Obstet. Gynecol. 2001, 97, 153–157. [Google Scholar] [CrossRef]
- Goff, B.A.; Kato, D.; Schmidt, R.A.; Ek, M.; Ferry, J.A.; Muntz, H.G.; Cain, J.M.; Tamimi, H.K.; Figge, D.C.; Greer, B.E. Uterine papillary serous carcinoma: Patterns of metastatic spread. Gynecol. Oncol. 1994, 54, 264–268. [Google Scholar] [CrossRef]
- Hui, P.; Kelly, M.; O’Malley, D.M. Minimal uterine serous carcinoma: A clinicopathological study of 40 cases. Mod. Pathol. 2005, 18, 75–82. [Google Scholar] [CrossRef]
- Boruta, D.M., 2nd.; Gehrig, P.A.; Groben, P.A.; Bae-Jump, V.; Boggess, J.F.; Fowler, W.C., Jr.; van Le, L. Uterine serous and grade 3 endometrioid carcinomas: Is there a survival difference? Cancer 2004, 101, 2214–2221. [Google Scholar] [CrossRef]
- Mutter, G.L. Endometrial intraepithelial neoplasia (EIN): Will it bringorder to chaos? The Endometrial Collaborative Group. Gynecol. Oncol. 2000, 76, 287–290. [Google Scholar] [CrossRef]
- Zheng, W.; Xiang, L.; Fadare, O.; Kong, B. A proposed model for endometrial serous carcinogenesis. Am. J. Surg. Pathol. 2011, 35, e1–e14. [Google Scholar] [CrossRef]
- Reid-Nicholson, M.; Iyengar, P.; Hummer, A.J.; Linkov, I.; Asher, M.; Soslow, R.A. Immunophenotypic diversity of endometrial adenocarcinomas: Implications for differential diagnosis. Mod. Pathol. 2006, 8, 1091–1100. [Google Scholar]
- Oliva, E. Unusual Variants of Endometrial Carcinoma: Histologic and Molecular Aspects; American Society of Clinical Oncology: Alexandria, VA, USA, 2004; pp. 303–308. [Google Scholar]
- Kuhn, E.; Wu, R.C.; Wu, G.; Guan, B.; Zhang, J.; Wang, Y.; Song, L.; Yuan, X.; Wei, L.; Roden, R.B.; et al. Genome-wide analyses of uterine serous carcinoma identify pathway aberrations involving cyclinE-Fbxw7, PI3K and p53. J. Natl. Cancer Inst. 2012, in press. [Google Scholar]
- Santin, A.D.; Bellone, S.; Gokden, M.; Palmieri, M.; Dunn, D.; Agha, J.; Roman, J.J.; Hutchins, L.; Pecorelli, S.; O’Brien, T.; et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin. Cancer Res. 2002, 8, 1271–1279. [Google Scholar]
- Santin, A.D.; Bellone, S.; Siegel, E.R.; Palmieri, M.; Thomas, M.; Cannon, M.J.; Kay, H.H.; Roman, J.J.; Burnett, A.; Pecorelli, S. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): A major prognostic indicator in uterine serous papillary cancer. Am. J. Obstet. Gynecol. 2005, 192, 813–818. [Google Scholar] [CrossRef]
- Villella, J.A.; Cohen, S.; Smith, D.H.; Hibshoosh, H.; Hershman, D. HER-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. Int. J. Gynecol. Cancer 2006, 16, 1897–1902. [Google Scholar] [CrossRef]
- Hayes, M.P.; Ellenson, L.H. Molecular alterations in uterine serous carcinoma. Gynecol. Oncol. 2010, 116, 286–289. [Google Scholar] [CrossRef]
- Jones, S.; Wang, T.L.; Shih, I.M.; Mao, T.L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A., Jr.; Vogelstein, B.; et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010, 6001, 228–231. [Google Scholar]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010, 16, 1532–1543. [Google Scholar]
- Guan, B.; Mao, T.L.; Panuganti, P.K.; Kuhn, E.; Kurman, R.J.; Maeda, D.; Chen, E.; Jeng, Y.M.; Wang, T.L.; Shih, I.M. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am. J. Surg. Pathol. 2011, 35, 625–632. [Google Scholar] [CrossRef]
- McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E. Subtype-specific mutation of PPP2R1A in endometrial and ovarian carcinomas. J. Pathol. 2011, 223, 567–573. [Google Scholar] [CrossRef]
- Boruta, D.M., 2nd.; Gehrig, P.A.; Fader, A.N.; Olawaiye, A.B. Management of women with uterine papillary serous cancer: A Society of Gynecologic Oncology (SGO) review. Gynecol. Oncol. 2009, 115, 142–153. [Google Scholar] [CrossRef]
- Zheng, W.; Yi, X.; Fadare, O.; Liang, S.X.; Martel, M.; Schwartz, P.E.; Jiang, Z. The oncofetal protein IMP3: A novel biomarker for endometrial serous carcinoma. Am. J. Surg. Pathol. 2008 32, 304–315.
- Bristow, R.E.; Zerbe, M.J.; Rosenshein, N.B.; Grumbine, F.C.; Montz, F.J. Stage IVB endometrial carcinoma: The role of cytoreductive surgery and determinants of survival. Gynecol. Oncol. 2000, 78, 85–91. [Google Scholar] [CrossRef]
- Acharya, S.; Hensley, M.L.; Montag, A.C.; Fleming, G.F. Rare uterine cancers. Lancet Oncol. 2005, 6, 961–971. [Google Scholar] [CrossRef]
- Kelly, M.G.; O’malley, D.M.; Hui, P.; McAlpine, J.; Yu, H.; Rutherford, T.J.; Azodi, M.; Schwartz, P.E. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol. Oncol. 2005, 98, 353–359. [Google Scholar] [CrossRef]
- Levenback, C.; Burke, T.W.; Silva, E.; Morris, M.; Gershenson, D.M.; Kavanagh, J.J.; Wharton, J.T. Uterine papillary serous carcinoma (UPSC)treated with cisplatin, doxorubicin, and cyclophosphamide(PAC). Gynecol. Oncol. 1992, 46, 317–321. [Google Scholar] [CrossRef]
- Price, F.V.; Chambers, S.K.; Carcangiu, M.L.; Kohorn, E.I.; Schwartz, P.E.; Chambers, J.T. Intravenous cisplatin, doxorubicin, and cyclophosphamide in the treatment of uterine papillary serous carcinoma (UPSC). Gynecol. Oncol. 1993, 51, 383–389. [Google Scholar] [CrossRef]
- Hoskins, P.J.; Swenerton, K.D.; Pike, J.A.; Wong, F.; Lim, P.; Acquino-Parsons, C.; Lee, N. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: A phase study. J. Clin. Oncol. 2001, 19, 4048–4053. [Google Scholar]
- Zanotti, K.M.; Belinson, J.L.; Kennedy, A.W.; Webster, K.D.; Markman, M. The use of paclitaxel and platinum-based chemotherapy in uterine papillary serous carcinoma. Gynecol. Oncol. 1999, 74, 272–277. [Google Scholar] [CrossRef]
- Goff, B.A. Uterine papillary serous carcinoma: What have we learned over the past quarter century? Gynecol. Oncol. 2005, 98, 341–343. [Google Scholar] [CrossRef]
- National Cancer Institute. Phase III randomized study of doxorubicin, cisplatin, paclitaxel, and filgrastim (G-CSF) versus carboplatin and paclitaxel in patients with stage III or IV or recurrent endometrial cancer. Available online: http://www.cancer.gov/clinicaltrials/search/view?cdrid=305940&version=HealthProfessional/ (accessed on 28 July 2012).
- Sutton, G.; Axelrod, J.H.; Bundy, B.N.; Roy, T.; Homesley, H.; Lee, R.B.; Gehrig, P.A.; Zaino, R. Adjuvant whole abdominal irradiation in clinical stages I and II papillary serous or clear cell carcinoma of the endometrium: A phase II study of the Gynecologic Oncology Group. Gynecol. Oncol. 2006, 100, 349–354. [Google Scholar] [CrossRef]
- Fader, A.N.; Starks, D.; Gehrig, P.A.; Secord, A.A.; Frasure, H.E.; O’Malley, D.M.; Tuller, E.R.; Rose, P.G.; Havrilesky, L.J.; Moore, K.N.; et al. UPSC Consortium. An updated clinicopathologic study of early-stage uterine papillary serous carcinoma (UPSC). Gynecol. Oncol. 2009, 115, 244–248. [Google Scholar] [CrossRef]
- Nakayama, K.; Nagai, Y.; Ishikawa, M.; Aoki, Y.; Miyazaki, K. Concomitant postoperative radiation and chemotherapy following surgery was associated with improved overall survival in patients with FIGO stages III and IV endometrial cancer. Int. J. Clin. Oncol. 2010, 15, 440–446. [Google Scholar] [CrossRef]
- Alvarez Secord, A.; Havrilesky, L.J.; Bae-Jump, V.; Chin, J.; Calingaert, B.; Bland, A.; Rutledge, T.L.; Berchuck, A.; Clarke-Pearson, D.L.; Gehrig, P.A. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol. Oncol. 2007, 107, 285–291. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nakayama, K.; Nakayama, N.; Ishikawa, M.; Miyazaki, K. Endometrial Serous Carcinoma: Its Molecular Characteristics and Histology-Specific Treatment Strategies. Cancers 2012, 4, 799-807. https://doi.org/10.3390/cancers4030799
Nakayama K, Nakayama N, Ishikawa M, Miyazaki K. Endometrial Serous Carcinoma: Its Molecular Characteristics and Histology-Specific Treatment Strategies. Cancers. 2012; 4(3):799-807. https://doi.org/10.3390/cancers4030799
Chicago/Turabian StyleNakayama, Kentaro, Naomi Nakayama, Masako Ishikawa, and Kohji Miyazaki. 2012. "Endometrial Serous Carcinoma: Its Molecular Characteristics and Histology-Specific Treatment Strategies" Cancers 4, no. 3: 799-807. https://doi.org/10.3390/cancers4030799




